Abstract
The existence of a common precursor for endothelial and hemopoietic cells, termed the hemangioblast, has been postulated since the beginning of the century. Recently, deletion of the endothelial-specific vascular endothelial growth factor receptor 2 (VEGFR2) by gene targeting has shown that both endothelial and hemopoietic cells are absent in homozygous null mice. This observation suggested that VEGFR2 could be expressed by the hemangioblast and essential for its further differentiation along both lineages. However, it was not possible to exclude the hypothesis that hemopoietic failure was a secondary effect resulting from the absence of an endothelial cell microenvironment. To distinguish between these two hypotheses, we have produced a mAb directed against the extracellular domain of avian VEGFR2 and isolated VEGFR2+ cells from the mesoderm of chicken embryos at the gastrulation stage. We have found that in clonal cultures, a VEGFR2+ cell gives rise to either a hemopoietic or an endothelial cell colony. The developmental decision appears to be regulated by the binding of two different VEGFR2 ligands. Thus, endothelial differentiation requires VEGF, whereas hemopoietic differentiation occurs in the absence of VEGF and is significantly reduced by soluble VEGFR2, showing that this process could be mediated by a second, yet unidentified, VEGFR2 ligand. These observations thus suggest strongly that in the absence of the VEGFR2 gene product, the precursors of both hemopoietic and vascular endothelial lineages cannot survive. These cells therefore might be the initial targets of the VEGFR2 null mutation.
Endothelial and hemopoietic cells of the avian embryonic yolk sac develop from aggregates of mesodermal cells, the hemangioblastic clusters (1, 2). These aggregates are present at the border of the embryonic and extraembryonic area from the beginning of the second day of the incubation period (E2), around the 1-somite stage (ss). They subsequently mature and form blood islands, the external cells of which flatten and differentiate into endothelial cells, while the internal cells of the cluster form hemopoietic cells. In the chicken embryo, blood islands are first detected at the 5-ss in the most external part of the yolk sac (1). The evidence for blood island formation comes from observations on living chicken blastoderms, made at the beginning of the century (1, 2). Endothelial and hemopoietic cells in the blood islands were proposed to originate from a common precursor, termed the hemangioblast (2), based on their simultaneous emergence. However, the existence of this cell remains to be formally proven.
Recently, the development of the endothelial cell lineage of vertebrate embryos has been shown to depend on receptor tyrosine kinases. Five endothelial-specific receptor tyrosine kinases are known to date and include the receptors tek and tie, and the vascular endothelial growth factor (VEGF) receptors 1–3 (3, 4). Inactivation of four of these five receptor tyrosine kinases (tek, tie, VEGFR1, and VEGFR2) by homologous recombination has shown that the function of each receptor is essential for the development of the embryonic vascular system, but that each receptor acts on different aspects or phases of endothelial differentiation (5–7). In particular, mice deficient for VEGFR2 die between embryonic days 8.5 and 9.5 due to lack of endothelial and hemopoietic cells in their yolk sacs (6). This suggested that VEGFR2 could be expressed by the hemangioblast and essential for its further differentiation along both lineages. However, the possibility that hemopoietic failure was secondary due to absence of endothelial cells was not excluded.
VEGF receptors are characterized by the presence of seven extracellular Ig-like domains, a single transmembrane domain and an intracellular tyrosine kinase domain, the activity of which is critical for transmission of the signal produced by VEGF binding (3, 4). VEGF is a secreted polypeptide mitogenic for endothelial cells and able to induce vascular permeability (8). Two closely related growth factors, VEGF-B and VEGF-C, were recently identified (9–11). Both of these factors are mitogenic for endothelial cells in culture. Their biological actions are, however, not yet fully understood. VEGFR1 binds VEGF and the related growth factor PlGF (12). VEGFR2 binds VEGF and VEGF-C. Finally, VEGFR3 binds only VEGF-C (10, 11).
We and others have previously shown that of the three VEGFRs, VEGFR2 is expressed very early during development (13–15). VEGFR2 transcripts are abundant in the mesoderm, which exits from the posterior primitive streak during gastrulation (13). Quail–chicken transplantations and vital dye injections have shown that these early mesodermal cells not only participate in formation of the embryonic mesoderm, but also migrate to the extraembryonic area where they will form the first blood islands (16, 17). In the blood islands, receptor transcripts are restricted to endothelial cells and are not present in hemopoietic cells at any developmental stage (13–15). We were intrigued by the early expression of VEGFR2 in the mesoderm and decided to further characterize the cells that turn on this gene at these early stages, by isolating them in semisolid clonal cultures and examining their developmental potentialities in different environmental conditions. The aim was to determine if at one stage of their ontogeny these cells possess the capacity to differentiate along both the hemopoietic and endothelial lineages, thus corresponding to the definition of the postulated hemangioblast.
For this purpose, we prepared recombinant VEGFR2, raised mAbs directed against the extracellular domain of the receptor, and sorted out the VEGFR2-expressing cells from the mesoderm of the early chicken gastrula. We report here that in appropriate clonal culture conditions, the VEGFR2+ cells differentiate along either the endothelial or the hemopoietic pathways, depending on the presence or absence of VEGF. As observed in vivo, VEGFR2 is maintained only on the endothelial cell type. Mixed colonies containing representatives of both cell lineages have not been observed, indicating that the bipotential precursor subsequently generates either cell type exclusively.
MATERIALS AND METHODS
Production of Recombinant VEGFR2-Fc Protein.
A 2.2-kb SmaI/SspI restriction fragment of the quail Quek1/VEGFR2 cDNA (positions 42–2241 in the published sequence, ref. 18), encoding the ATG, signal peptide, and seven extracellular Ig-loops, was cloned into a modified MO90 vector (kindly provided by Shin-ichi Nishikawa, Kyoto University, Japan), in frame with the Fc part of human Ig. Parent MO90 vector, containing the human CD4 extracellular domain fused to the Fc part, was used as a control. Twenty micrograms of these constructs was transfected per 10-cm dish of COS-7 cells using the DEAE-dextran method. Cells were grown in DMEM⋅F12 (GIBCO) supplemented with 0.5% Neutridoma (Boehringer Mannheim) and antibiotics. Culture medium was harvested 3 and 6 days after transfection, and the fusion proteins purified on protein-A Sepharose columns (Bioprocessing, Princeton, NJ). Culture supernatant (800 ml) was applied, washed with 20 column volumes of PBS (10 ml), and eluted with five 1-ml portions of 10 mM HCl into Eppendorff tubes prefilled with 200 ml of 0.2 M phosphate buffer containing protease inhibitors. Individual fractions were assayed for protein content by SDS/PAGE, and the peak fraction (fraction 2), containing about 1 mg of protein, was retained.
mAb Production.
A BALB/c mouse was immunized by intrasplenic injection with 50 μg of VEGFR2-Fc protein in PBS mixed with Freund’s complete adjuvant (1:1) and boosted four times by intraperitoneal injection. Splenocytes were fused with SP2/O mouse myeloma as described (19). Undiluted supernatants from confluent wells were tested for secreted antibodies by ELISA on 96-well plates (Dynatec) coated with 0.1 μg/ml VEGFR2-Fc or CD4-Fc. Positive hybridomas were cloned by the limiting dilution technique and subcloned twice. For Western blotting, proteins were run on a 7.5% reducing SDS/PAGE and blotted onto nitrocellulose membrane. The blot was probed with hybridoma supernatant or anti-Fc mAb (Sigma), followed by treatment with alkaline phosphatase-coupled goat anti-mouse Ig (Promega), and coloration according to manufacturer’s instructions.
Cell Sorting.
Sixty outbred JA57 chicken embryos were incubated for 22 hr at 38°C. Posterior area (PA) cells were dissociated by pipetting with a finely pulled Pasteur pipette in Ca2+Mg2+-free PBS at 25°C. The cell suspension was centrifuged for 5 min (150 × g), and the pellet resuspended in alpha medium (GIBCO/BRL). Eight to 10 × 105 cells were routinely obtained. Cells were incubated for 30 min at 25°C with anti-VEGFR2 hybridoma supernatant. After washing in alpha medium/3% fetal calf serum, cells were incubated for 30 min with phycoerythrin-conjugated goat anti-mouse IgG1 (Southern Biotechnology Associates) and washed twice in medium/ fetal calf serum. The cell suspension was diluted in PBS and filtered over a nylon tissue before analysis and sorting on a FACStar (Becton Dickinson). Dead cells and doublets were eliminated by using a high forward and orthogonal light scattering window. After sorting, cells were collected in tubes containing 5% BSA.
Cell Culture and Staining.
Between 800 and 2,000 cells were seeded in 0.5 ml of serum-free medium prepared as described (20) and clotted by the addition of citrated bovine plasma (GIBCO, 10% vol/vol) and thrombin (1 unit/ml, Produits Roche, France). Cultures were scaled down to 2 cells in 0.1 ml for the limiting dilution assay. Benzidine dihydrochloride (Sigma) staining of erythroid colonies was performed as described (21). CD41/CD61 staining using the 11C3 mAb (22) and mAb 118 staining was performed as described (23). DiI-Ac-LDL (acetylated low density lipoprotein labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate; Biomedical Technologies, Stoughton, MA) uptake was assayed according to the manufacturer’s instructions as previously described (24). Antibody staining of cryostat sections and whole-mount in situ hybridizations were carried out as described (25). Antisense riboprobes were synthesized from a 4.2-kb VEGFR2 and 4.2-kb VEGFR3 cDNA (18).
RESULTS
Production of mAbs Directed Against the Extracellular Domain of VEGFR2.
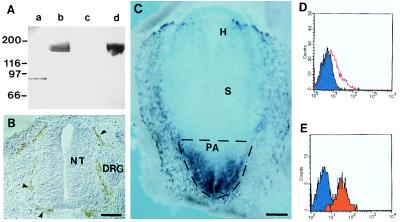
We constructed an expression vector encoding a fusion protein between the extracellular domain of the quail Quek1/VEGFR2 cDNA (18) and the Fc portion of human IgG1 (designated VEGFR2-Fc), and then produced and purified the fusion protein as described above. This protein was used for immunization of a mouse and hybridoma production. Hybridoma supernatants were screened by ELISA for reactivity with VEGFR2-Fc, and the absence of nonspecific reactivity with a control protein containing the extracellular domain of human CD4 fused to the Fc portion of human IgG1 (CD4-Fc). After cloning, eight hybridomas were retained which specifically reacted with the VEGFR2-Fc protein in Western blots (Fig. 1A). In vivo, these mAbs stained endothelial cells (Fig. 1B), and cells present in the newly formed mesoderm, reproducing the expression pattern of VEGFR2 previously determined by in situ hybridization (13). The cell-sorting experiments described below were performed with one of these mAbs (4H11), termed anti-VEGFR2, of IgG1 subclass.
Figure 1.
Production of mAbs recognizing avian VEGFR2 and cell sorting of PA mesodermal cells. (A) Western blot with control CD4-Fc protein (10 μg, lanes a and c) and VEGFR2-Fc protein (10 μg, lanes b and d) was probed with anti-VEGFR2 (4H11) hybridoma supernatant (lanes c and d) and with anti-Fc mAb (lanes a and b). Anti-VEGFR2 recognizes only VEGFR2-Fc (lane d). (B) Immunohistochemistry with anti-VEGFR2 mAb on cryostat sections (20 μm) of an E4 chicken embryo. Section at the trunkal level showing anti-VEGFR2+ endothelial cells of the perineural vascular plexus (arrowheads). NT, neural tube; DRG, dorsal root ganglion. (Bar: 75 μm.) (C) Whole-mount in situ hybridization of a quail embryo at the 1-ss with a VEGFR2 antisense riboprobe. Note abundant positive cells in the mesoderm of the PA. H, headfold; S, somite (out of focus). The stippled region of the embryo was dissected. (Bar: 417 μm.) (D and E) Flow cytometry of VEGFR2+ cells from the PA. Fluorescence histogram showing anti-VEGFR2 labeling (red) before (D) and after (E) cell sorting.
Sorting of VEGFR2+ Cells from the PA.
VEGFR2+ cells are abundant in the mesoderm of the PA of the embryo, exiting from the primitive streak of chicken embryos at presomitic and early ss (Fig. 1C). These mesodermal cells subsequently colonize the yolk sac (16, 17). In the yolk sac, VEGFR2+ cells aggregate in clusters, which then differentiate into blood islands, characterized by a vascular endothelium surrounding hemopoietic cells (1, 2). Once a blood island has formed, VEGFR2 expression is restricted to the endothelial cells (13). The first blood islands appear in the external and posterior part of the yolk sac at the 5-ss (1). Thus, to examine their developmental potentials, VEGFR2+ cells were isolated before they generated blood islands from the PA of embryos at presomitic to 3-ss (Fig. 1C). PA were dissected without separation of the three germ layers, dissociated mechanically into single cells, and then labeled with anti-VEGFR2 (see Materials and Methods). FACScan analysis showed that the percentage of anti-VEGFR2+ cells ranged from 15% to 20% in different experiments (Fig. 1D). These cells were sorted by FACS, and their purity was determined by reanalysis of positive cells. Around 95% purity was routinely obtained (Fig. 1E).
In Vitro Culture of Sorted Cells.
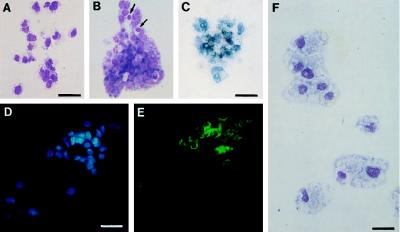
Sorted cells were cultured for 72 hr in the absence or presence of VEGF in semisolid medium (containing 10% bovine plasma, BSA, transferrin, and lipids; ref. 20) to allow clonal analysis. In the absence of VEGF, VEGFR2+ cells differentiated into hemopoietic colonies, containing 10 to 200 cells. These colonies belonged to the erythroblast-thromboblast lineage, as judged by May–Grünwald–Giemsa (MGG) staining (Fig. 2 A and B). Furthermore, benzidine staining indicated that 59% of colonies contained erythroblasts (Fig. 2C), whereas the thrombocytic lineage marker CD41/61 (22) labeled 36% of the colonies (Fig. 2 D and E). Other hemopoietic surface antigens such as the glycoprotein BEN or MEP 21 (23, 26) were expressed as well (not shown). None of these markers, including c-kit, which labels early chicken hemopoietic progenitors (27), were expressed by PA cells at the time they were removed from the embryo (not shown).
Figure 2.
Pheno types of hemopoietic cells obtained from VEGFR2+ PA cells. (A and B) MGG staining of hemopoietic colonies of thrombocyte (A) and thromboblast/erythroblast (B) type. Arrows in B point to two erythrocytes. (Bar: 25 μm.) (C) Benzidine-positive colony belonging to the erythrocytic lineage. (Bar: 21 μm.) (D) Hoechst nuclear stain and (E) CD 41/61 staining of a thromboblastic colony. (Bar: 26 μm.) (F) MGG staining of macrophages obtained in the presence of fibroblast-conditioned medium. (Bar: 18 μm.)
To determine whether other hemopoietic lineages could develop from VEGFR2+ PA cells, we tested fibroblast-conditioned medium, which promotes the development of myeloid colony-forming cells (23). In these conditions, the differentiation of macrophage progenitors was induced (Fig. 2F).
Addition of 50 ng/ml VEGF to the cultures reduced the number of hemopoietic colonies and induced the formation of colonies containing cells with a different morphology (Figs. 3A and 4A). These colonies consisted of 5 to 40 elongated cells, which tended to form networks and were strikingly different from the compact hemopoietic colonies composed of rounded cells (Fig. 3A). The VEGF-induced colonies consisted of endothelial cells, as shown by labeling with four specific markers. VEGFR2 and VEGFR3, both of which specifically label endothelial cells during early developmental stages (13, 18) were expressed by the endothelial, but not by the hemopoietic colonies (Fig. 3 B and C). Furthermore, the endothelial colonies incorporated DiI-Ac-LDL, characteristic of endothelial cells and macrophages (24) (Fig. 3 D and E). Endothelial clones also reacted with the mAb 118, which recognizes endothelial cells and basement membranes (28) (Fig. 3 F and G).
Figure 3.
Phenotypes of colonies obtained in the presence of VEGF. (A) Typical appearance of a culture. MGG staining of two colonies, one hemopoietic (HC) of erythroblast/thromboblast phenotype, the other (EC) of endothelial phenotype. (Bar: 28 μm.) (B–G) VEGF-induced colonies consist of endothelial cells. (B) Anti-VEGFR2 mAb staining followed by alkaline phosphatase-coupled secondary antibody. (Bar: 40 μm.) (C) In situ hybridization with a VEGFR3 antisense riboprobe. (Bar: 16 μm.) (D) Hoechst nuclear stain and (E) DiI-Ac-LDL uptake of an endothelial cell colony. (Bar: 32 μm.) (F) Hoechst nuclear stain and (G) mAb 118 immunoreactivity. (Bar: 53 μm.)
Figure 4.
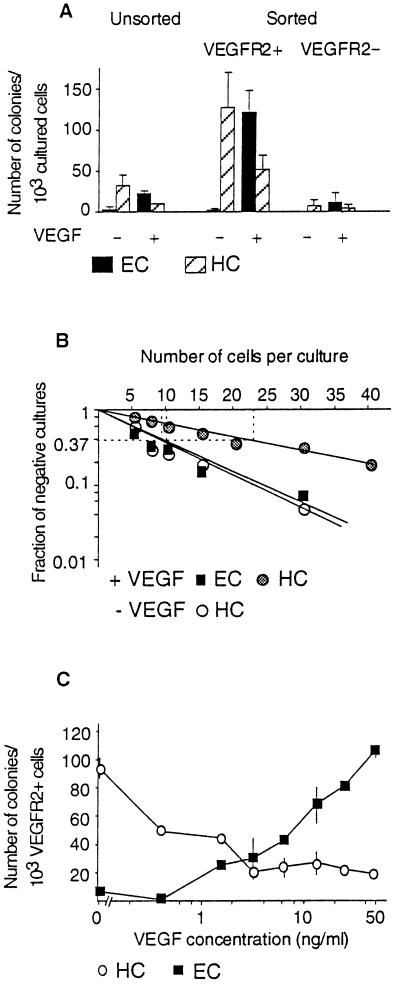
(A) Colony-forming cells (CFC) of the PA are enriched in the VEGFR2+ population as compared with unsorted or VEGFR2− cells. HC, hemopoietic colonies; EC, endothelial colonies. Each column represents the mean number of CFC calculated per 1 × 103 cells obtained in three independent sorting experiments ± SD. (B) Limiting dilution analysis of VEGFR2+ cells. One of two experiments showing similar results. The frequency of colony-initiating progenitors at 37% negative wells was, according to the Poisson analysis, 1/22 for HC and 1/10 for EC in the presence of VEGF and 1/11 for HC obtained without VEGF. (C) Dose-dependent stimulation of endothelial cell development by VEGF. Values represent the mean ± SD from two experiments.
Thus, in these culture conditions, VEGFR2+ cells of the early PA mesoderm gave rise to endothelial and hemopoietic colonies at the exclusion of any other cell type. Quantitative analyses were performed by staining the cultures with MGG and recording the number of colonies of both types. One thousand cells of the VEGFR2+ FACS-sorted population were cultured either according to the standard procedure or in a limiting dilution assay (see Materials and Methods).
In the absence of VEGF, 120(±40) hemopoietic colonies were obtained (Fig. 4A), and in the dilution assay, the frequency of hemopoietic colonies able to develop was found to be in the order of 1 in 10 cells (Fig. 4B). In the presence of 50 ng/ml VEGF, the number of hemopoietic colonies was reduced to 50 (±10), whereas 117 (±25) endothelial clones were recorded in these conditions (Fig. 4A). Limiting dilution analysis confirmed that the frequency of endothelial precursors was 1 in 10 cells, whereas the frequency of hemopoietic precursors was reduced to 1 in 22 cells in the presence of VEGF (Fig. 4B).
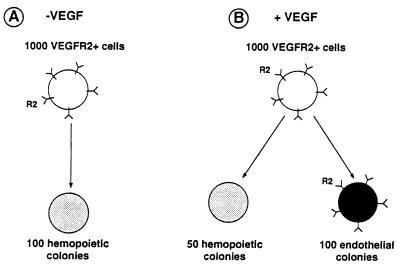
It was noticeable that hemopoietic colonies formed in both the presence and absence of VEGF (Fig. 5). In medium containing VEGF, the cloning efficiency was increased, and endothelial colonies appeared. The emergence of endothelial colonies was correlated with a decrease in the number of hemopoietic colonies (Fig. 5).
Figure 5.
Schematic representation of the results. One thousand VEGFR2+ cells cultured in the absence (A) or presence (B) of VEGF give rise to endothelial and hemopoietic colonies as indicated. Endothelial colonies only maintain VEGFR2 expression.
Cells of the VEGFR2 negative population tested in the same conditions yielded very few colonies of both types (Fig. 4A), which might correspond to VEGFR2+ cells that escaped the sort. Unsorted cells yielded both types of colonies, but with a frequency 5-fold lower than the VEGFR2+ population cultured in similar conditions (Fig. 4A). This is consistent with the 20% of VEGFR2+ cells in the total cell population. Thus, hemopoietic and endothelial progenitors present in the PA segregate with the VEGFR2+ population.
We next determined if the VEGF-response was dose–dependent (Fig. 4C). The number of VEGF-induced endothelial colonies was maximal at a concentration of 50 ng/ml (Fig. 4C). Higher VEGF concentrations (100 ng/ml) did not increase the response (not shown). In the presence of lower concentrations of VEGF, the number of endothelial colonies gradually decreased, whereas the number of hemopoietic colonies increased (Fig. 4C). Although the number of colonies obtained could vary between experiments, a general rule emerged: in a given experiment, the number of hemopoietic colonies obtained without addition of VEGF was equal to the number of endothelial colonies obtained in the presence of an optimal dose of VEGF. Lower doses of VEGF produced intermediate numbers of both types of colonies.
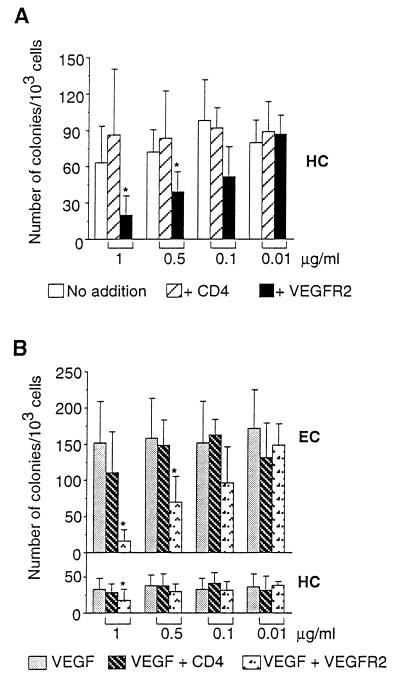
Thus, VEGFR2+ cells differentiate into either endothelial or hemopoietic cells. Endothelial differentiation requires VEGF and hemopoietic differentiation proceeds in the absence of exogenous growth factors (Fig. 5). This led us to question the mechanism triggering hemopoietic differentiation of the VEGFR2+ precursors. The phenotype of VEGFR2 knockout mice (6) had suggested that hemopoietic differentiation depended on the receptor. We thus tested this hypothesis in our culture system. Sorted VEGFR2+ cells were cultured in the presence of different concentrations of soluble VEGFR2-Fc protein. Human CD4-Fc protein was used as a negative control. VEGFR2-Fc inhibits hemopoietic colony formation in a dose-dependent manner (Fig. 6A). At 1 μg/ml, colony formation was reduced to 20% with respect to control levels, whereas at 0.5 μg/ml, hemopoietic colony formation was reduced to about 50% of control levels (Fig. 6A).
Figure 6.
(A) Soluble VEGFR2 inhibits hemopoietic colony formation. One thousand VEGFR2+ cells were cultured without growth factor addition and in the presence of the indicated concentrations of soluble VEGFR2-Fc protein or CD4-Fc protein. Values represent the mean ± SD from three experiments. Statistical analysis was performed using the paired Student’s t test. ∗ indicates P ≤ 0.05. (B) Soluble VEGFR2 inhibits VEGF-induced colony formation. One thousand VEGFR2+ cells were cultured in the presence of 50 ng/ml VEGF and the indicated concentrations of soluble VEGFR2-Fc protein or CD4-Fc protein. Values represent the mean ± SD from four (1 μg/ml and 0.1 μg/ml) or three experiments. Statistical analysis was performed as in A. HC, hemopoietic colonies, EC, endothelial colonies.
In the presence of 50 ng/ml VEGF, VEGFR2-Fc significantly inhibited hemopoietic colony formation only at the highest dose used (1 μg/ml) (Fig. 6B). Endothelial colony formation was significantly inhibited at 1 and 0.5 μg/ml VEGFR2-Fc (Fig. 6B). Thus, VEGFR2 is involved in the induction of both hemopoietic and endothelial differentiation from receptor-positive mesodermal precursors of the PA.
DISCUSSION AND CONCLUSIONS
The main finding of this study is that early mesodermal VEGFR2+ cells give rise to hemopoietic cell colonies, which lose expression of the receptor. If they are provided with the ligand VEGF, the same mesodermal VEGFR2+ cells also can give rise to endothelial cell colonies, which maintain receptor expression (Fig. 5).
Despite the fact that the hemopoietic cells lose VEGFR2 expression, this receptor is required for their differentiation, as shown by the fact that addition of soluble VEGFR2 to the culture medium reduces hemopoietic differentiation in a dose–dependent manner. The most likely explanation for this observation is to assume the existence of a VEGFR2 ligand in the culture medium, which is titrated out by the soluble receptor. Although the identity of this ligand remains unknown, it may either be present in the plasma used for the semisolid cultures or be produced by the VEGFR2+ cells themselves.
The known VEGFR2 ligand VEGF is unlikely to be responsible for hemopoietic differentiation. Addition of VEGF to the culture medium reduces hemopoietic differentiation in a dose–dependent manner, while inducing the emergence of the endothelial cell lineage. However, the possibility remains that at low doses and/or for short periods of time VEGF would be able to ensure the survival and differentiation of hemopoietic cells and that the culture medium would contain enough VEGF to trigger this effect. This possibility can be ruled out, however, given the results of the targeted deletion of the VEGF gene in mice. Although these mice die at embryonic days 9–10 due to insufficient formation of vascular endothelium, they show hemopoietic differentiation (29, 30). The two-ligand hypothesis therefore seems more likely. A possibility may be that VEGF-C (10, 11) could be responsible for triggering hemopoiesis in the early mesodermal VEGFR2+ progenitors.
The culture medium we used allows the development of different types of hemopoietic colonies from VEGFR2+ cells of the early mesoderm with a significant level of clonal efficiency. In contrast, this medium does not support growth of bone-marrow hemopoietic stem cells, because bone-marrow-derived hemopoietic colonies can arise only if the appropriate cytokines are added (20, 23). These early mesodermal hemopoetic stem cells are therefore of a type undescribed to date. In chimeric mice resulting from the association of normal and VEGFR2−/− morulas, no hemopoietic cells were derived from the mutant cells (F. Shalaby, J. Ho, W. Stanford, K.-D. Fischer, A. Bernstein, and J. Rossant, personal communication). This observation may imply that the early mesodermal VEGFR2+ cells are the progenitors of the whole contingent of hemopoetic stem cells, those functioning in the yolk sac as well as those emerging later on in the embryo and functioning throughout adult life. Altogether, these results rule out the possibility that failure of hemopoiesis in VEGFR2−/− mice (6) could be secondary due to the lack of endothelial cells.
How do these observations fit with the proposed notion of a common precursor for endothelial and hemopoietic cells, the hemangioblast? We show that a population of VEGFR2+ cells, isolated from the early mesoderm of gastrulating chicken embryos, can give rise to both cell types. However, one VEGFR2+ cell can give rise to either one or the other. Mixed colonies containing both hemopoietic and endothelial cells were not observed in our cultures. This could indicate that the VEGFR2+ population is heterogenous and contains two precursors, one for hemopoietic and one for endothelial cells. The alternative hypothesis is that the sorted VEGFR2+ population is homogenous and composed of true hemangioblasts able to follow two distinct differentiation pathways (hemopoietic and endothelial) depending on two growth factors competing for the same receptor. This cell can engage along only one of the two pathways, thus excluding the formation of mixed colonies. We cannot so far distinguish between these two hypotheses, but we know that both phenotypes develop from a cell the fate of which is determined by a single receptor. In the absence of this receptor, the early mesodermal precursors die (6), demonstrating that the VEGFR2 ligand(s) is essential for their survival.
Acknowledgments
We thank Drs. J. Folkman, B. Imhof, S. Ben Sasson, and K. Eichmann for critical reading of the manuscript; Drs. S.-I. Nishikawa and T. Kunisada for the MO90 vector and helpful advice; Drs. B. Imhof, S. Jeurissen, and P. Quéré for antibodies; F. Cormier, R. Lahav, and E. Dupin for discussions; H. San Clemente for computer drawings; A. Lehmann for technical assistance; and F. Viala and S. Gournet for photographic work. This work was supported by the Centre National de la Recherche Scientifique, the Association pour la Recherche sur le Cancer, the Ligue Nationale Française Contre le Cancer, and the Fondation pour la Recherche Médicale Français. A.E. was supported by the European Economic Community.
ABBREVIATIONS
- PA
posterior area
- ss
somite stage
- VEGFR
vascular endothelial growth factor receptor
- MGG
May–Grünwald–Giemsa
References
- 1.Sabin F R. Contributions to Embryology. Vol. 9. Carnegie Institution; 1920. , No. 36, pp. 214–262. [Google Scholar]
- 2.Murray P D F. Proc R Soc (London) Ser B. 1932;11:497–521. [Google Scholar]
- 3.Mustonen T, Alitalo K. J Cell Biol. 1995;129:895–898. doi: 10.1083/jcb.129.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shibuya M. Adv Cancer Res. 1995;67:281–316. doi: 10.1016/s0065-230x(08)60716-2. [DOI] [PubMed] [Google Scholar]
- 5.Fong G H, Rossant J, Gertsenstein M, Breitman M L. Nature (London) 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- 6.Shalaby F, Rossant J, Yamaguchi T P, Gertsenstein M, Wu X-F, Breitman M L, Schuh A C. Nature (London) 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 7.Sato T, Tozawa J, Deutsch U, Wolburg-Buchholz K, Fujiwara J, Gendron-Maguire M, Gridley T, Wolburg H, Risau W, Qin J. Nature (London) 1995;376:70–74. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- 8.Neufeld G, Tessler S, Gitay-Goren H, Cohen T, Levi B Z. Prog Growth Factor Res. 1994;5:89–97. doi: 10.1016/0955-2235(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 9.Olofsson B, Pajusola K, Kaipainen A, von Euler G, Joukov V, Saksela O, Orpana A, Petterson R F, Alitalo K, Eriksson V. Proc Natl Acad Sci USA. 1996;93:2576–2581. doi: 10.1073/pnas.93.6.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkinnen N, Alitalo K. EMBO J. 1996;15:290–298. [PMC free article] [PubMed] [Google Scholar]
- 11.Lee J, Gray A, Yuan J, Luoh S M, Avraham H, Wood W I. Proc Natl Acad Sci USA. 1996;93:1988–1992. doi: 10.1073/pnas.93.5.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park J E, Cjen H H, Winer J, Houck K A, Ferrara N. J Biol Chem. 1994;269:25646–25654. [PubMed] [Google Scholar]
- 13.Eichmann A, Marcelle C, Breant C, Le Douarin N M. Mech Dev. 1993;42:33–48. doi: 10.1016/0925-4773(93)90096-g. [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi T P, Dumont D J, Conlon R A, Breitman M L, Rossant J. Development (Cambridge, UK) 1993;118:489–498. doi: 10.1242/dev.118.2.489. [DOI] [PubMed] [Google Scholar]
- 15.Dumont D J, Fong G-H, Puri M C, Gradwohl G, Alitalo K, Breitman M L. Dev Dyn. 1995;203:80–92. doi: 10.1002/aja.1002030109. [DOI] [PubMed] [Google Scholar]
- 16.Nicolet G. J Embryol Exp Morphol. 1970;23:79–91. [PubMed] [Google Scholar]
- 17.Garcia-Martinez V, Alvarez I S, Schoenwolff G C. J Exp Zool. 1993;267:431–442. doi: 10.1002/jez.1402670409. [DOI] [PubMed] [Google Scholar]
- 18.Eichmann A, Marcelle C, Breant C, Le Douarin N. Gene. 1996;174:3–8. doi: 10.1016/0378-1119(96)00159-x. [DOI] [PubMed] [Google Scholar]
- 19.Köhler G, Milstein C. Eur J Immunol. 1976;6:511–515. doi: 10.1002/eji.1830060713. [DOI] [PubMed] [Google Scholar]
- 20.Cormier F, Dieterlen-Lièvre F. Development (Cambridge, UK) 1988;102:279–285. doi: 10.1242/dev.102.2.279. [DOI] [PubMed] [Google Scholar]
- 21.Palis J, McGrath K E, Kingsley P. Blood. 1995;86:156–163. [PubMed] [Google Scholar]
- 22.Lacoste-Eleaume A S, Bleux C, Quéré P, Coudert F, Corbel C, Kanellopoulos-Langevin C. Exp Cell Res. 1994;213:198–209. doi: 10.1006/excr.1994.1191. [DOI] [PubMed] [Google Scholar]
- 23.Corbel C, Cormier F, Pourquie O, Bluestein H. Exp Cell Res. 1992;203:91–99. doi: 10.1016/0014-4827(92)90043-8. [DOI] [PubMed] [Google Scholar]
- 24.Flamme I, Risau W. Development (Cambridge, UK) 1992;116:435–439. doi: 10.1242/dev.116.2.435. [DOI] [PubMed] [Google Scholar]
- 25.Pourquie O, Fan C-M, Coltey M, Hirsinger E, Watanabe Y, Bréant C, Francis-West P, Brickell P, Tessier-Lavigne M, Le Douarin N M. Cell. 1996;84:461–471. doi: 10.1016/s0092-8674(00)81291-x. [DOI] [PubMed] [Google Scholar]
- 26.McNagny K M, Lim F, Grieser S, Graf T. Leukemia. 1992;6:975–984. [PubMed] [Google Scholar]
- 27.Vainio O, Dunon D, Aïssi F, Dangy J-P, McNagny K M, Imhof B A. J Cell Biol. 1996;135:1655–1668. doi: 10.1083/jcb.135.6.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeurissen S H M, Vervelde L, Janse E M. Poultry Sci Rev. 1994;5:183–207. [Google Scholar]
- 29.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Nature (London) 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 30.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea K S, Powell-Braxton L, Hillan K J, Moore M W. Nature (London) 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]