Abstract
In 1924, Spemann and Mangold demonstrated the induction of Siamese twins in transplantation experiments with salamander eggs. Recent work in amphibian embryos has followed their lead and uncovered that cells in signalling centres that are located at the dorsal and ventral poles of the gastrula embryo communicate with each other through a network of secreted growth-factor antagonists, a protease that degrades them, a protease inhibitor and bone-morphogenic-protein signals.
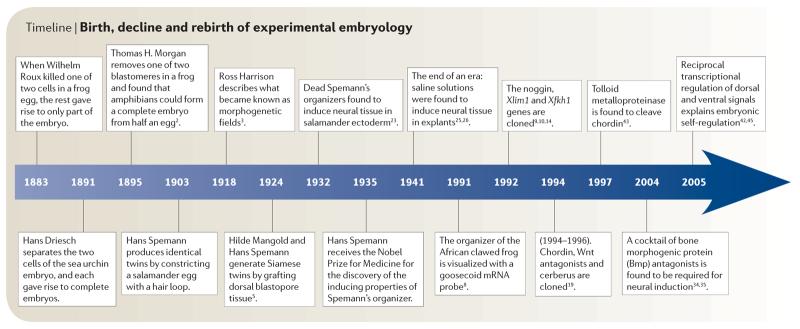
When an embryo is cut in half, it can self-regulate to regenerate the missing part (FIG. 1). The field of experimental embryology originated in 1883 when Roux killed one of two cells in a frog embryo with a hot needle and found that the rest gave rise to only part of the embryo, usually a right or a left half 1 (TIMELINE). However, in 1891, Driesch separated the two first blastomeres of the sea urchin embryo and found that each was able to self-regulate and give rise to complete, although smaller, embryos1. In 1895, Thomas Hunt Morgan — who before becoming a geneticist was an experimental embryologist — repeated Roux's experiment and showed that if one of the two blastomeres is gently pipetted out of a frog embryo (instead of killing it and leaving it in place), amphibians too could self-regulate and give rise to a complete embryo from half an egg2.
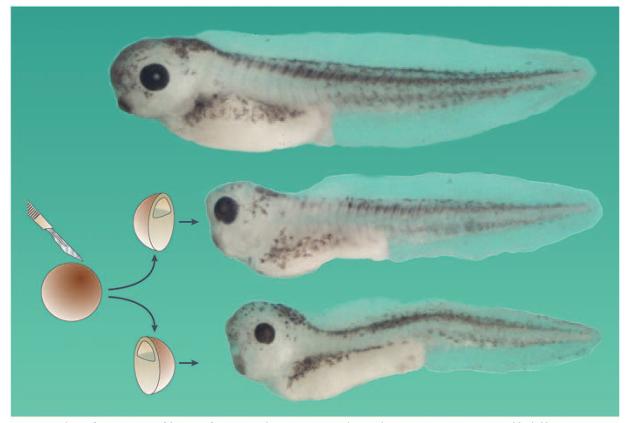
Figure 1. Embryonic self-regulation.
The entire early embryo constitutes a self-differentiating morphogenetic field, in which cells communicate with each other over great distances. This is demonstrated by experiments such as the one shown here, in which an African clawed frog (Xenopus laevis) embryo was cut into two halves at the blastula stage. If it is ensured that both halves contain part of the dorsal organizer region, two perfect identical twins are obtained (an intact sibling is shown at the top of the figure). In humans, identical twins are found in 3 out of 1,000 live births, and arise most frequently by the spontaneous separation of the inner cell mass of the mammalian blastula into two, followed by self-regulation60. The ultimate example of self-regulation is provided by another mammal, the nine-banded armadillo, in which every blastocyst gives rise to four genetically identical siblings50. Note that each twin is longer than just half the length of the intact sibling, which represents yet another effort to regulate towards the normal pattern. Both half-embryos shown here are derived from the same blastula.
Timeline.
Birth, decline and rebirth of experimental embryology
In 1903, Hans Spemann used a baby-hair loop (from his own daughter) to subdivide the cleaving amphibian (salamander) egg into two halves. If the half-embryo contained part of the future blastopore dorsal lip (the region where involution of the mesoderm starts), it formed a perfectly well-proportioned tadpole1 (FIG. 1). In 1918, the great American embryologist Ross Harrison carried out another remarkable experiment: if the forelimb field in the mesoderm of a salamander embryo was cut in half and transplanted into the flank of a host embryo, each half could induce the formation of a complete limb, not just half a limb3. The part of the embryo where this phenomenon takes place was called the ‘self-differentiating morphogenetic field’.
Self-regulation, as defined by these early experimental embryologists, is one of the most interesting and mysterious properties of embryos. What are the molecular mechanisms that explain the intrinsic tendency of the embryo to regulate towards the whole? Here, I recount the story of the birth, decline and revival of amphibian experimental embryology, and how recent studies have uncovered a molecular pathway of interacting extracellular proteins that explains how embryonic self-regulation works. This short review focuses on the advances made in amphibians, although great strides have also been made in other model systems, such as the fruitfly (Drosophila melanogaster), chick (Gallus gallus) and zebrafish (Danio rerio), as detailed elsewhere4. Rather than providing a comprehensive overview of the entire field of early development, this short timeline presents a personal account of the status of experimental embryology, a field that a century ago was at the very front of biological research.
Gene-fishing in Spemann's organizer
The starting point for understanding self-regulation was provided by the most famous experiment in embryology, the Spemann–Mangold organizer (hereafter referred to as Spemann's organizer) graft with salamander eggs5 (BOX 1). This experiment established the present view that animal development results from a succession of cell–cell inductions in which groups of cells, or organizing centres, signal the differentiation of their neighbours. The dorsal-lip graft induced neighbouring cells to adopt the normal pattern of tissue types, so that a Siamese twin was formed. In 1988, a memoir recounting the heyday of experimental embryology in the Spemann laboratory was published by Viktor Hamburger6. At least in my case, the modern revival of studies into Spemann's organizer can be traced to this little book, which retold the excitement of discovering the inductive powers of the organizer. Hamburger was 88 years old at the time — clearly, it is never too late to impact the scientific thinking of others.
Box 1. The Spemann–Mangold organizer experiment in 1924.
The photograph shows that when a small region of the embryo, the dorsal lip (albino cells in this case), is grafted to the opposite (ventral) side of a host gastrula embryo (see embryo on the left; note that the host's dorsal lip can also be seen), the resulting Xenopus laevis tadpole develops a Siamese twin 3 days later. X. laevis is an African clawed frog that is favoured in modern research because it lays eggs year-round. Hilde Mangold (neé Proescholdt), a graduate student with Hans Spemann at Freiburg University, Germany, used salamander eggs of species that differed in their pigmentation. Because the fate of the transplanted cells could therefore be traced during development, Spemann and Mangold5 were able to demonstrate that the graft became notochord, yet induced neighbouring cells to change fates. These neighbouring cells adopted differentiation pathways that were more dorsal, and produced tissues such as the central nervous system, somites and kidneys. Note that the transplanted cells ‘organize’ a perfect dorsal–ventral and antero–posterior pattern in the induced tissues. The Spemann–Mangold experiment firmly established the key importance of cell–cell inductions during animal development. Hilde Proescholdt married embryologist Otto Mangold, had a baby boy, and died tragically a few months later at the age of only 26, just before her landmark paper was published. For photographs of Hans Spemann and Hilde Mangold and a re-enactment of their transplantation experiment as carried out by the author, see Supplementary information S1 (movie). The image is reproduced, with permission, from REF. 19 © (2004) Annual Reviews.
In our own laboratory, studies on Spemann's organizer were approached by cloning its molecular components: cDNA libraries were generated from manually dissected dorsal blastopore lips from the African clawed frog (Xenopus laevis), which were first screened for homeobox sequences. Homeobox genes encode DNA-binding proteins that are involved in the control of development in fruitflies, and we had discovered years earlier that vertebrates, namely the African clawed frog, contained homeobox genes of the Hox type7. In 1991, our team succeeded in isolating a homeobox gene, goosecoid (Gsc), that was specifically expressed in Spemann's organizer8. The first in situ hybridizations of Gsc mRNA, constituted a truly memorable event, because Gsc mRNA demarcated, very specifically, tissue belonging to Spemann's organizer. Since its discovery almost three-quarters of a century earlier, the existence of Spemann's organizer had been deduced from its inductive effects after transplantation, but the expression pattern of Gsc now allowed us to visualize, for the first time, that the Spemann's organizer existed as a distinct molecular entity8.
A few months later, the groups of Igor Dawid and Milan Jamrich reported that genes encoding two other transcription factors, Xlim1 and Xfkh1/Hnf3β, were also expressed in the organizer region of the African clawed frog9,10. Importantly, microinjection of Gsc mRNA into ventral cells caused the formation of twinned axes, which indicated that the Gsc gene was part of the molecular machinery that leads to the activity of Spemann's organizer8. Because Gsc encodes a DNA-binding protein, we proposed that it might, in turn, activate the expression of secreted signalling proteins that execute the cell-differentiation changes in neighbouring cells. In time this was, in fact, found to be the case11,12. The Gsc gene was subsequently isolated in many other species, and became a widely used marker for comparative studies of gastrulation13.
In 1992, Richard Harland reported the isolation of the first secreted protein that was expressed in Spemann's organizer14. He and his colleagues used a different method — the functional screening for molecules that changed embryonic development after overexpression. In this expression-cloning procedure, pools of cDNAs were grown, transcribed in vitro with bacteriophage SP6 RNA polymerase and microinjected into African clawed frog embryos. Those with biological activity were then sub-selected until a single clone was identified. Many interesting genes have been isolated using this powerful method15. Microinjected noggin mRNA was found to induce neural tissue in explants of ectodermal cells16. It was subsequently found that follistatin, yet another secreted molecule that is specific to Spemann's organizer, was also a neural inducer17.
Spemann's organizer secretes many antagonists
Many secreted proteins that are specific to Spemann's organizer have been isolated from the African clawed frog gastrula through extensive molecular screens. The expectation was that new growth factors would be identified, but, instead, Spemann's organizer was discovered to signal mainly by secreting a cocktail of bone morphogenic protein (Bmp; these proteins are members of the transforming growth factor (Tgf)β superfamily) and Wnt antagonists18,19. The new components and molecular mechanisms that scientists had hoped to find were discovered in abundance, but the fact that these new components were antagonists of growth factors was a great surprise. Of all the organizer molecules shown in BOX 2, only anti-dorsalizing morphogenic protein (Admp) is a growth factor. Admp is a member of the Bmp family that is expressed in the dorsal organizer20,21. This localization is paradoxical because Bmps, including Admp, induce ventral development and also because Spemann's organizer normally arises in a region of low Bmp signalling (see below).
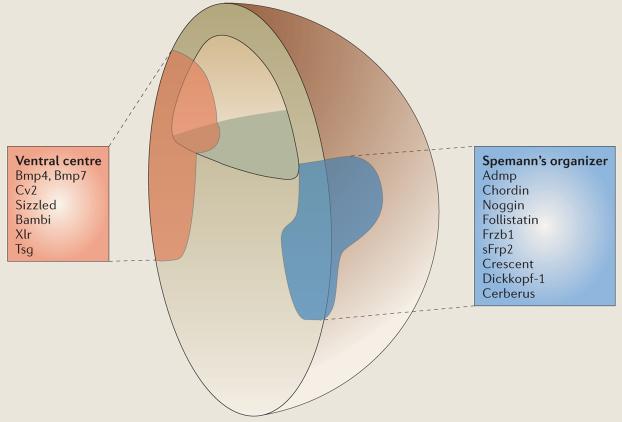
Box 2. Proteins secreted by the dorsal and ventral signalling centres in the gastrula.
The factors from Spemann's organizer chordin, noggin and follistatin are bone morphogenic protein (Bmp) antagonists, whereas Frzb1, secreted frizzled-related protein-2 (sFrp2), crescent and dickkopf-1 are Wnt antagonists19. Cerberus is a multivalent antagonist of Wnt, Bmps and transforming growth factor (Tgf)β–nodal signalling that induces head structures; its discovery led to the realization that head development is induced by anterior endoderm52,53. Most of these secreted antagonists work by binding to growth factors in the extracellular space, thereby preventing them from binding to membrane receptors. Dickkopf-1 works in a different way54: it binds to the Wnt transmembrane co-receptor low-density-lipoprotein-receptor-related protein-5/6 (Lrp5/6) and, together with another transmembrane protein that is known as kremen, induces endocytosis of the Wnt co-receptor and depletes it from the cell surface55. Anti-dorsalizing morphogenic protein (Admp) is a Bmp-like molecule that is, paradoxically, transcribed in regions where Bmp levels are low.
Chordin, a molecule with a key role in embryonic self-regulation, has an intricate mechanism of action. It contains four cysteine-rich domains that serve as Bmp-binding modules in many extracellular proteins that are involved in Bmp–Tgfβ signalling18,19. Chordin binds both to Bmp and to twisted-gastrulation (Tsg), forming a diffusible ternary complex that cannot bind to Bmp receptors18,56. Bmps are probably bound to Tsg most of the time, so that the overall effect of Tsg in zebrafish (Danio rerio) is to promote Bmp signalling, as shown by loss-of-function experiments57,58. Chordin activity is regulated by tolloids (known as xolloids in the African clawed frog (Xenopus laevis)) — zinc metalloproteinases that have pro-Bmp effects because they cleave chordin at two specific sites — which release Bmp that can then signal through Bmp receptors43.
Ventral-centre proteins19 include: first, the growth factors Bmp4 and Bmp7; second, crossveinless-2 (Cv2), a molecule that contains five Bmp-binding modules similar to those of chordin; third, sizzled, a molecule with the structure of an sFrp that functions as a feedback inhibitor of Bmp signalling46-48 indirectly by binding to and inhibiting metalloproteinases that degrade the Bmp antagonist chordin45; fourth, bambi (Bmp and activin membrane-bound inhibitor), a natural dominant-negative Bmp receptor that lacks the catalytic intracellular domain59; fifth, xolloid-related (Xlr), a zinc metalloproteinase that cleaves chordin very effectively43,44; and sixth, Tsg, a protein that binds both to chordin and Bmp56.
The ventral-centre factors
The realization is now emerging that a second signalling centre is formed in the ventral side of the gastrula19 (BOX 2). At early stages of development, the expression of ventral-centre genes tends to be diffuse and therefore the existence of this signalling centre was overlooked. The ventral-centre region marks the highest levels of signalling by Bmp4 and Bmp7. These growth factors bind to membrane Bmp receptors, which subsequently phosphorylate the end of a transcription factor known as Smad1, causing it to migrate into the nucleus and activate certain subsets of genes. Ventral-centre genes are coordinately activated by high Smad1 activity, and are part of what had been designated earlier as the Bmp4 synexpression group (that is, a group of genes that are coordinately activated during development)22. What is striking is that many components that are expressed in the ventral centre, and that are transcribed when Bmp signals are high, have counterparts in the dorsal centre, in which they are transcribed when Bmp signals are low. So, ventral Bmp4 and Bmp7 are matched dorsally by Admp, and ventral crossveinless-2 (Cv2) and sizzled are matched dorsally by chordin and crescent, respectively. This arrangement, in which proteins of similar biochemical activities are expressed at opposite poles of the embryo, provides a molecular framework for understanding embryonic self-regulation.
Bmp inhibition and neural induction
The demise of Spemann's organizer
One of the properties of the Spemann's organizer experiment that captured the imagination of embryologists was that dorsal-lip mesoderm induced the development of a complete central nervous system (CNS), the most complex and intricate of all organ systems. Spemann distinguished this phenomenon as the ‘primary embryonic induction’1. Eventually, however, the fixation with neural induction would bring the edifice of experimental embryology down. In 1932, it was discovered that boiled (and, therefore, dead) tissue from Spemann's organizer could induce neural tissue if sandwiched between layers of ectoderm or implanted into the blastula cavity23. Identifying the chemical substance that caused neural induction became the Holy Grail and attracted some of the best names in embryology such as J. Needham, C. Waddington, J. Brachet and J. Holtfreter. Many ‘heterologous inducers’ were found in tissue extracts, in purified fractions such as ribosomes (nucleoproteins), in fatty acids and sterols, and in clearly non-physiological substances such as methylene blue and even sand particles24. These discoveries became a funeral march for the field, as researchers realized that ectodermal explants of the salamander (but not the African clawed frog) embryo could be caused to differentiate into neural tissue very easily. The final blow came in the 1940s when Barth and Holtfreter discovered that ectodermal cells of Amblystoma maculatum (a close relative of the axolotl, or Mexican salamander) could differentiate into neural tissue without the need for any organizer or inducing substance, simply by culturing explants in sub-optimal saline solutions25,26. This brought an era of research to an end. By the time I was a student in the 1970s, it was common to hear comments such as “Spemann's organizer set developmental biology back by 50 years.”
The revival of Spemann's organizer
Experimental embryology had a renaissance when the endogenous factors from Spemann's organizer were cloned. Surprisingly, it was discovered that the neural induction by chordin, noggin and follistatin could be counteracted by the injection of Bmp4 mRNA27. Subsequently, in biochemical assays, these three antagonists were found to bind Bmp4 directly28-30.
Antisense morpholino oligos provide a powerful technology that allows the depletion of individual gene products31. Using these gene-specific inhibitors, it was found that chordin expression was absolutely required for the inductive activity of organizer transplants, but not for CNS formation in intact embryos32. In the mouse (Mus musculus), the knockout of both noggin and chordin is required before defects in forebrain development become apparent33. In the African clawed frog, triple knockdown of follistatin, chordin and noggin34, or of cerberus, chordin and noggin35, caused a catastrophic loss of CNS structures. So, the endogenous cocktail of Bmp antagonists that is secreted by tissue from Spemann's organizer is required for neural induction.
And what about the heterologous neural inducers that had paralysed the field for many decades? Work in the African clawed frog had shown that neural induction could also be obtained by simply dissociating ectodermal cells for a few hours36,37. Recently, it has been discovered that cell dissociation triggers the sustained activation of mitogen-activated protein kinase (Mapk), which, in turn, causes the phosphorylation of Smad1 at sites that inhibit the activity of this transcription factor38. In the course of normal development, phosphorylation of Smad1 by Mapk transmits signals from receptor tyrosine kinases such as fibroblast growth factor (Fgf) and insulin-like growth factor (Igf) receptors39,40. Mapk activation counteracts the activating effects of Bmp signalling on Smad1, thereby inhibiting epidermal fate and enhancing neural differentiation. Mapk is activated by many non-specific cellular stimuli — its activation probably explains the effects of heterologous inducers38.
Molecular self-regulation
Ubiquitous neural induction
Embryonic development has in-built redundancy to ensure that the process is error-free and reproducible from individual to individual. When Bmp2, Bmp4 and Bmp7 were knocked down simultaneously, African clawed frog embryos displayed larger dorsal structures but were found to still retain a significant dorsal–ventral pattern41. Surprisingly, in embryos that lacked Spemann's organizer (which were generated using tricks such as irradiating the egg with ultraviolet light or surgically preparing ventral half-embryos) the effects were much greater, with the depletion of Bmp2, Bmp4 and Bmp7 causing massive brain differentiations that lacked a dorsal–ventral pattern. This suggested that Spemann's organizer itself was a source of signals that compensated for the loss of Bmps. A main component of this signal was found to be the dorsal Bmp molecule Admp; indeed, the quadruple knockdown of Admp, Bmp2, Bmp4 and Bmp7 caused the entire ectoderm to become CNS tissue and eliminated epidermal-cell differentiation42 (FIG. 2a-d). So, when dorsal and ventral Bmp signals are depleted, the self-regulating morphogenetic field collapses and ubiquitous neural induction ensues.
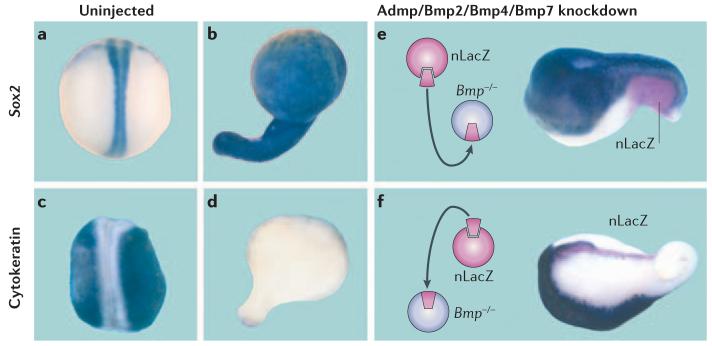
Figure 2. Ubiquitous neural differentiation: epidermal differentiation can be restored by transplantation of either a dorsal or a ventral centre.
Embryos were microinjected with four anti-Bmp (bone morphogenic protein) antisense morpholino oligos (against Admp (anti-dorsalizing morphogenic protein), Bmp2, Bmp4 and Bmp7) and stained for a neural (Sox2, top row) or an epidermal marker (cytokeratin, bottom row). a | An uninjected embryo that was stained for Sox2 mRNA and shows the normal central nervous system (CNS). b | After Bmp depletion, the entire embryo becomes covered uniformly by CNS tissue. c | Cytokeratin mRNA is abundantly expressed in skin. d | Cytokeratin mRNA disappears in Bmp-depleted embryos. e | When a ventral centre is grafted onto Bmp-depleted embryos (transplanted cells marked in red with the nuclear LacZ (nLacZ) lineage marker) the dorsal–ventral pattern is restored in part, even at a great distance from the graft. f | Surprisingly, the dorsal centre, or Spemann's organizer, also rescues the pattern: note that epidermis is induced, but at a considerable distance from the grafted tissue (the transplanted Spemann's organizer elongates because it gives rise to notochord). These experiments demonstrate: first, that Bmp inhibition causes neural induction and second, that the embryo has dorsal and ventral sources of Bmp signals. Reproduced, with permission, from REF. 42 © (2005) Elsevier.
Transplantation of wild-type tissue into these Bmp-depleted embryos showed that both the ventral and dorsal centres can serve as sources of Bmps that diffuse over considerable distances in the embryo and trigger changes in cell differentiation42, as shown in FIG. 2e,f. This double gradient of Bmp signals that emanate from opposite poles of the embryo assures the robustness of dorsal–ventral-pattern formation.
Cell–cell communication
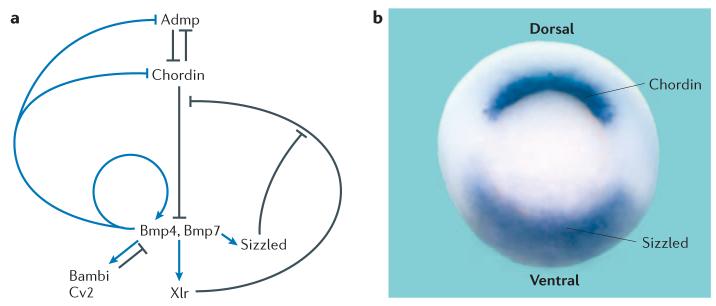
FIGURE 3 shows how a network of extracellular proteins regulates embryonic dorsal–ventral patterning. Genes from Spemann's organizer are transcribed when Bmp levels are low, whereas ventral-centre genes require high levels of Bmps for transcription. Self-regulation can be explained by this ‘see-saw’ of reciprocal transcriptional regulation42. As Bmp levels are lowered, transcription of Admp increases dorsally, which leads to compensation because Admp has Bmp-signalling activity. As Bmp-signalling levels increase, Bmp antagonists — such as bambi and sizzled — are transcribed in the ventral centre, where they function as negative-feedback regulators42. Although Admp is produced dorsally, it is unable to signal in this location because it binds to chordin. Admp only signals on the ventral side once chordin is cleaved by the xolloid-related (Xlr) metalloproteinase42,43, which is produced by the ventral centre44.
Figure 3. A network of interacting secreted proteins regulates dorsal–ventral cell communication.
a | The dorsal centre secretes chordin and anti-dorsalizing morphogenic protein (Admp), whereas the ventral centre secretes bone morphogenic protein (Bmp)4 and Bmp7, bambi, crossveinless-2 (Cv2), sizzled and the xolloid-related (Xlr) metalloproteinase. Self-regulation of the dorsal–ventral pattern is mediated by dorsal and ventral proteins that are under reciprocal transcriptional regulation by Bmp signals42,45. Arrows in black indicate direct protein–protein interactions, arrows in blue indicate transcriptional-regulation steps that are mediated by the transcription factor Smad1, which is activated by Bmp signals. b | In the gastrula embryo, chordin mRNA is expressed in the dorsal blastopore lip and sizzled is expressed in the ventral blastopore lip. The circular blastopore closes during gastrulation, eventually giving rise to the anus.
It has recently been discovered that sizzled antagonizes Bmp signalling by an indirect molecular mechanism: it is a competitive inhibitor of the Xlr metalloproteinase45. Although sizzled has the structure of a secreted frizzled-related protein (sFrp, a type of Wnt inhibitor) — and was therefore expected to be a Wnt antagonist — its loss-of-function phenotype in African clawed frog46 and zebrafish47,48 embryos was very similar to that of chordin. The Xlr enzyme has a similar chemical affinity (a dissociation constant (Kd) of ∼20 nM) for its substrate chordin and for its inhibitor sizzled45. Remarkably, the embryonic dorsal–ventral pattern is controlled by modulating the relative levels of these two proteins in the extracellular space.
Many organ-forming morphogenetic fields — such as those that are required for limb, lens, eye and heart growth — are formed during animal development3,49, and the reciprocal transcriptional regulation of signalling molecules might provide a general paradigm for understanding how they too self-regulate after experimental perturbations to form normal structures.
In the amphibian gastrula, dorsal and ventral signalling centres serve as sources of Bmps and their antagonists. Interestingly, the key step that controls the ongoing conversation between dorsal and ventral cells in the developing embryo is the regulation of the activity of the Xlr metalloproteinase that degrades chordin. Another remarkable characteristic of this network of self-regulating proteins is that it works largely by direct protein–protein inter actions in the extracellular milieu (FIG. 3).
Future prospects
The field of experimental embryology has a long and distinguished history. For most of the twentieth century, its guiding light was the Spemann's organizer experiment. The discipline hit a snag with the spurious discovery of heterologous inducers, and became dormant for about 40 years, during which time the genetics approach of T. H. Morgan dominated research50. The advent of gene cloning has revived experimental embryology. So much so, that we now have a molecular framework for one of the most mysterious problems in developmental biology: how do hundreds (or thousands) of cells in a morphogenetic field communicate to each other their relative positions, so that a perfect identical twin can be formed?
The African clawed frog embryo is a favourable biological model because the three main techniques of experimental biology can be applied to it. First, transplantation of cells to a new environment is possible, which allows their full regulatory potential to be challenged. Second, biochemistry with purified proteins can easily be applied in this system, which allows chemical affinities to be determined and components to be quantified in the embryo. Third, the loss of function of individual genes, which can presently be achieved by non-genetic means, can be applied to many genes simultaneously. The beauty of Spemann's experimental legacy has permeated all model systems. Together with the genetic approaches that are possible in zebrafish and mice, and the manipulations that are feasible in the chick embryo, we can expect much progress in the coming years. The problem of how cells communicate with each other over long ranges to regulate a field of differentiating cells is largely unsolved and still remains in its infancy.
Another avenue that is worth exploring is to use computer modelling to understand, in quantitative terms, how a network of proteins that interact with each other self-regulates. The mathematician Alan Turing proposed in 1952 that the formation of stable patterns during development might arise from an activator and an inhibitor that originate from the same source but that diffuse at different rates51. It seems possible that such activator–inhibitor pairs are provided dorsally by Admp–chordin and ventrally by Xrl–sizzled42,45. Understanding how the regulatory circuit shown in FIG. 3a works in detail will challenge systems biology. However, each component can be obtained as a pure recombinant protein, its binding affinities determined, and its gene products specifically depleted in the embryo of the African clawed frog. It appears that the discipline of experimental embryology is not quite dead yet, and that its rich heritage will continue to guide investigations into the molecular mechanisms by which cells communicate with each other for a long time into the future.
Supplementary Material
Acknowledgements
I would like to thank the multiple generations of postdoctoral and graduate students that helped unravel the Spemann's organizer in our laboratory. B. Reversade, H. Kuroda and H. Lee generously provided material for the figures. Many thanks also to the members of the University of California, Los Angeles (UCLA) Embryology Club, which celebrates its twentieth anniversary this year, for providing the atmosphere that made developing these ideas possible. Our work is supported by the Norman Sprague Endowment, a National Institutes of Health MERIT Award and the Howard Hughes Medical Institute.
Footnotes
Competing interests statement
The author declares no competing financial interests.
DATABASES
The following terms in this article are linked online to:
Entrez Gene:
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=gene
Gsc | noggin | Xflh1
UniProtKB: http://us.expasy.org/uniprot
Bmp4 | Bmp7 | chordin | Cv2 | follistatin | sizzled
FURTHER INFORMATION
Eddy De Robertis' laboratory:
http://www.hhmi.ucla.edu/derobertis/
Xenbase: a X. laevis web resource: http://www.xenbase.org/
The origins of Entwicklungsmechanik: http://www.devbio.com/article.php?ch=3&id=15
SUPPLEMENTARY INFORMATION
See online article: S1 (movie)
Access to this interactive links box is free online.
References
- 1.Spemann H. Embryonic Development and Induction. Yale Univ.; New Haven: 1938. [Google Scholar]
- 2.Morgan TH. Half embryos and whole embryos from one of the first two blastomeres. Anat. Anz. 1895;10:623–638. [Google Scholar]
- 3.Harrison RG. Experiments on the development of the fore-limb of Amblystoma, a self-differentiating equipotential system. J. Exp. Zool. 1918;25:413–461. [Google Scholar]
- 4.Stern CD, editor. Gastrulation. Cold Spring Harbor Laboratory; New York: 2004. [Google Scholar]
- 5.Spemann H, Mangold H. Induction of embryonic primordia by implantation of organizers from a different species. Roux's Arch. Entw. Mech. 1924;100:599–638. [Google Scholar]
- 6.Hamburger V. The Heritage of Experimental Embryology: Hans Spemann and the Organizer. Oxford Univ.; Oxford, UK; 1988. [Google Scholar]
- 7.Carrasco AE, McGinnis W, Gehring WJ, De Robertis EM. Cloning of an X. laevis gene expressed during early embryogenesis coding for a peptide region homologous to Drosophila homeotic genes. Cell. 1984;37:409–414. doi: 10.1016/0092-8674(84)90371-4. [DOI] [PubMed] [Google Scholar]
- 8.Cho KWY, Blumberg B, Steinbeisser H, De Robertis EM. Molecular nature of Spemann's organizer: the role of the Xenopus homeobox gene goosecoid. Cell. 1991;67:1111–1120. doi: 10.1016/0092-8674(91)90288-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taira M, Jamrich M, Good PJ, Dawid IB. The LIM domain-containing homeo box gene Xlim-1 is expressed specifically in the organizer region of Xenopus gastrula embryos. Genes Dev. 1992;6:356–366. doi: 10.1101/gad.6.3.356. [DOI] [PubMed] [Google Scholar]
- 10.Dirksen ML, Jamrich M. A novel, activin-inducible, blastopore lip-specific gene of Xenopus laevis contains a fork head DNA-binding domain. Genes Dev. 1992;6:599–608. doi: 10.1101/gad.6.4.599. [DOI] [PubMed] [Google Scholar]
- 11.Niehrs C, Keller R, Cho KWY, De Robertis EM. The homeobox gene goosecoid controls cell migration in Xenopus embryos. Cell. 1993;72:491–503. doi: 10.1016/0092-8674(93)90069-3. [DOI] [PubMed] [Google Scholar]
- 12.Sasai Y, Lu B, Steinbeisser H, Geissert D, Gont LK, De Robertis EM. Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell. 1994;79:779–790. doi: 10.1016/0092-8674(94)90068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Robertis EM. In: Gastrulation. Stern CD, editor. Cold Spring Harbor Laboratory; New York: 2004. pp. 581–589. [Google Scholar]
- 14.Smith WC, Harland RM. Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell. 1992;70:829–840. doi: 10.1016/0092-8674(92)90316-5. [DOI] [PubMed] [Google Scholar]
- 15.Harland R. Neural induction. Curr. Opin. Genet. Dev. 2002;10:357–362. doi: 10.1016/s0959-437x(00)00096-4. [DOI] [PubMed] [Google Scholar]
- 16.Lamb TM, et al. Neural induction by secreted polypeptide noggin. Science. 1993;262:713–718. doi: 10.1126/science.8235591. [DOI] [PubMed] [Google Scholar]
- 17.Hemmati-Brivanlou A, Kelly OG, Melton DA. Follistatin, an antagonist of activin, is expressed in the Spemann organizer and displays direct neuralizing activity. Cell. 1994;77:283–295. doi: 10.1016/0092-8674(94)90320-4. [DOI] [PubMed] [Google Scholar]
- 18.De Robertis EM, Larraín J, Oelgeschläger M, Wessely O. The establishment of Spemann's organizer and patterning of the vertebrate embryo. Nature Rev. Genet. 2000;1:171–181. doi: 10.1038/35042039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Robertis EM, Kuroda H. Dorsal–ventral patterning and neural induction in Xenopus embryos. Annu. Rev. Cell Dev. Biol. 2004;20:285–308. doi: 10.1146/annurev.cellbio.20.011403.154124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moos M, Jr., Wang S, Krinks M. Anti-dorsalizing morphogenetic protein is a novel TGF-β homolog expressed in the Spemann organizer. Development. 1995;121:4293–4301. doi: 10.1242/dev.121.12.4293. [DOI] [PubMed] [Google Scholar]
- 21.Dosch R, Niehrs C. Requirement for anti-dorsalizing morphogenetic protein in organizer patterning. Mech. Dev. 2000;90:195–203. doi: 10.1016/s0925-4773(99)00245-2. [DOI] [PubMed] [Google Scholar]
- 22.Niehrs C, Pollet N. Synexpression groups in eukaryotes. Nature. 1999;402:483–487. doi: 10.1038/990025. [DOI] [PubMed] [Google Scholar]
- 23.Bautzman H, Holtfreter J, Spemann H, Mangold O. Versuche zur Analyse der Induktionsmittel in der Embryonalentwicklung. Naturwissenschaften. 1932;20:971–974. [Google Scholar]
- 24.Holtfreter J, Hamburger V. In: Analysis of Development. Willier BH, Weiss PA, Hamburger V, editors. W. B. Saunders; Philadelphia: 1955. pp. 230–296. [Google Scholar]
- 25.Barth LG. Neural diffferentiation without organizer. J. Exp. Zool. 1941;87:371–384. [Google Scholar]
- 26.Holtfreter J. Neural differentiation of ectoderm through exposure to saline solution. J. Exp. Zool. 1944;95:307–343. [Google Scholar]
- 27.Sasai Y, Lu B, Steinbeisser H, De Robertis EM. Regulation of neural induction by the chd and BMP-4 antagonistic patterning signals in Xenopus. Nature. 1995;376:333–336. doi: 10.1038/376333a0. [DOI] [PubMed] [Google Scholar]
- 28.Piccolo S, Sasai Y, Lu B, De Robertis EM. Dorsoventral patterning in Xenopus: inhibition of ventral signals by direct binding of Chordin to BMP-4. Cell. 1996;86:589–598. doi: 10.1016/s0092-8674(00)80132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zimmerman LB, De Jesús-Escobar JM, Harland RM. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996;86:599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]
- 30.Thompson TB, Lerch TF, Cook RW, Woodruff TK, Jardetzky TS. The structure of the follistatin:activin complex reveals antagonism of both Type I and Type II receptor binding. Dev. Cell. 2005;9:535–543. doi: 10.1016/j.devcel.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 31.Heasman J. Morpholino oligos: making sense of antisense? Dev. Biol. 2002;243:209–214. doi: 10.1006/dbio.2001.0565. [DOI] [PubMed] [Google Scholar]
- 32.Oelgeschläger M, Kuroda H, Reversade B, De Robertis EM. Chordin is required for the Spemann organizer transplantation phenomenon in Xenopus embryos. Dev. Cell. 2003;4:219–230. doi: 10.1016/s1534-5807(02)00404-5. [DOI] [PubMed] [Google Scholar]
- 33.Bachiller D, et al. The organizer secreted factors Chordin and Noggin are required for forebrain development in the mouse. Nature. 2000;403:658–661. doi: 10.1038/35001072. [DOI] [PubMed] [Google Scholar]
- 34.Khokha MK, Yeh J, Grammer TC, Harland RM. Depletion of three BMP antagonists from Spemann's organizer leads to a catastrophic loss of dorsal structures. Dev. Cell. 2005;8:401–411. doi: 10.1016/j.devcel.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 35.Kuroda H, Wessely O, Robertis EM. Neural induction in Xenopus: requirement for ectodermal and endomesodermal signals via Chordin, Noggin,β-catenin, and Cerberus. PLoS Biol. 2004;2:623–633. doi: 10.1371/journal.pbio.0020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grunz H, Tacke L. Neural differentiation of Xenopus laevis ectoderm takes place after disaggregation and delayed reaggregation without inducer. Cell Differ. Dev. 1989;28:211–217. doi: 10.1016/0922-3371(89)90006-3. [DOI] [PubMed] [Google Scholar]
- 37.Wilson PA, Hemmati-Brivanlou A. Induction of epidermis and inhibition of neural fate by Bmp-4. Nature. 1995;376:331–333. doi: 10.1038/376331a0. [DOI] [PubMed] [Google Scholar]
- 38.Kuroda H, Fuentealba L, Ikeda A, Reversade B, De Robertis EM. Default neural induction: neuralization of dissociated Xenopus cells is mediated by Ras/MAPK activation. Genes Dev. 2005;19:1022–1027. doi: 10.1101/gad.1306605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Massagué J. Integration of Smad and MAPK pathways: a link and a linker revisited. Genes Dev. 2003;17:3023–3028. doi: 10.1101/gad.1167003. [DOI] [PubMed] [Google Scholar]
- 40.Stern CD. Neural induction: old problem, new findings, yet more questions. Development. 2005;132:2007–2021. doi: 10.1242/dev.01794. [DOI] [PubMed] [Google Scholar]
- 41.Reversade B, Kuroda H, Lee H, Mays A, De Robertis EM. Depletion of Bmp2, Bmp4, Bmp7 and Spemann organizer signals induces massive brain formation in Xenopus embryos. Development. 2005;132:3381–3392. doi: 10.1242/dev.01901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reversade B, De Robertis EM. Regulation of ADMP and BMP2/4/7 at opposite embryonic poles generates a self-regulating morphogenetic field. Cell. 2005;123:1147–1160. doi: 10.1016/j.cell.2005.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Piccolo S, et al. Cleavage of Chordin by the Xolloid metalloprotease suggests a role for proteolytic processing in the regulation of Spemann organizer activity. Cell. 1997;91:407–416. doi: 10.1016/s0092-8674(00)80424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dale L, Evans W, Goodman SA. Xolloid-related: a novel BMP1/Tolloid-related metalloprotease is expressed during early Xenopus development. Mech. Dev. 2002;119:177–190. doi: 10.1016/s0925-4773(02)00359-3. [DOI] [PubMed] [Google Scholar]
- 45.Lee HX, Ambrosio AL, Reversade B, De Robertis EM. Embryonic dorsal–ventral signaling: secreted frizzled-related proteins as inhibitors of tolloid proteinases. Cell. 2006;124:147–159. doi: 10.1016/j.cell.2005.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Collavin L, Kirschner MW. The secreted Frizzled-related protein Sizzled functions as a negative feedback regulator of extreme ventral mesoderm. Development. 2003;130:805–816. doi: 10.1242/dev.00306. [DOI] [PubMed] [Google Scholar]
- 47.Yabe T, et al. Ogon/Secreted Frizzled functions as a negative feedback regulator of Bmp signaling. Development. 2003;130:2705–2716. doi: 10.1242/dev.00506. [DOI] [PubMed] [Google Scholar]
- 48.Martyn U, Schulte-Merker S. The ventralized ogon mutant phenotype is caused by a mutation in the zebrafish homologue of Sizzled, a secreted Frizzled-related protein. Dev. Biol. 2003;260:58–67. doi: 10.1016/s0012-1606(03)00221-5. [DOI] [PubMed] [Google Scholar]
- 49.De Robertis EM, Morita EA, Cho KWY. Gradient fields and homeobox genes. Development. 1991;112:669–678. doi: 10.1242/dev.112.3.669. [DOI] [PubMed] [Google Scholar]
- 50.Morgan TH. Embryology and Genetics. Columbia Univ.; New York: 1934. [Google Scholar]
- 51.Turing AM. The chemical basis of morphogenesis. Phil. Trans. Royal Soc. 1952;237:37–72. [Google Scholar]
- 52.Bouwmeester T, Kim SH, Sasai Y, Lu B, De Robertis EM. Cerberus is a head-inducing secreted factor expressed in the anterior endoderm of Spemann's organizer. Nature. 1996;382:595–601. doi: 10.1038/382595a0. [DOI] [PubMed] [Google Scholar]
- 53.Beddington RSP, Robertson EJ. Axis development and early asymmetry in mammals. Cell. 1999;96:195–209. doi: 10.1016/s0092-8674(00)80560-7. [DOI] [PubMed] [Google Scholar]
- 54.Glinka A, et al. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- 55.Mao B, et al. Kremen proteins are Dickkopf receptors that regulate Wnt–β-catenin signalling. Nature. 2002;417:664–667. doi: 10.1038/nature756. [DOI] [PubMed] [Google Scholar]
- 56.Oelgeschläger M, Larraín J, Geissert D, De Robertis EM. The evolutionarily conserved BMP-binding protein Twisted gastrulation promotes BMP signalling. Nature. 2000;405:757–763. doi: 10.1038/35015500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Little SC, Mullins MC. Twisted gastrulation promotes BMP signaling in zebrafish dorsal–ventral axial patterning. Development. 2004;131:5825–5835. doi: 10.1242/dev.01464. [DOI] [PubMed] [Google Scholar]
- 58.Xie J, Fisher S. Twisted gastrulation enhances BMP signaling through chordin dependent and independent mechanisms. Development. 2005;132:383–391. doi: 10.1242/dev.01577. [DOI] [PubMed] [Google Scholar]
- 59.Onichtchouk D, et al. Silencing of TGF-β signalling by the pseudoreceptor BAMBI. Nature. 1999;401:480–485. doi: 10.1038/46794. [DOI] [PubMed] [Google Scholar]
- 60.Sadler TW. Langman's Medical Embryology. 9th edn Lippincott Williams & Wilkins; Baltimore: 2004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.