Abstract
Context/Objectives
The diagnosis of Zollinger-Ellison syndrome (ZES) requires secretin testing in 60%. Even with secretin the diagnosis may be difficult because variable responses occur and 6–30% have negative testing. The basis for variability or negative responses is unclear. It is unknown if the tumor density of secretin receptors or the presence of a secretin-receptor-variant, which can act as a dominant-negative, are important. The aim of this study was to investigate these possibilities.
Patients/Methods
Secretin-receptor and variant mRNA expression was determined in gastrinomas using real-time-PCR from 54 ZES patients. Results were correlated with Western blotting, secretin-receptor immunohistochemistry, with gastrin-provocative-test results and tumoral/clinical/laboratory features.
Results
Secretin-receptor mRNA was detectible in all gastrinomas but varied 132-fold with a mean of 0.89±0.12 molecules/β-actin. Secretin-receptor PCR results correlated closely with Western blotting (r=0.95,p<0.0001) and receptor-immunohistochemistry (p=0.0015, r=0.71). The variant was detected in all gastrinomas but levels varied 102-fold and were 72-fold lower than the total. Secretin-receptor levels correlated with variant levels, Δsecretin, but not Δcalcium and with tumor location, but not growth, extent or clinical responses. Variant levels did not correlate with the Δsecretin. Detailed analysis provides no evidence variant expression modified the secretin-receptor response or accounted for negative tests.
Conclusions
Secretin-receptor and secretin-receptor-variant expression occur in all gastrinomas. Because the expression of the total but not variant correlated with the secretin results and no evidence for dominant negative activity of the variant was found, our results suggest the total-secretin-receptor density is an important determinant of the secretin test response.
Key terms: Zollinger-Ellison syndrome, neuroendocrine tumor, gastrinoma, secretin test
Introduction
The diagnosis of Zollinger-Ellison syndrome(ZES) can be difficult in >60% of patients because their fasting serum gastrin levels overlap with other conditions causing hyperchlorhydria/hypergastrinemia(1). The secretin test has become a mainstay for ZES diagnosis in this group of patients(2–4). Secretin test results in >800 patients are reported(4) and criteria of positivity extensively studied(2–6). However, no studies address the variability of up to a 4000-fold difference in gastrin responses to secretin in different ZES patients(4) or why 20–35% have negative responses(3,4).
A recent case report(7) described one of three patients with ZES whose gastrinoma showed a large amount of a particular secretin-receptor-variant transcript that could function as a dominant negative in transfected cells. It was proposed(7) this effect might account for the patient’s negative secretin test. At present it is unknown whether the abundance of the secretin-receptor, the secretin-receptor-variant or their ratio could be factors in determining the variability of the secretin-stimulated gastrin response in different ZES patients or the occurrence of a positive test. To address these questions we assessed the secretin-receptor, secretin-receptor-variant transcript and their ratio in gastrinomas from 54 ZES patients and correlated these with clinical/laboratory/tumoral features as well as the secretin- and calcium-test responses.
Materials and Methods
Patients, Tumors and Clinical Variables
Fifty-four ZES patients seen at NIH between 1989–2005 were included. The protocol was approved by the Clinical Research Committee-NIDDK and all patients gave informed consent. The diagnosis of ZES and MEN1 were established as previously reported(8–10). MEN1 patients had DNA testing for mutations in the MEN1 gene. Seven patients had mutations: E363del, 672del CC/N and 512delC. Preoperative fasting gastrin, secretin(2 units/kg) and calcium (Ca=5 mg/kg/hX 3 hours) tests were performed (4). Studies assessing basal acid output (BAO), maximal acid output (MAO), and drug control of acid secretion were performed(11). Conventional imaging [CT, MRI, ultrasound], angiography with secretin-stimulation/gastrin sampling and somatostatin receptor scintigraphy(SRS) were performed(12,13) to locate the primary and/or extent. Each patient underwent an exploratory laparotomy for attempted cure(14). Patients were reassessed postoperatively(15,16). Disease-free status and relapse was defined as outlined previously(16,17). In patients not disease-free, annual imaging studies (CT, MRI, ultrasound, SRS) and, if the results were unclear, angiography provided the basis for assessment of tumor growth/progression(18). For each patient the number and size of each measurable tumor were determined by imaging modalities. Tumor growth rate was calculated as the percent volume increase per month(18). Patients were classified as having nonaggressive ZES if there was no growth or <25% increase in volume/month either with or without liver metastases at all yearly evaluations and if initially there was not extensive metastatic disease.
Tumor samples and cDNA preparation
Tumors were snap-frozen and stored at −70°C. Tumor mRNA was extracted from 5-tm cryosections after analyzing an adjacent slide to determine >80% of the section contained tumor(19). Total RNA was extracted utilizing the RNeasy Mini-Kit (Qiagen Inc., Santa Clarita, CA) from two or three adjacent slides. Random hexamer-primed first strand complementary DNA was prepared with RT (RNA PCR Kit; Applied Biosystems, Foster City, CA).
Real-Time PCR
PCR was done using SYBR Green (Applied Biosystems 2X SYBR® GREEN PCR Mater Mix, Warrington, United Kingdom) and Stratagene Mx3000P Multiplex Quantitative PCR Systems (La Jolla, CA.) Primers for both the total and a secretin-receptor-variant (missing exon 3) were selected through analysis of the secretin-receptor mRNA sequence (Genbank accession no. U28281), and were based on the gene structure described by Po-ki Ho (20). The total-secretin-receptor primer (product-201bp) was selected from the 3′end of the mRNA sequence. The sense and anti-sense sequences of the total-secretin-receptor primer were as follows: [sense-5′-CTGGCCAGGTCCACTCTC-3′(nucleotides 1126–1143), antisense-5′-CCATTGCTGCCACTTCTTCT-3′ (nucleotides 1307–1326)]. The secretin-receptor-variant primers (product-118bp) were designed so that the sense primer spanned the end of exon 2 and the beginning of exon 4; [sense-: 5′-CCCGACTATGTGACGTGCTA-3′(nucleotides 194-213), antisense-5′-AACAAGGAACCTGGCACTGG-3′(nucleotides 292-419)]. The abundance of the total-secretin-receptor mRNA was determined in all 54 gastrinomas but because of the low expression of the variant-receptor more concentrated cDNA samples were required in the PCR reaction and sufficient material was available from 35 gastrinomas. The specificity of all the primers was assured by their size and their localization in unique sequences across spliced regions of the gene. The PCR reactions were carried out in a final volume of 25 μL. Patient samples were screened for the expression of β-actin as a reference gene (19). The reaction mixture included the SYBR Green PCR buffer (12.5 μL), H20 (5.5 μL) and 300 nmol/L of the specific primer((6 μL) and was run in duplicate. The PCR was conducted with 40 cycles, which were within the linear amplification range. The PCR began with a cycle of 94 °C for 10 min, followed by 40 cycles of 94 °C for 50 sec(denaturing), 62 °C for 50 sec(annealing), and 72 °C for 50 sec(elongation.) A final extension period of 94 °C for 5 min concluded the amplification. In all experiments, dilutions of standard were included, which was human pancreas cDNA (BioChain Institute, Inc., lot no. A901107, Hayward, CA.)
Western Blot
Pooled tissue from cryosections containing >80% tumor were lysed in lysis buffer (50 mM Tris/HCl pH 7.5, 150 mM NaCl, 1% Triton X-100, 1% deoxycholate, 0.1% sodium azide, 1 mM EGTA, 0.4 mM EDTA, 0.2 mM sodium orthovanadate, 1 mM PMSF, and one protease inhibitor tablet per 10 ml). Equal amounts of total protein were analyzed by SDS-PAGE and Western-blotting using rabbit anti-Secretin-receptor antibody (AbD Serotec, Oxford, UK) or mouse anti-beta actin antibody (SantaCruz Biotechnologies, Santa Cruz, CA). The intensity of the protein bands was measured using Kodak ID-Image Analysis.
Immunohistochemistry(IHC)
Formalin-fixed, paraffin-embedded tissue samples from 26 patients were analyzed by IHC for secretin-receptor and gastrin. Slides were deparaffinized in xylene and re-hydrated in graded alcohol. Antigen-retrieval was achieved by boiling slides in 10 Mm Tris (pH 10.0) and keeping them at sub-boiling temperature for 10 min. Endogenous peroxidase was blocked by incubation in 3% hydrogen peroxide solution for 10 min. Sections were stained using the RTU Vectastain Universal Quick kit and the DAB Substrate Kit (Vector Laboratories, Burlingame, CA) according to the manufacturer’s recommendations. Rabbit anti-gastrin polyclonal antibody (Chemicon, Temecula, Ca) (1:1000) and rabbit anti-Secretin receptor antibody (AbD Serotec, Oxford, UK) (1:50) were used. Slides incubated with an isotype-matched immunoglobulin were used as negative control. Sections were counterstained with Haematoxylin (Vector Laboratories), mounted and analyzed by two independent blinded investigators. Staining intensity for secretin-receptor was graded 0–3 as previously described(19) and the percentage of positive cells was graded 1–4 corresponding to <25%, 25–50%, 50–75% and >75%, so that the maximal score was 7.
Statistical Analysis
Values were expressed as mean±SEM. Discontinuous variables were compared using the Fisher exact test and for continuous variables the Mann-Whitney U test (two variables), the Kruskal-Wallis test or an ANOVA with the Bonferroni Dunn test as a post hoc test. Correlation coefficients were calculated using least-squares analysis. All calculations were performed using the statistical program Statview 5 (SAS Institute, Cary, NC).
Results
The clinical/laboratory characteristics of the 54 ZES patients resemble those in other large series (9,11) in having almost equal frequency in both sexes, mean age in the fifth decade, long-disease duration, elevated fasting serum gastrin level and secretin-stimulated gastrin level and preoperative basal/maximal acid outputs (Table 1). Similar to recent series (9), duodenal primaries were more frequent than pancreatic, and primary tumors in lymph nodes as well as other sites were found (9,17). In approximately one-third of patients the tumor was confined to the primary site. In contrast, 22% of patients developed liver metastases at some time (Table 1). As reported recently, approximately one-third had a gastrinoma with an aggressive postoperative course (16) and approximately 45% of the patients had a long-term cure (15,17).
Table 1.
Clinical characteristics, laboratory values and tumor characteristics in the patients studied
| Characteristic | Number (%) |
|---|---|
| Patients | 54 |
| Male | 26 (48%) |
| Age at surgery (yrs) | |
| Mean ± SE | 49.7 ± 1.4 |
| [Range] | [15–75] |
| Duration onset to surgery (yrs)a | |
| Mean ± SE | 8.6 ± 0.8 |
| [Range] | [0.2–25] |
| Duration of disease (yrs) | |
| Mean ± SE | 16.6 ± 1.0 |
| [Range] | [2.4–37.0] |
| Fasting serum gastrin (pg/ml)b | |
| Mean ± SE | 4900 ± 2200 |
| [Range] | [170–110000] |
| Secretin (pg/ml)c | |
| Mean ± SE | 9800 ± 3700 |
| [Range] | [67–158000] |
| BAO (mEq/hr)d | |
| Mean ± SE | 45.0 ± 3.3 |
| [Range] | [3.6–99.5] |
| MAO (mEq/hr)d | |
| Mean ± SE | 69.4 ± 4.9 |
| [Range] | [18–136] |
| MEN1 present | 9 (17%) |
| Primary tumor locatione | |
| Duodenum | 32 (59%) |
| Pancreas | 8 (15%) |
| Lymph nodef | 7 (13%) |
| Othersg | 5 (9%) |
| Unknown | 3 (6%) |
| Tumor extent at surgeryh | |
| Primary only | 17 (31%) |
| Primary + lymph node | 36 (67%) |
| Metastatic lymph node onlyi | 3 (6%) |
| Liver metastases any time | 12 (22%) |
| Aggressive diseasej | 17 (31%) |
| Postoperative cure | |
| Immediately | 34 (63%) |
| Last follow-up | 24 (44%) |
Duration of disease was defined as the time from onset of continuous symptoms attributable to Zollinger-Ellison syndrome until surgery as described previously (4,16).
Fasting serum gastrin was determined preoperatively.
ΔSecretin was determined preoperatively (n=48) as the increase in fasting serum gastrin (pg/ml) with bolus secretin injection (2 clinical units/Kg) over the preinjection level (4).
BAO and MAO were determined preoperatively (11). Shown are the BAO and MAO from patients without previous gastric acid-reducing surgery [BAO (n=48), MAO (n=38)].
One patient had two primary tumor locations, a duodenal and pancreatic primary.
A lymph node primary was as defined previously with only a gastrinoma in a lymph node found at surgery and the patient was disease-free (22,47).
Other primary tumors include: liver (n=1); bile duct (n=2); nonsmall cell lung cancer (n=1); and omentum (n=1).
Each patient is in only one of the three categories.
Metastatic lymph node only was defined as finding gastrinoma in lymph node(s) without a primary tumor and the patient was not disease-free
Aggressive disease refers to patients postresection in whom new lesions, liver metastases or tumor growth on imaging developed as defined in Methods.
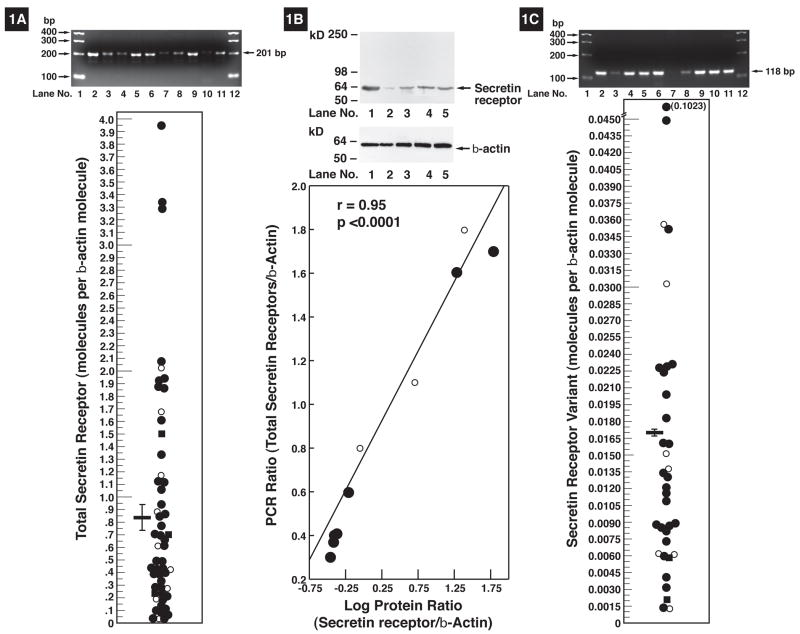
Total-secretin-receptor mRNA abundance varied greatly in different gastrinomas (Fig. 1), however, it was detectible in all(Fig. 1A). Control studies demonstrated that the PCR products resulted from tumor mRNA because total-secretin-receptor primers spanned a long intron and gave no amplification with genomic DNA. The total-secretin mRNA levels varied 132-fold, from 0.03 to 3.95 molecules/β-actin molecule(Fig. 1A) with a mean of 0.89 ± 0.12/β-actin molecule(Fig. 1A).
Figure 1.
Secretin-receptor mRNA expression in gastrinomas. (Left panel). Total secretin-receptor mRNA expression. (Top left panel) PCR result for total-secretin-receptor mRNA expression in gastrinomas of 10 patients showing variable amounts of the 201 bp PCR product. (left bottom panel). Distribution of the total-secretin-receptor mRNA expression in gastrinomas from 54 patients with ZES. All values are expressed as the number of total-secretin-receptor molecules per ~β-actin molecules in the gastrinoma. Each point is the mean value from one patient’s gastrinoma. All patient gastrinoma values are the mean of at least three determinations. The horizontal and vertical lines indicate the mean ± SE for all 54 patients (0.89 ± 0.12). (Middle panel). Secretin protein expression in gastrinomas. (Top panel). Western plot of secretin receptor protein expression in 5 gastrinomas. (Bottom). Correlation of secretin protein expression and total-secretin-receptor mRNA assessed by PCR from 10 gastrinomas. Results are expressed as the amount present compared to β-actin. (Right panel). Secretin-receptor-variant mRNA expression in gastrinomas. (Top panel) PCR result for secretin-receptor-variant mRNA expression in gastrinomas from 10 patients showing the variable amounts of the 118 bp PCR product. (Bottom panel). Distribution of the secretin-receptor-variant mRNA expression in gastrinomas from 35 patients with ZES. All values are expressed as the number of secretin-receptor-variant molecules per ~ β-actin molecules in the gastrinoma determined as described in Methods. Each point is the mean value from one patient’s gastrinoma. All patient values are the mean of at least three PCR determinations. The horizontal and vertical lines indicate the mean ± SE for all patients (0.017 ± 0.003). The different symbols indicate the source of the tumor analyzed in each case. Symbols: ●Primary-sporadic, ○Primary-MEN1, ■LN-sporadic, □LN-MEN1, ▲Liver metastases
Because only small amounts of tumor were available from most patients, a PCR analysis was the only means able to assess secretin-receptor abundance in most patients. To be certain the secretin-receptor mRNA expression correlated with secretin-receptor protein expression, the results of PCR and Western blotting were compared in 10 patients with sufficient tissue to perform each. (Fig. 1B). The results of the two analyses demonstrated a very close correlation(r=0.95, p<0.0001). To further confirm this association, immunohistochemistry (IHC) for secretin-receptor and gastrin was performed in 26 gastrinomas where formalin-fixed paraffin-embedded tissue samples were available. All tumors showed positive staining for secretin receptor. The vast majority of cells (>75%) staining positive for gastrin also showed expression of secretin-receptor, with only two tumors showing <50% of tumor cells expressing secretin-receptor. The average secretin-receptor score was 5.5 (range:3–7) and we found a highly significant (r=0.71, p=0.0015) correlation between the IHC-score and the total-secretin-receptor mRNA expression.
To assess variant-receptor mRNA expression specific primers were designed that would only produce a product if exon 3 was deleted. Control studies demonstrated this primer set produced a single 118 bp amplification product, which was seen only in tissues known to contain the secretin-receptor (3 separate pancreatic cDNA’s) and not in tissues, which do not contain secretin receptors (placenta, lung cancer). Secretin-receptor-variant expression was detected in all gastrinomas with its levels varying 102-fold from 0.001 to 0.102 molecules/β-actin(Fig. 1C) with a mean±SEM level of 0.017 ±0.003(Fig. 1C). For the different gastrinomas the total-secretin-receptor had a 72 ±12 –fold greater level of expression than the secretin-receptor-variant with levels ranging from 10.5-fold to 301-fold. The total-secretin-receptor expression level showed a highly significant (r=0.65, p<0.0001) direct correlation with the level of the secretin-receptor-variant(Fig. 2D1).
Increased age (21), MEN1 (16), short-disease duration (9,16), female gender (21), or high hormone levels or its effects (i.e., in gastrinomas –BAO, MAO) (21) are associated with a poor prognosis in gastrinomas and/or neuroendocrine tumors. However, none of these characteristics correlated with the magnitude of either the total or variant-secretin-receptor expression or their ratio (Table 2). Similarly, other clinical factors such as race or need for previous gastric-acid reducing surgery, which was frequently performed in patients with more severe disease, did not correlated with the expression levels of the total and secretin-receptor-variant or their ratio (Table 2). However, a history of ulcer disease correlated with the magnitude of the variant, but not the magnitude of the total-secretin-receptor or variant/total ratio(Table 2).
Table 2.
Effect of various demographic, clinical and laboratory features on total-secretin-receptor and variant secretin-receptor mRNA and their ratio.
| Total-secretin-receptor (molecules per β-actin)1,2 | Secretin-receptor-variant (molecules per β-actin)1,2 | Secretin-receptor-variant/Total Ratio2 | ||||
|---|---|---|---|---|---|---|
| Variable3 | Variable present | Variable absent | Variable present | Variable absent | Variable present | Variable absent |
| Male gender | 0.69±0.13 | 0.97±0.18 | 0.019±0.002 | 0.015±0.002 | 0.030±0.005 | 0.021±0.003 |
| MEN1 present | 0.81±0.23 | 0.84±0.13 | 0.015±0.005 | 0.017±0.004 | 0.019±0.004 | 0.027±0.009 |
| Age (yrs) | ||||||
| Diagnosis>47 | 0.74±0.18 | 0.93±0.13 | 0.017±0.006 | 0.017±0.002 | 0.031±0.005 | 0.020±0.004 |
| Onset>42 | 0.77±0.19 | 0.89±0.12 | 0.018±0.006 | 0.016±0.003 | 0.032±0.005 | 0.020±0.004 |
| Surgery>51 | 0.86±0.20 | 0.81±0.11 | 0.018±0.006 | 0.015±0.002 | 0.030±0.005 | 0.021±0.009 |
| Caucasian race | 0.83±0.13 | 0.83±0.20 | 0.017±0.001 | 0.017±0.001 | 0.024±0.003 | 0.032±0.008 |
| Disease duration (yrs) | ||||||
| Onset to 1st evaluation>5 | 0.65±0.10 | 1.01±0.19 | 0.015±0.002 | 0.019±0.006 | 0.027±0.005 | 0.024±0.009 |
| Onset to diagnosis>3 | 0.77±0.19 | 0.89±0.12 | 0.016±0.002 | 0.018±0.006 | 0.031±0.005 | 0.020±0.003 |
| Ulcer History | 0.78±0.13 | 0.96±0.21 | 0.021±0.004 | 0.008±0.001** | 0.030±0.004 | 0.016±0.003 |
| BAO>35 mEq/h4 | 0.94±0.18 | 0.59±0.13 | 0.019±0.006 | 0.013±0.004 | 0.026±0.004 | 0.027±0.007 |
| MAO>51 mEq/h4 | 0.83±0.17 | 0.73±0.18 | 0.020±0.006 | 0.012±0.005 | 0.024±0.004 | 0.030±0.009 |
| Fasting gastrin>720 pg/ml | 0.72±0.12 | 0.94±0.18 | 0.012±0.002 | 0.020±0.006 | 0.023±0.004 | 0.029±0.005 |
| Previous acid-reducing5 surgery | 0.94±0.30 | 0.82±0.12 | 0.014±0.001 | 0.017±0.003 | 0.020±0.004 | 0.027±0.003 |
Total-secretin or variant secretin-receptor mRNA expressed was determined by rt-PCR as described in Methods and expressed per molecule of β-actin in the gastrinoma.
Total-secretin-receptor data are from 54 gastrinomas, secretin-receptor-variant and ratio from 35 gastrinomas.
The median value of the individual variable was used to divide the total patient group.
BAO (n=47) and MAO (n=38) from patients without previous gastric acid-reducing surgery were analyzed.
(p<0.02)
Five patients had previous acid-reducing surgery [(vagotomy/drainage (n=2) and partial gastrectomy (n=3)].
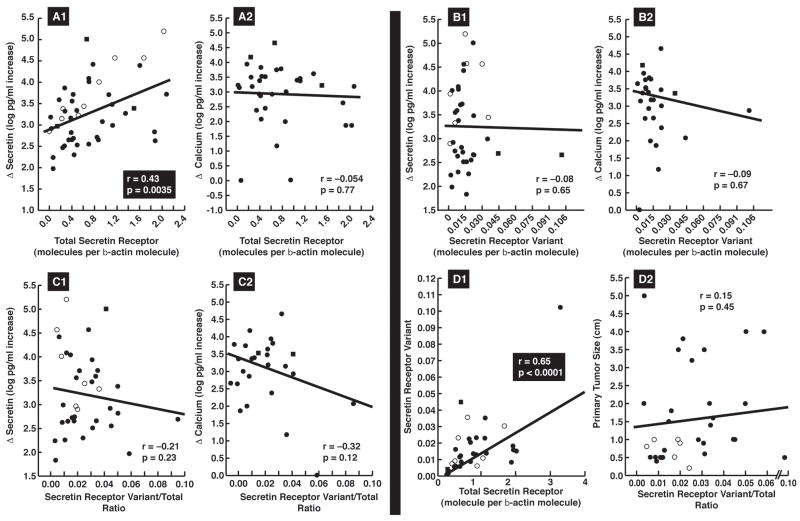
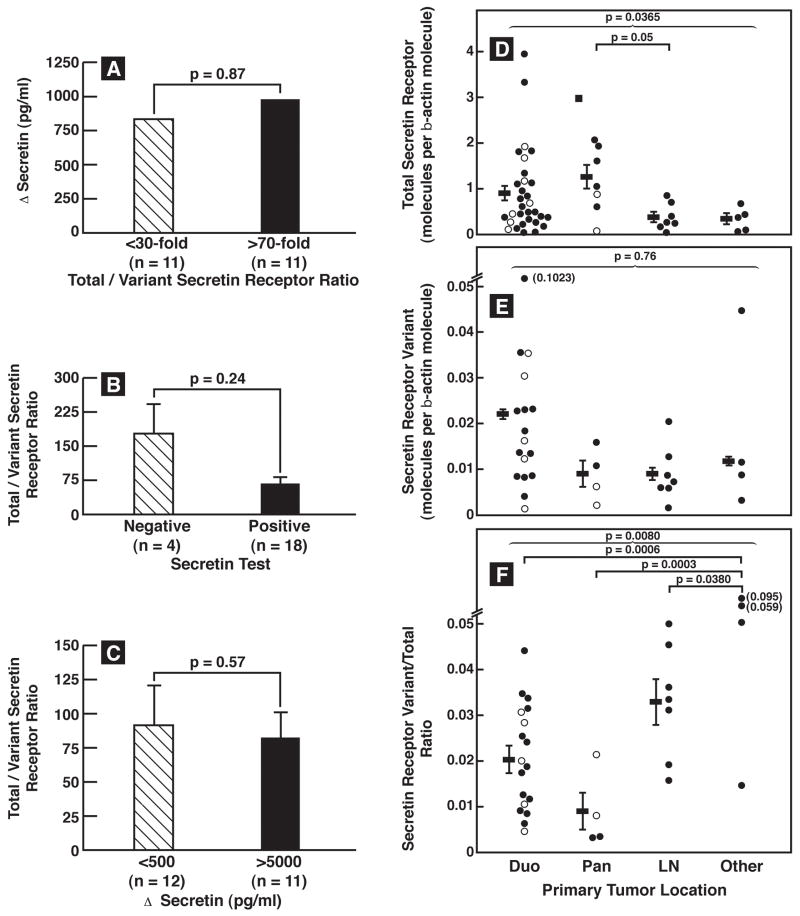
The median Δsecretin was 1566 pg/ml(range- 67–158000 pg/ml), preoperatively. All but 4 patients (92%) had a positive test using the historical criterion of ≥200 pg/ml (22) or all but 2 patients (96%) using the recently proposed more sensitive criterion of ≥120 pg/ml (4). The median Δcalcium was 1620 pg/ml(range-9–46000 pg/ml) with 77% having a positive test using the criterion of ≥395 pg/ml increase (3,4). The magnitude of the increase with secretin, but not with calcium, directly correlated with the total-secretin-receptor expression (p=0.0035)(Fig. 2A1,A2). The mean total-secretin-receptor mRNA expression level did not differ in the four patients with a negative secretin test from those with a positive test (1.00 ± 0.53 vs. 0.82± 0.11 molecules/β-actin) suggesting factors other than total-secretin-receptor expression were responsible for these patient’s negative test. The secretin-receptor-variant examined in this study is proposed to function as a dominant-negative (23,24). To examine this possibility in more detail we studied the effect of differing amount of the Δsecretin-receptor-variant on secretin-stimulated gastrin-release from different gastrinomas (Fig. 1B). No correlation existed between the absolute amount of secretin-receptor-variant or the total/variant ratio and the magnitude of Δsecretin or Δcalcium(Fig. 2B,C). The magnitude of the Δsecretin did not differ for gastrinomas with a high (>70-fold) or low (<30-fold) ratio of total to variant secretin-receptor(Fig. 3A) and conversely the total to variant secretin-receptor ratio did not differ with gastrinomas with Δsecretin <500 pg/ml compare to those with >5000 pg/ml Δsecretin (Fig. 3C). Lastly, it was proposed that sufficiently high amounts of secretin-receptor-variant could cause a negative secretin test(7). In our study 4 patients had a negative secretin test, and we found no significant difference between their total to variant secretin-receptor ratio compared to the patients with positive secretin tests(Fig. 3B).
Figure 2.
Correlation of total-secretin-receptor and secretin-variant expression and their ratio with results of secretin and calcium provocative testing (A, B, C) and with each other (D1) or with tumor size (D2)., Each dot indicates the results from a gastrinoma from one patient and each value is the mean of at least three separate PCR determinations. Correlation coefficients and significance were determined by least-square analysis.). The different symbols indicate the source of the tumor analyzed in each case. Symbols: ● Primary-sporadic, ○Primary-MEN1, ■LN-sporadic, □LN-MEN1, ▲Liver metastases
Figure 3.
Comparison of total-secretin and variant secretin-receptor expression or their ratio in gastrinomas with the positivity or magnitude of the secretin response or the tumor location. (Left Panel). The top panel compares the median Δsecretin in patients with gastrinomas that had <30-fold to those that had >70 fold greater amounts of total compared to secretin-receptor-variant abundance. The middle panel compares the total to variant secretin-receptor ratio in patients with or without a positive secretin test (i.e. >200 pg/ml increase). The lower panel compares the total to variant secretin-receptor ratio in gastrinomas from patients who had <500 pg/ml increase or >5000 pg/ml increase on secretin. (Right panel). Tumor location was determined at surgery. For the total-secretin-receptor expression results are from 31 duodenal, 7 pancreatic, 7 primary lymph node and 5 primary gastrinomas in other locations. For the variant and ratio results from 17 duodenal, 4 pancreatic, 7 lymph node primary and 4 primary gastrinomas in other locations are shown. Each dot represents the value from one gastrinoma in the indicated location and is a mean of at least 3 separate PCR determinations. Horizontal and vertical lines show the mean ± SEM for each primary tumor group.). The different symbols indicate the source of the tumor analyzed in each case. Symbols: ● Primary-sporadic, ○Primary-MEN1, ■LN-sporadic, □LN-MEN1, ▲Liver metastases
Abbreviations: Duod, duodenum; Panc, pancreas; Prim, primary; LN, lymph node.
Tumor extent, liver metastases, disease-free status postresection, occurrence of relapse, postoperative tumor growth-pattern or survival, did not correlation with total or variant-secretin-receptor expression or their ratio(Table 3, Fig. 2D2). However, both gastrinoma size and location had an effect on at least one of these three variables(Table 3, Fig. 3D,E,F). Specifically, the total-secretin-receptor expression was 2.5-times higher for tumors located in the pancreas/duodenum, whereas the secretin-receptor-variant levels were 2-times higher in duodenal than nonduodenal tumors(Table 3, Fig. 3D,E). This resulted in a 2.3-fold higher secretin-receptor-variant to total-secretin-receptor ratio in primary tumors outside the duodenum or pancreas (Table 3, Fig. 3F). The secretin-receptor-variant was 2.3–4 times more frequent in small gastrinomas than in large primaries(Table 3). The secretin-receptor-variant to total-secretin-receptor ratio was significantly higher in gastrinomas outside the duodenum or pancreas (p=0.0004, Fig. 3F).
Table 3.
Effect of tumoral characteristics on expression variant and their ratio of total-secretin-receptor and secretin-receptor-variant in gastrinomas.
| Total-secretin-receptor (molecules per β-actin)1 | Secretin-receptor-variant (molecules per β-actin)1 | Secretin-receptor-variant/Total Ratio | ||||
|---|---|---|---|---|---|---|
| Variable present | Variable absent7 | Variable present | Variable absent | Variable present | Variable absent | |
| Tumor variable | ||||||
| Pancreas/duo location8 | 0.98±0.14 | 0.37±0.08** | 0.017±0.003 | 0.012±0.004 | 0.018±0.002 | 0.041±0.007*** |
| Duo location | 0.94±0.16 | 0.66±0.14 | 0.022±0.001 | 0.011±0.03** | 0.020±0.003 | 0.032±0.006 |
| Tumor extent9 | ||||||
| Primary only | 0.68±0.15 | 0.90±0.15 | 0.012±0.003 | 0.020±0.005 | 0.031±0.006 | 0.022±0.003 |
| Primary + LN mets | 0.93±0.15 | 0.68±0.18 | 0.020±0.004 | 0.012±0.003 | 0.022±0.003 | 0.031±0.006 |
| Tumor size | ||||||
| Primary<1 cm | 0.98±0.17 | 0.68±0.14 | 0.023±0.005 | 0.010±0.002** | 0.023±0.005 | 0.029±0.005 |
| Primary >2.9 cm | 0.52±0.22 | 0.90±0.13 | 0.006±0.002 | 0.020±0.004*** | 0.031±0.008 | 0.024±0.004 |
| Largest tumor>3 cm | 0.71±0.17 | 0.88±0.14 | 0.012±0.003 | 0.019±0.004 | 0.030±0.007 | 0.024±0.006 |
| Cured immediate post op10 | 0.87±0.16 | 0.77±0.19 | 0.017±0.005 | 0.016±0.003 | 0.027±0.003 | 0.024±0.006 |
| Cured last follow-up10 | 0.87±0.20 | 0.80±0.12 | 0.020±0.006 | 0.014±0.003 | 0.028±0.004 | 0.024±0.05 |
| Relapsed post cure11 | 0.86±0.25 | 0.83±0.13 | 0.008±0.002 | 0.018±0.004 | 0.026±0.008 | 0.026±0.003 |
| Developed liver mets | 0.92±0.22 | 0.81±0.13 | 0.011±0.004 | 0.018±0.004 | 0.022±0.007 | 0.027±0.009 |
| Postop tumor growth12 | 0.81±0.16 | 0.85±0.15 | 0.017±0.008 | 0.016±0.002 | 0.020±0.005 | 0.029±0.004 |
| Postop aggressive growth12 | 0.78±0.18 | 0.86±0.14 | 0.020±0.001 | 0.015±0.002 | 0.020±0.006 | 0.028±0.004 |
| Died follow-up | 0.32±0.16 | 0.88±0.012 | 0.009±0.007 | 0.017±0.003 | 0.025±0.017 | 0.026±0.003 |
Abbreviations: Duo, duodenum; LN, lymph node; postop, postoperative; mets, metastases.
Total-secretin-receptor data are from 54 gastrinomas, secretin-receptor-variant and ratio from 35 gastrinomas
(p<0.02);
(p<0.01).
Tumor location was determined at surgical exploration and comparisons is made of either duodenal alone or duodenal and pancreas location compared to other sites (14,17).
Patient disease-free as defined in Methods immediately post resection or at last follow-up.
Relapse refers to patient who was initially disease-free post resection and during follow-up had a recurrence (15,17). . as recommend
Tumor growth and aggressive tumor growth were determined by follow-up imaging studies as defined in Methods (18).
Discussion
Since the initial description of secretin’s ability to increase gastrin levels in ZES(25), the secretin test was used in ZES diagnosis(2,4). Although some studies suggest the increased secretin response in ZES represents an exaggerated normal response(4,5,26), recent studies demonstrate secretin stimulates gastrin-release through secretin receptors on gastrinomas. Secretin can stimulate gastrin-release from isolated gastrinoma cells (4,27,28,28,29), stimulate adenylate cyclase activation in these cells (29) and secretin receptors occur on gastrinomas(7,24).
Many studies have examined the gastrin changes postsecretin in ZES patients (2,4) and proposed criteria diagnostic for ZES(2,4), however there is almost no information on the factors that determine the markedly variable secretin-stimulated gastrin change that occurs between patients (i.e. 0 to 4000-fold in one recent review(4)) or explains the nondiagnostic increase in 13% (mean 11 series, [range, 0–30%]) of ZES patients(3,4). Recently it was shown in one gastrinoma(7) and a number of pancreatic ductal adenocarcinomas(23) that secretin-receptor-variants occur. In transfected cells one secretin-receptor-variant which lacks exon 3 encoding amino acids 44-79 in the amino terminus, could function as a dominant-negative(7,23). It was proposed that the dominant-negative action might account for the negative secretin test in the ZES patient studied(7). At present whether the secretin receptor abundance or the proportion of variant actually contributes to the magnitude of the secretin-stimulated gastrin response is unclear. This has occurred because it is difficult to perform pathological-clinical correlations on gastrinomas because of their low incidence and small sizes(1,16). In the present study the mean size of the duodenal gastrinomas, which were the majority, was 3 mm and in most cases only a few microscopic slides of tissue were available. Therefore, a PCR approach was used for the secretin receptor mRNA. Western blotting in a limited number of tumors with more tissue showed a close correlation (r=0.94, p<0.001) between the expression of the mRNA as did the receptor protein as did secretin-receptor immunohistochemistry (p=0.0015, r=0.71). This result supported the validity of this approach and is similar to recent studies with somatostatin receptor expression in neuroendocrine tumors which show a close correlation between mRNA expression and receptor expression(30,31). The present study using this approach was undertaken to address the above-unanswered questions.
A number our results support the conclusion that secretin-receptor is expressed in all gastrinomas and the expression level is at least one determinant of the secretin-stimulated gastrin release response. First, in all 54 gastrinomas, secretin-receptor mRNA levels was detected and in all 26 gastrinomas by immunohistochemistry. Second, 15% were pancreatic gastrinomas, with the remaining primarily duodenal, and a lesser number from other locations. These results demonstrate all gastrinomas express secretin-receptors, even though gastrinomas in different locations are reported to have different origins(32). Third, secretin-receptor level showed a highly significant direct correlation (p=0.0035) with the maximal secretin-stimulated gastrin release, suggesting secretin-receptor density on gastrinomas was an important variable in determining the maximal gastrin-release with secretin. The finding that secretin-receptor expression did not correlate with maximal calcium-stimulated release shows the association was not just co-incidental or due to a non-related factor causing increased release. Furthermore, it is unlikely this difference is due to one stimulant having a direct effect and the other an indirect effect on the gastrinoma, because recent studies report that similar to secretin, calcium-stimulated gastrin release from gastrinomas is due to a direct action on the gastrinomas(27,33–35). Fourth, in the four patients with negative secretin tests each had total-secretin-receptor mRNA present in their gastrinoma, demonstrating that not the presence or absence of secretin-receptors determines a positive secretin test result, but other factors such as secretin-receptor amount, coupling to secretory pathways or gastrin-storage could be responsible for the negative secretin test in these patients.
The present study revealed no clinical/laboratory features of ZES patients correlated with the abundance of total-secretion-receptor. This result is consistent with findings in a recent study(4) demonstrating no clinical/laboratory features in 830 ZES patients from the NIH and literature, correlated with the secretin test result either with its positivity or magnitude of response. In terms of tumoral features, an association was found between the gastrinoma primary location and total-secretin-receptor mRNA abundance in the gastrinoma, with duodenal/pancreatic tumors having higher levels than nonduodenal-pancreatic tumors. A number of studies report secretin can have either growth stimulatory(36,37) or inhibitory effects(23,38) in pancreatic, gastric or colonic tumors. Our finding that there is no correlation of total-secretin-receptor expression and growth of gastrinomas, suggests it is unlikely secretin in having a potent growth stimulatory or inhibitory effect on the tumor itself.
Alternatively-spliced forms of various G protein-coupled receptors as well as other receptors are described which can have important clinical implications(39–44). In some cases they can alter signaling by the wild-type receptor by functioning as a dominant-negative. Such splice-variant behavior has been described for receptors for GHRH(45), calcitonin(46), vasopressin(41), alpha-adrenergic agents(44), thyroid hormones(40), and recently for one secretin receptor variant(7,23). This secretin-receptor-variant(7,23) lacking expression of the third exon encoding for amino acid residues 44-79 in the amino terminus(7,23) occurred in 1 of 3 gastrinomas(7), in 4 pancreatic adenocarcinomas cell lines(23) and 3 primary pancreatic adenocarcinomas(23). In these studies the secretin-receptor-variant(7,23) did not alter cell signaling when present alone but functioned as a dominant-negative likely by hetero-dimerizing with the wild-type receptor(23). This dominant-negative ability was proposed as a possible mechanism causing a negative secretin test in one patient who had a high level of expression of the secretin-variant-receptor in his gastrinoma (i.e. 70% of the total-secretin-receptor transcript)(7). A number of our results support the conclusion that this secretin-receptor-variant is expressed in all gastrinomas, although its level is low in most tumors, and that its presence neither modifies the ability of the wild-type receptor to stimulate gastrin release, nor is it a cause of the negative secretin test which occurred in a subset of patients. First, secretin-receptor-variant mRNA expression levels could be quantitated in all gastrinomas. Second, the total-secretin-receptor transcript had a mean level of expression 72-fold higher that the variant receptor. This means the variant receptor accounted for mean of 1.4% of the total-secretin transcript with a range from 0.4 to 11% in different gastrinomas. This result demonstrates that the occurrence of the variant accounting for 70% of the total-secretin-receptor transcript in a gastrinoma as reported in the original case discussed above(7) is rare. Third, neither the expression of the secretin-receptor-variant nor the ratio of the variant to the total-secretin-receptor transcript correlated with the magnitude of secretin-stimulated gastrin release. Fourth, the ratio of total to variant secretin-receptor expression did not differ between patients whose gastrinomas showed a low or high gastrin release with the secretin test or between patients with a positive or negative secretin test. These results suggest that in most patients with a negative secretin test some other mechanism than the level of secretin-variant receptor expression accounts for the negative study. It also supports the conclusion that the level of the variant expression in gastrinomas does not account for the marked variability of the secretin test results from one patient to the next. One likely explanation for our failure to see an effect of the variant receptor on secretin-stimulated gastrin release in our patients, in contrast to the previous case report(7), is the marked differences in level of variant-receptor expression found in the gastrinomas in these two studies. The highest variant receptor level we detected was 6-fold lower than that reported in the case report (11% vs. 70% of total transcript, respectively) and our mean variant receptor level was 50 –fold lower than that reported in the case report(7) (i.e. 1.4% vs. 70%).
Not only did the magnitude of the secretin-receptor-variant or its percentage of the total-secretin transcript not correlate with secretin-stimulated gastrin release, it also was not influenced by most clinical/laboratory variables. Although no differences were seen between MEN1 and non-MEN1 patients the number of MEN1 patients was small(N=9) and further study of this group is warranted. Primary tumors not located in the duodenum or pancreas had a higher percentage of total, but not variant-receptor and small gastrinomas had a 2.3–4-times higher level of the variant receptor. At present the pathogenetic factors responsible for these differences are unknown and could include different origins of the tumors, differences in differentiation, or difference influences of local tissue factors. Even though these tumoral differences for the total and variant-receptor expression occurred they unfortunately did not help to differentiate the 25% of patients with tumors with aggressive growth or the development of liver metastases from those with nonaggressive growth, which is one of the most important clinical differentiations that need to be made in the long-term management of these patients(16,21).
Acknowledgments
This research is partially supported by the Intramural Research Program of the NIDDK and NEI, NIH
Abbreviations
- BAO/MAO
basal/maximal acid output
- bp
base pair
- CT
computed tomography
- Δcalcium, Δmeal, Δsecretin
maximal increase in serum gastrin with these tests
- IHC
immunohistochemistry
- MEN1
multiple endocrine neoplasia type 1
- MRI
magnetic resonance imaging
- PCR
polymerase chain reaction
- PPI
proton pump inhibitor
- SRS
somatostatin receptor scintigraphy
- ZES
Zollinger-Ellison syndrome
Footnotes
DISCLOSURE STATEMENT: The authors have nothing to disclose
“This is an un-copyedited author manuscript copyrighted by The Endocrine Society. This may not be duplicated or reproduced, other than for personal use or within the rule of “Fair Use of Copyrighted Materials” (section 107, Title 17, U.S. Code) without permission of the copyright owner, The Endocrine Society. From the time of acceptance following peer review, the full text of this manuscript is made freely available by The Endocrine Society at http://www.endojournals.org/. The final copy edited article can be found at http://www.endojournals.org/. The Endocrine Society disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties. The citation of this article must include the following information: author(s), article title, journal title, year of publication and DOI.”
Reference List
- 1.Jensen RT, Doppman JL, Gardner JD. Gastrinoma. In: Go VLW, Brooks FA, DiMagno EP, Gardner JD, Lebenthal E, Scheele GA, editors. The Exocrine Pancreas: Biology, Pathobiology and Disease. 1. New York: Raven Press; 1986. pp. 727–744. [Google Scholar]
- 2.McGuigan JE, Wolfe MM. Secretin injection test in the diagnosis of gastrinoma. Gastroenterology. 1980;79:1324–1331. [PubMed] [Google Scholar]
- 3.Deveney CW, Deveney KS, Jaffe BM, Jones RS, Way LW. Use of calcium and secretin in the diagnosis of gastrinoma (Zollinger-Ellison syndrome) Ann Intern Med. 1977;87:680–686. doi: 10.7326/0003-4819-87-6-680. [DOI] [PubMed] [Google Scholar]
- 4.Berna MJ, Hoffmann KM, Long SH, Serrano J, Gibril F, Jensen RT. Serum gastrin in Zollinger-Ellison syndrome: II. Prospective study of gastrin provocative testing in 309 patients from the National Institutes of Health and comparison with 2229 cases from the literature. Evaluation of diagnostic criteria, proposal of new criteria and correlations with clinical and tumoral features. Medicine (Baltimore) 2006;85:331–364. doi: 10.1097/MD.0b013e31802b518c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brady CE., III Secretin provocation test in the diagnosis of Zollinger-Ellison syndrome. Am J Gastroenterol. 1991;86:129–134. [PubMed] [Google Scholar]
- 6.Lamers CB, Van Tongeren JHM. Comparative study of the value of calcium, secretin, and meal stimulated increase in serum gastrin in the diagnosis of the Zollinger-Ellison syndrome. Gut. 1977;18:128–134. doi: 10.1136/gut.18.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding WQ, Kuntz S, Bohmig M, Wiedenmann B, Miller LJ. Dominant negative action of an abnormal secretin receptor arising from mRNA missplicing in a gastrinoma. Gastroenterology. 2002;122:500–511. doi: 10.1053/gast.2002.31039. [DOI] [PubMed] [Google Scholar]
- 8.Gibril F, Schumann M, Pace A, Jensen RT. Multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome. A prospective study of 107 cases and comparison with 1009 patients from the literature. Medicine. 2004;83:43–83. doi: 10.1097/01.md.0000112297.72510.32. [DOI] [PubMed] [Google Scholar]
- 9.Roy P, Venzon DJ, Shojamanesh H, Abou-Saif A, Peghini P, Doppman JL, Gibril F, Jensen RT. Zollinger-Ellison syndrome: clinical presentation in 261 patients. Medicine. 2000;79:379–411. doi: 10.1097/00005792-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Benya RV, Metz DC, Venzon DJ, Fishbeyn VA, Strader DB, Orbuch M, Jensen RT. Zollinger-Ellison syndrome can be the initial endocrine manifestation in patients with multiple endocrine neoplasia-type 1. Am J Med. 1994;97:436–444. doi: 10.1016/0002-9343(94)90323-9. [DOI] [PubMed] [Google Scholar]
- 11.Roy P, Venzon DJ, Feigenbaum KM, Koviack PD, Bashir S, Ojeaburu JV, Gibril F, Jensen RT. Gastric secretion in Zollinger-Ellison syndrome. Correlation with clinical expression, tumor extent and role in diagnosis--a prospective NIH study of 235 patients and a review of 984 cases in the literature. Medicine (Baltimore) 2001;80:189–222. doi: 10.1097/00005792-200105000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Termanini B, Gibril F, Reynolds JC, Doppman JL, Chen CC, Stewart CA, Sutliff V, Jensen RT. Value of somatostatin receptor scintigraphy: A prospective study in gastrinoma of its effect on clinical management. Gastroenterology. 1997;112:335–347. doi: 10.1053/gast.1997.v112.pm9024287. [DOI] [PubMed] [Google Scholar]
- 13.Thom AK, Norton JA, Doppman JL, Chang R, Miller DL, Jensen RT. Prospective study of the use of intraarterial secretin injection and portal venous sampling to localize duodenal gastrinomas. Surgery. 1992;112(6):1002–1008. [PubMed] [Google Scholar]
- 14.Sugg SL, Norton JA, Fraker DL, Metz DC, Pisegna JR, Fishbeyn V, Benya RV, Shawker TH, Doppman JL, Jensen RT. A prospective study of intraoperative methods to diagnose and resect duodenal gastrinomas. Ann Surg. 1993;218:138–144. doi: 10.1097/00000658-199308000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fishbeyn VA, Norton JA, Benya RV, Pisegna JR, Venzon DJ, Metz DC, Jensen RT. Assessment and prediction of long-term cure in patients with Zollinger-Ellison syndrome: the best approach. Ann Intern Med. 1993;119:199–206. doi: 10.7326/0003-4819-119-3-199308010-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu F, Venzon DJ, Serrano J, Goebel SU, Doppman JL, Gibril F, Jensen RT. Prospective study of the clinical course, prognostic factors and survival in patients with longstanding Zollinger-Ellison syndrome. J Clin Oncol. 1999;17:615–630. doi: 10.1200/JCO.1999.17.2.615. [DOI] [PubMed] [Google Scholar]
- 17.Norton JA, Alexander HR, Fraker DL, Venzon DJ, Jensen RT. Does the use of routine duodenotomy (DUODX) affect rate of cure, development of liver metastases or survival in patients with Zollinger-Ellison syndrome (ZES)? Ann Surg. 2004;239:617–626. doi: 10.1097/01.sla.0000124290.05524.5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sutliff VE, Doppman JL, Gibril F, Yu F, Serrano J, Venzon DJ, Jensen RT. Growth of newly diagnosed, untreated metastatic gastrinomas and predictors of growth patterns. J Clin Oncol. 1997;15:2420–2431. doi: 10.1200/JCO.1997.15.6.2420. [DOI] [PubMed] [Google Scholar]
- 19.Furukawa M, Raffeld M, Mateo C, Sakamoto A, Moody TW, Venzon DJ, Serrano J, Jensen RT. Increased expression of insulin-like growth factor I (IGF-1) and/or its receptor (IGF-1R) in gastrinomas is associated with low curability, increased growth and development of metastases. Clin Canc Res. 2005;11(9):3233–42. doi: 10.1158/1078-0432.CCR-04-1915. [DOI] [PubMed] [Google Scholar]
- 20.Ho PK, Fong RS, Kai HS, Lau EH, Ngan ES, Cotton CU, Chow BK. The human secretin receptor gene: genomic organization and promoter characterization. FEBS Lett. 1999;455:209–214. doi: 10.1016/s0014-5793(99)00864-9. [DOI] [PubMed] [Google Scholar]
- 21.Jensen RT. Natural history of digestive endocrine tumors. In: Mignon M, Colombel JF, editors. Recent advances in pathophysiology and management of inflammatory bowel diseases and digestive endocrine tumors. Paris, France: John Libbey Eurotext Publishing Co.; 1999. pp. 192–219. [Google Scholar]
- 22.Coussens CM, Williams JM, Ireland DR, Abraham WC. Tyrosine phosphorylation-dependent inhibition of hippocampal synaptic plasticity. Neuropharmacology. 2000;39:2267–2277. doi: 10.1016/s0028-3908(00)00087-3. [DOI] [PubMed] [Google Scholar]
- 23.Ding WQ, Cheng ZJ, McElhiney J, Kuntz SM, Miller LJ. Silencing of secretin receptor function by dimerization with a misspliced variant secretin receptor in ductal pancreatic adenocarcinoma. Cancer Res. 2002;62:5223–5229. [PubMed] [Google Scholar]
- 24.Korner M, Hayes GM, Rehmann R, Zimmermann A, Friess H, Miller LJ, Reubi JC. Secretin receptors in normal and diseased human pancreas: marked reduction of receptor binding in ductal neoplasia. Am J Pathol. 2005;167:959–968. doi: 10.1016/S0002-9440(10)61186-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Isenberg JI, Walsh JH, Passaro E, Jr, Moore EW, Grossman ME. Unusual effect of secretin on serum gastrin, serum calcium and gastric acid secretion in a patient with suspected Zollinger-Ellison syndrome. Gastroenterology. 1972;62:626–631. [PubMed] [Google Scholar]
- 26.Brady CE, Utts SJ, Dev J. Secretin provocation in normal and duodenal ulcer subjects. Is the gastrin rise in Zollinger-Ellison syndrome paradoxic or exaggeration? Dig Dis Sci. 1987;32:232–238. doi: 10.1007/BF01297046. [DOI] [PubMed] [Google Scholar]
- 27.Elouaer-Blanc L, Sobhani I, Ruszniewski P, Duet M, Lehy T, Mignon M, Bonfils S, Lewin MJ. Gastrin secretion by gastrinoma cells in long-term culture. Am J Physiol. 1988;255:G596–G602. doi: 10.1152/ajpgi.1988.255.5.G596. [DOI] [PubMed] [Google Scholar]
- 28.Gower WR, Jr, Ellison EC, Knierim TH, Elkhammas EA, O’Dorisio TM, Fabri PJ. Gastrinoma in vitro: morphological and physiological studies of primary cell cultures. Gastroenterology. 1990;98:936–954. doi: 10.1016/0016-5085(90)90018-v. [DOI] [PubMed] [Google Scholar]
- 29.Imamura M, Adachi H, Takahashi K, Noguchi M, Mizutani N, Nakagawa M, Tobe T. Gastrin release from gastrinoma cells stimulated with secretin. Dig Dis Sci. 1982;27:1130–1136. doi: 10.1007/BF01391453. [DOI] [PubMed] [Google Scholar]
- 30.Raggi CC, Maggi M, Renzi D, Calabro A, Bagnoni ML, Scaruffi P, Tonini GP, Pazzagli M, De Bernardi B, Bernini G, Serio M, Orlando C. Quantitative determination of sst2 gene expression in neuroblastoma tumor predicts patient outcome. J Clin Endocrinol Metab. 2000;85:3866–3873. doi: 10.1210/jcem.85.10.6904. [DOI] [PubMed] [Google Scholar]
- 31.Papotti M, Croce S, Macri L, Funaro A, Pecchioni C, Schindler M, Bussolati G. Correlative immunohistochemical and reverse transcriptase polymerase chain reaction analysis of somatostatin receptor type 2 in neuroendocrine tumors of the lung. Diagn Mol Pathol. 2000;9:47–57. doi: 10.1097/00019606-200003000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Passaro E, Jr, Howard TJ, Sawicki MP, Watt PC, Stabile BE. The origin of sporadic gastrinomas within the gastrinoma triangle: a theory. Arch Surg. 1998;133:13–16. doi: 10.1001/archsurg.133.1.13. [DOI] [PubMed] [Google Scholar]
- 33.Bold RJ, Lowry PS, Ishizuka J, Battey JF, Townsend CM, Jr, Thompson JC. Bombesin stimulates the in vitro growth of a human gastric cancer cell line. J Cell Physiol. 1994;161:519–525. doi: 10.1002/jcp.1041610315. [DOI] [PubMed] [Google Scholar]
- 34.Ahlman H, Ahlund L, Dahlstrom A, Theodorsson E. Expression of gastrointestinal endocrine tumours in culture systems. Arch Histol Cytol. 1989;52(Suppl):233–240. doi: 10.1679/aohc.52.suppl_233. [DOI] [PubMed] [Google Scholar]
- 35.Woltering EA, Ellison EC, O’Dorisio TM, Hitchcock C, Roberts K, Stephens R, Sparks J, Carey LC. Gastrin release from dispersed gastrinoma cells: effects of calcium and calcium ionophore (A23187) J Surg Res. 1985;39:331–337. doi: 10.1016/0022-4804(85)90111-8. [DOI] [PubMed] [Google Scholar]
- 36.Edwards BF, Redding TW, Schally AV. The effect of gastrointestinal hormones on the incorporation of tritiated thymidine in the pancreatic adenocarcinoma cell line (WD PaCa) Int J Pancreatol. 1989;5:191–201. doi: 10.1007/BF02924419. [DOI] [PubMed] [Google Scholar]
- 37.Ohyama K. [Effects of anti-tumor drugs and gastrointestinal hormones on the growth of pancreatic duct cell adenocarcinoma in homologous transplanted animal models] Hokkaido Igaku Zasshi. 1985;60:206–216. [PubMed] [Google Scholar]
- 38.Tanaka J, Yamaguchi T, Takahashi T, Ogata N, Bando K, Koyama K. Regulatory effects of gastrin and secretin on carcinomas of the stomach and colon. Tohoku J Exp Med. 1986;148:459–460. doi: 10.1620/tjem.148.459. [DOI] [PubMed] [Google Scholar]
- 39.Kilpatrick GJ, Dautzenberg FM, Martin GR, Eglen RM. 7TM receptors: the splicing on the cake. Trends Pharmacol Sci. 1999;20:294–301. doi: 10.1016/s0165-6147(99)01355-3. [DOI] [PubMed] [Google Scholar]
- 40.Ando S, Sarlis NJ, Krishnan J, Feng X, Refetoff S, Zhang MQ, Oldfield EH, Yen PM. Aberrant alternative splicing of thyroid hormone receptor in a TSH-secreting pituitary tumor is a mechanism for hormone resistance. Mol Endocrinol. 2001;15:1529–1538. doi: 10.1210/mend.15.9.0687. [DOI] [PubMed] [Google Scholar]
- 41.Sarmiento JM, Anazco CC, Campos DM, Prado GN, Navarro J, Gonzalez CB. Novel down-regulatory mechanism of the surface expression of the vasopressin V2 receptor by an alternative splice receptor variant. J Biol Chem. 2004;279:47017–47023. doi: 10.1074/jbc.M410011200. [DOI] [PubMed] [Google Scholar]
- 42.Edwards SW, Tan CM, Limbird LE. Localization of G-protein-coupled receptors in health and disease. Trends Pharmacol Sci. 2000;21:304–308. doi: 10.1016/s0165-6147(00)01513-3. [DOI] [PubMed] [Google Scholar]
- 43.Kim HJ, Woo IS, Kang ES, Eun SY, Kim HJ, Lee JH, Chang KC, Kim JH, Seo HG. Identification of a truncated alternative splicing variant of human PPARgamma1 that exhibits dominant negative activity. Biochem Biophys Res Commun. 2006;347:698–706. doi: 10.1016/j.bbrc.2006.06.147. [DOI] [PubMed] [Google Scholar]
- 44.Chen S, Lin F, Xu M, Hwa J, Graham RM. Dominant-negative activity of an alpha(1B)-adrenergic receptor signal-inactivating point mutation. EMBO J. 2000;19:4265–4271. doi: 10.1093/emboj/19.16.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grosse R, Schoneberg T, Schultz G, Gudermann T. Inhibition of gonadotropin-releasing hormone receptor signaling by expression of a splice variant of the human receptor. Mol Endocrinol. 1997;11:1305–1318. doi: 10.1210/mend.11.9.9966. [DOI] [PubMed] [Google Scholar]
- 46.Seck T, Pellegrini M, Florea AM, Grignoux V, Baron R, Mierke DF, Horne WC. The delta e13 isoform of the calcitonin receptor forms a six-transmembrane domain receptor with dominant-negative effects on receptor surface expression and signaling. Mol Endocrinol. 2005;19:2132–2144. doi: 10.1210/me.2004-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Norton JA, Alexander HA, Fraker DL, Venzon DJ, Gibril F, Jensen RT. Possible primary lymph node gastrinomas: occurrence, natural history and predictive factors: A prospective study. Ann Surg. 2003;237:650–659. doi: 10.1097/01.SLA.0000064375.51939.48. [DOI] [PMC free article] [PubMed] [Google Scholar]