Abstract
Berberine, a botanical alkaloid used to control blood glucose in type 2 diabetes in China, has been reported to activate AMPK recently. However, it is not clear how AMPK is activated by berberine. In this study, activity and action mechanism of berberine were investigated in vivo and in vitro. In dietary obese rats, berberine increased insulin sensitivity after five week administration. Fasting insulin and HOMA-IR were decreased by 46% and 48% in the rats, respectively. In cell lines including 3T3-L1 adipocytes, L6 myotubes, C2C12 myotubes and H4IIE hepatocytes, berberine was found to increase glucose consumption, 2-deoxy-glucose uptake and to a less degree 3-O-methyl-glucose (3-OMG) uptake independently of insulin. The insulin-induced glucose uptake was enhanced by berberine in the absence of change in IRS-1 (S307/312), Akt, P70S6 and ERK phosphorylation. AMPK phosphorylation was increased by berberine at 0.5 h and the increase remained for at least 16 h. Aerobic and anaerobic respiration were determined to understand the mechanism of berberine action. The long-lasting phosphorylation of AMPK was associated with persistent elevation in AMP/ATP ratio and reduction in oxygen consumption. An increase in glycolysis was observed with a rise in lactic acid production. Berberine exhibited no cytotoxicity, and protected plasma membrane in L6 myotubes in the cell culture. These results suggest that berberine enhances glucose metabolism by stimulation of glycolysis, which is related to inhibition of glucose oxidation in mitochondria. Berberine-induced AMPK activation is likely a consequence of mitochondria inhibition that increases AMP/ATP ratio.
Keywords: Type 2 diabetes, Insulin sensitivity, Oxygen consumption, Mitochondrial function, AMPK
Berberine is a botanical alkaloid in the roots and bark of several plants including Coptis chinensis French, an ancient Chinese herb, which has been used to treat diabetes for thousands of years in China. Berberine is the main active compound of the herb. In addition to the metabolic activities, berberine has a well-established antimicrobial activities in the control of infection by bacteria, viruses, fungi, protozoans and helminthes (8, 14). It is an over-the-counter drug for the treatment of gastrointestinal infections in China. In 1980’, the hypoglycemic effect of berberine was found when berberine was used to treat diarrhea in diabetic patients in China (13). Since then, berberine has been used as an anti-hyperglycemic agent by many physicians in China. There are many clinical reports about the hypoglycemic action of berberine in Chinese literature.
Regarding mechanism of berberine action, we found that berberine increased glucose metabolism in cultured cells and this activity was comparable to that observed for metformin in 2002 (20). This activity was confirmed later in other studies and AMPK was proposed to mediate the metabolic activities of berberine (2, 3, 9, 22). In 2006, it was reported that berberine was able to activate AMPK for inhibition of lipid synthesis in human hepatocytes (2). Berberine was reported to reduce body weight and improve glucose metabolism in animal models of metabolic syndrome (9). Berberine was found to induce phosphorylation of AMPK in 3T3-L1 adipocytes and L6 myotubes, and AMPK was proposed to explain the insulin sensitizing effect of berberine (9). Activation of AMPK pathway by berberine was also observed by two other groups (3, 22). Although AMPK is activated by berberine, the metabolic conditions that lead to this effect require further investigation. Berberine was shown to inhibit citric acid cycle in isolated mitochondria (11, 12, 1). However, it is not clear if this activity can be observed in living cells for AMPK activation.
In this study, the metabolic activity of berberine was examined in diabetic rats and the action mechanism was investigated in cellular models. The result suggests that berberine stimulates glucose metabolism through induction of glycolysis. Activation of AMPK by berberine is related to an increase in AMP/ATP ratio. Inhibition of glucose oxidation in mitochondria may contribute to the AMP/ATP ratio increase and AMPK activation.
RESEARCH DESIGN AND METHODS
Reagents
DMEM, 3-isobutyl-l-methyl-xanthine (IBMX), dexamethasone, insulin solution, 2-deoxy-D-glucose, 3-O-methyl-D-glucopyranose and Cytochalasin B were purchased from Sigma Chemicals (St. Louis, MO). The fetal bovine serum (FBS) was purchased from ATALANTA Biologicals (Lawrenceville, GA). Glucose color reagent was purchased from RAICHEM (San Diego, CA). 2-deoxy-D-[3H]-glucose and 3-O-[methyl-14C]-D-glucose were purchased from Perkin Elmer (Boston, MA). Antibodies to Phospho-AKT (Thr308) and Phospho-p70 S6 Kinase (Thr389) were purchased from the Cell Signaling Technology (Danvers, MA 01923). Anti-goat IgG (HRP conjugated), antibodies to AKT1/2, GLUT4, and Phospho-IRS-1 (Tyr632) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Antibody to Phospho-IRS-1 (Ser307) was purchased from Upstate USA (Charlottesville, VA). Antibodies to α-Tubulin and GLUT1 were obtained from Abcam (Cambridge, MA). Horseradish peroxidase-conjugated anti-rabbit IgG or anti-mouse IgG were purchased from GE Healthcare UK Ltd (Buckinghamshire HP7 9NA, England). PVDF membrane for immunoblot was purchased from BIO-RAD (Hercules, CA). Protein assay kit was purchased from PIERCE (Rockford, IL). Berberine for animal experiments was from the Materia Medica Factory of Meitan County, Guizhou Province, China. Berberine for in vitro experiments was from Sigma Chemicals (St. Louis, MO).
Animal experiments
Male Wistar rats (Experimental Animals Center of Chinese Academic, Shanghai, China), 2~3 months in age and ~240 g in body weight, were housed individually in wire cages in a temperature-controlled room (22±1 °C) on a 12 h/12 h light/dark cycle. The animals were fed a high-fat diet (HFD) (lard, 59% of calories) with free access to food and water. The animal protocol was approved by the institutional board. After six months on HFD, the animals were divided into control (n=6) and berberine-treated (n=6) groups. Berberine was administrated at 125 mg/Kg twice a day at 10:00 and 22:00 by gastric gavage for 5 weeks. Control rats were gavaged with an equal volume of vehicle (distilled water). After 5-week treatment, intraperitoneal glucose tolerance test (IPGTT) was conducted between 9:00 and 11:00 on 10 hours fasted animals. Fasting blood was firstly collected by lacerating the tail vein before glucose was given at 2g/Kg body weight (40% glucose solution). Then blood samples were collected at 2 h. Fasting insulin was detected using LINGO Rat Insulin RIA Kit (ST. CHARLES, MO). The HOMA method was used to determine insulin resistance (HOMA-IR) (10).
Cells
The mouse fibroblast 3T3-L1 preadipocytes, rat skeletal myoblasts L6, mouse myoblasts C2C12 and rat hepatoma cell line H4IIE were purchased from the American Type Culture Collection (ATCC, Manassas, VA) and maintained in DMEM culture medium supplemented with 10% FBS, 4 mmol/L glutamine, and 50 mg/L gentamicin in an atmosphere of 5% CO2 at 37 °C. For adipogenesis, 3T3-L1 preadipocytes were grown into confluence in a 100-mmol/L plate, and then were treated with the adipogenic cocktail (5 μg/ml insulin, 0.5 mmol/L isobutylmethylxanthine and 10 μmol/L dexamethasone) for 4 days. This was followed by incubation in insulin-supplemented medium for additional 3 days. The normal medium was used at day 7 to maintain the adipocytes (6). For differentiation of myotubes, L6 cells were cultured in DMEM with 10% FBS for 24 h. The cells were then maintained in 2% FBS medium (αMEM) for 6 days to induce differentiation. The medium was changed every 48 h (15). For C2C12 cells, DMEM supplemented with 2% horse serum was used to induce differentiation. The inducing process was similar to that of L6 cells (16).
Glucose consumption
The cells were cultured in 96-well tissue culture plates. When experiments, the normal culture medium was replaced by DMEM supplemented with 0.25% bovine serum albumin (BSA). Then, the cells were treated with berberine and/or insulin (final concentration 100 nmol/L) for 24 hours. The glucose concentration in medium was determined by glucose oxidase method. Glucose of the wells with cells was subtracted from glucose of the blank wells to obtain the amount of glucose consumption (20).
Glucose uptake
3T3-L1 preadipocytes, L6 or C2C12 myoblasts were differentiated into mature adipocytes or myotubes in a 12-well plate. For 2-deoxy-glucose (or 3-O-methyl-glucose) uptake, after berberine treatment in serum-free DMEM with 0.25% BSA for 16 hours, the cells were treated with insulin (200 nmol/L) for 20 min at 37°C in PBS (1 ml/well). After washing in PBS, the cells were incubated in 1 ml PBS containing 0.1 mmol/L of 2-deoxy-D-glucose (or 3-O-methyl-D-glucose) and 1 μCi/ml 2-deoxy-D-3H-glucose (or 3-O-[methyl-14C]-D-glucose) for 5 min. Then, the cells were washed twice in ice-cold PBS, and lyzed in 0.4 ml of 1% sodium dodecyl sulfate. 3H-glucose (or 14C-glucose) content was determined in 4 ml of scintillant using a Beckman LS6500 scintillation counter. Nonspecific glucose uptake is measured in the presence of 20 μmol/L cytochalasin B and is subtracted from the total uptake to get specific glucose uptake (6,18).
Western blotting
Cells were treated with berberine in serum-free medium with 0.25% BSA. In some experiments insulin (200 nmol/L) was added 20 minutes before cell collection. The whole cell lysate was made by sonication in lysis buffer (1% Triton X-100, 50 mmol/L KCI, 25 mmol/L HEPES, pH 7.8, 10 μg/ml leupeptin, 20 μg/ml aprotinin, 125 μmol/L dithiothreitol, 1 mmol/L phenylmethylsulfonyl fluoride, 1 mmol/L sodium orthovanadate). The protein (100 μg) was boiled for 5 min, resolved in 7% mini-SDS-PAGE for 90 min at 100 volts, and blotted on the polyvinylidene difluoride membrane (Bio-Rad, Hercules, CA). After being pre-blotted in milk buffer for 30 minutes, the membrane was blotted with the first antibody for 2 h – 12 h and the secondary antibody for 30 minutes. The horseradish peroxidase-conjugated secondary antibodies were used with chemiluminescence reagent (PerkinElmer Life Sciences, Boston, MA) for generation of the light signal. To detect multiple signals from one membrane, the membrane was treated with a stripping buffer (0.5 mol/L NaOH) for 20 min after each cycle of blotting to remove the bound antibody. All of the experiments were conducted three times (5). ImageJ 1.37V was used to quantify the Western signals.
LDH-cytotoxicity assay
3T3-L1 adipocytes and L6 myotubes were cultured in 24-well plates. After berberine treatment for 24 h in serum-free DMEM supplemented with 0.25% BSA, lactate dehydragenase (LDH) concentration in the medium was detected with LDH-Cytotoxicity Assay Kit II (BioVision, Mountain View, CA).
Oxygen consumption
Oxygen consumption was performed in BD Oxygen Biosensor Systems (BD biosciences, Bedford, MA). After differentiation, the 3T3-L1 adipocytes or L6 myotubes were trypsinized and plated to a plate embedded with an oxygen-sensitive dye in DMEM culture medium supplemented with 10% FBS. After six hours, berberine and/or insulin (final concentration 100 nmol/L) were added to the medium. Fluorescence reading was taken at different times. The units of oxygen consumption result, normalized relative fluorescence units (NRFU), were obtained by dividing the fluorescence values of cells with the value of blank wells.
Adenine nucleotide contents assay
3T3-L1 adipocytes and L6 myotubes were cultured in 6-well plates. They were treated with 5 μmol/L berberine for 0.5 h or 16 h in serum-free medium with 0.25% BSA. Then ATP and AMP contents of the cells were measured using high-performance liquid chromatography (HPLC) described by Wynants et al (17). In brief, the cells were lysed in 0.6 N HClO4 and neutralized with 1 N KHCO3. HPLC analysis was developed on a HPLC system (Waters Delta 600, Waters Co., Milford, MA) consisting of a solvent delivery pump unit (Waters 600), an autosampler (Waters 717 plus), a UV-Vis diode array detector (Waters 2996 Photodiode Array Detector, 190 to 800 nm). The system was computer controlled and analyzed with the Empower software system (Waters Delta 600, Waters Co., Milford, MA). Separation was carried out using an Atlantis T3 column (5.0 μm, 4.6 × 150 mm I.D.; Waters Co) with guard column (4.6 × 20 mm I.D; Waters Co). The mobile phase consisted of (A) an aqueous buffer containing 0.15 M phosphoric acid (10 ml of 85% H3PO4 per liter) adjusted to pH 6.00 with ammonium hydroxide (approx 8 ml) and (B) a mixture of acetonitrile and methanol (50/50, v/v). The optimal mobile phase was set as below: 0~5 min, 100% A; 5~25 min, gradient to A/B = 90: 10; the flow rate was set as 0.7 ml/min and the wavelength was set at 259 nm. ATP and AMP appeared at 9.6 and 18.1 min, respectively.
Lactate assay
The cells were cultured in a 24-well plate and treated with berberine and/or insulin in serum-free DMEM supplemented with 0.25% BSA. The lactate concentration in the medium was detected by Lactate Assay Kit from Biomedical Research Service Center, University at Buffalo (Buffalo, NY).
Statistical analysis
Data are presented as means ± SEM. All experiments in cells were performed at least in triplicate. When a single comparison was performed, the significance of the differences between means was analyzed by student t test. When multiple comparisons were performed, the significance was analyzed by one-way ANOVA (SPSS 12.0). When a significant effect was found, differences between means were determined by Fisher’s Least Significant Difference post hoc test. The α level was set at 0.05.
RESULTS
Berberine improved insulin sensitivity in dietary obese rats
In this study, dietary obese rats were used as an animal model of insulin resistance. At six months on the high-fat diet, blood glucose was significantly elevated in the Wistar rats in fasting and fed conditions. With five-week treatment by berberine, both fasting blood glucose (FBG) and postprandial blood glucose (PBG) were significantly reduced in the dietary obese rats (Fig. 1A). FBG was reduced from 5.74 mmol/L to 5.39 mmol/L (P=0.035). PEG was reduced from 10.4 mmol/L to 7.6 mmol/L (P=0.039), which is close to the normal glucose level. Fasting insulin level was decreased by 46% (P=0.045; Fig. 1B). The insulin sensitivity was increased significantly by berberine as indicated by 48% reduction (P=0.036; Fig. 1C) in HOMA-IR.
Fig. 1.
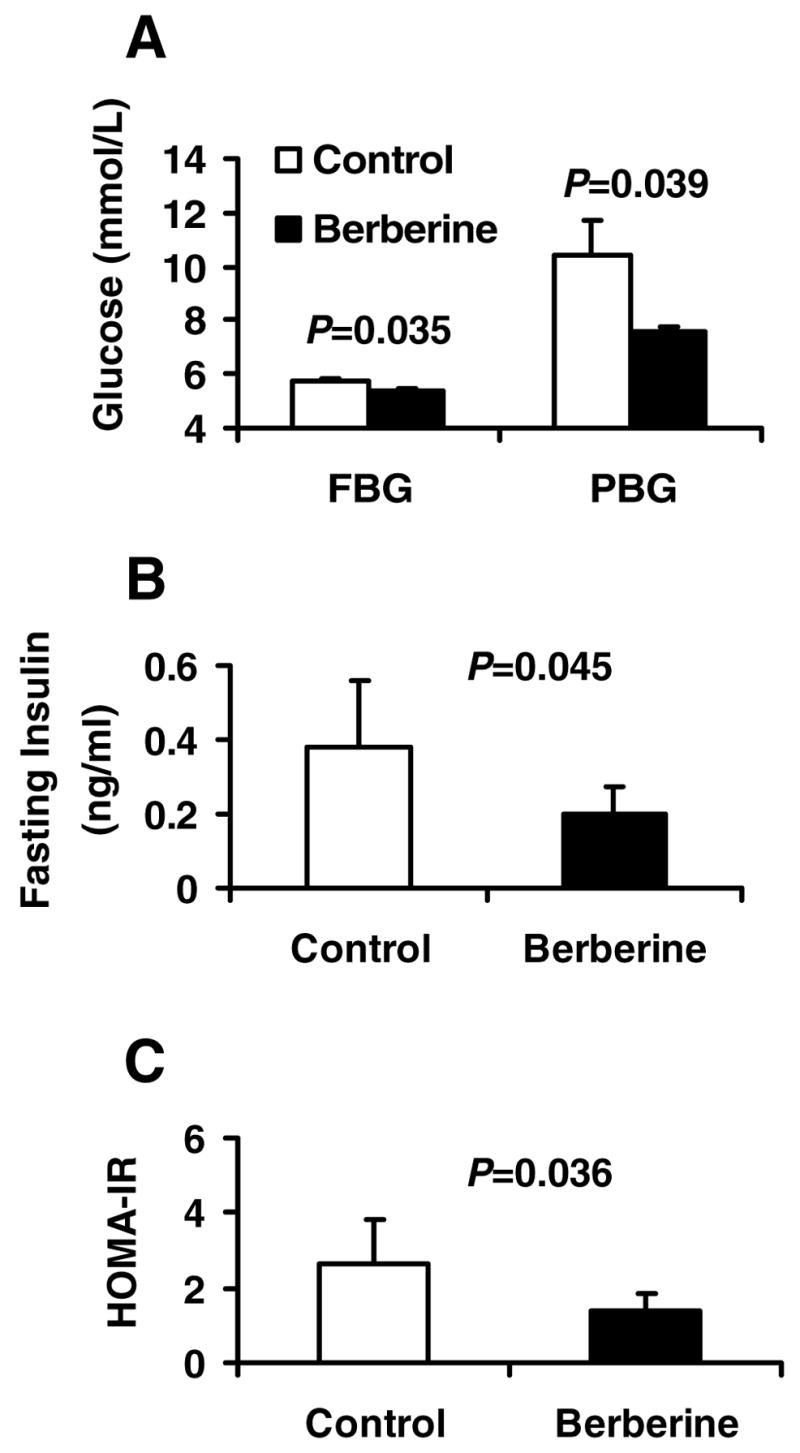
Berberine improved glucose metabolism in dietary obese rats. Twelve HFD fed rats were divided into control (n=6) and berberine-treated (n=6) groups. The treated rats were given berberine at 125 mg/Kg body weight twice a day by gastric gavage for 5 weeks. The control rats were gavaged with an equal volume of vehicle (distilled water). After 5-week treatment, intraperitoneal glucose tolerance test (IPGTT) was conducted after 10 hours fasting. A: Fasting blood glucose (FBG) and 2 hour postprandial blood glucose (PBG) determined during IPGTT in the rats. B: Fasting serum insulin levels in control and berberine-treated rats. C: HOMA-IR calculated by fasting serum insulin and FBG of the control and berberine treated rats.
Berberine improved glucose metabolism in vitro
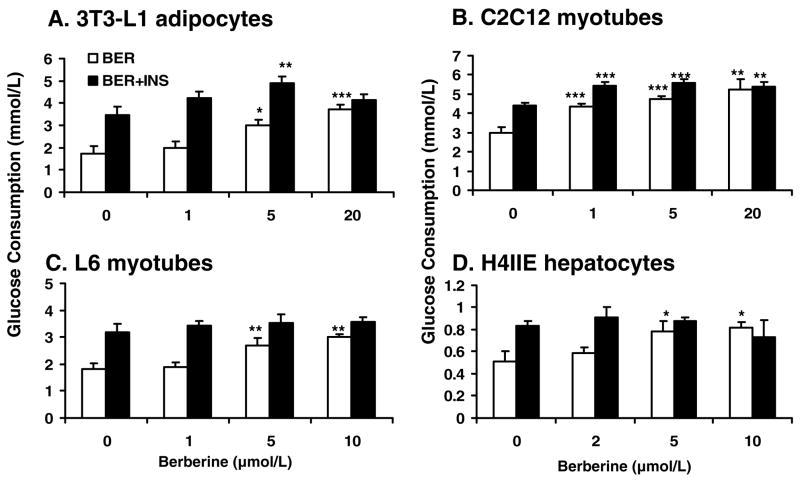
To investigate the mechanism of berberine action, glucose consumption was examined in several cell lines after berberine treatment. In 3T3-L1 adipocytes, berberine was found to increase glucose consumption in a dose-dependent manner (Fig. 2A). In the positive control, insulin (100 nmol/L) caused a two-fold increase in glucose consumption in 24 hours. A similar activity was observed for berberine in a dose-dependent manner. At concentrations between 5 μmol/L – 20 μmol/L, berberine increased glucose consumption by 72.9% – 113.7% in the absence of insulin (P<0.05-P<0.001). In the presence of insulin, insulin-induced glucose consumption was further increased by berberine, which led to 41.2% more increase at 5 μmol/L. (P<.01). This suggests that berberine and insulin had additive interaction in the induction of glucose consumption in 3T3-L1 adipocytes. A similar effect of berberine was observed in C2C12 cells, in which glucose consumption was increased by 74.0% with berberine treatment at 20 μmol/L (P<0.01; Fig. 2B). Interestingly, berberine exhibited a stronger effect over insulin in the muscle cells. Insulin by itself only led to 47% increment of glucose consumption in the same condition. In L6 myotubes and H4IIE hepatocytes, berberine also increased glucose consumption in a dose dependent manner (Fig. 2, C and D). At the concentration of 10 μmol/L, berberine led to 64.7% and 61.6% increment in glucose consumption in these two cell lines, which is comparable to the effects of insulin. However, berberine was unable to enhance insulin activity in L6 and H4IIE cells.
Fig. 2.
Berberine (BER) increased glucose consumption in a dose-dependent manner. The cells were cultured in a 96-well plate. When experiments, the normal culture medium was replaced by serum-free DMEM supplemented with bovine serum albumin at 0.25%. Then, the cells were treated with berberine for 24 hours. Insulin was used in the positive control (INS; final concentration 100 nmol/L). A: Glucose consumption in 3T3-L1 adipocytes. B: Glucose consumption in C2C12 myotubes. C: Glucose consumption in L6 myotubes. D: Glucose consumption in H4IIE hepatocytes. Data was expressed as means ± SEM (n=8). Compared with control (0 μmol/L): *P<0.05, **P<0.01, ***P<0.001
Berberine enhanced 2-deoxy-glucose uptake in vitro
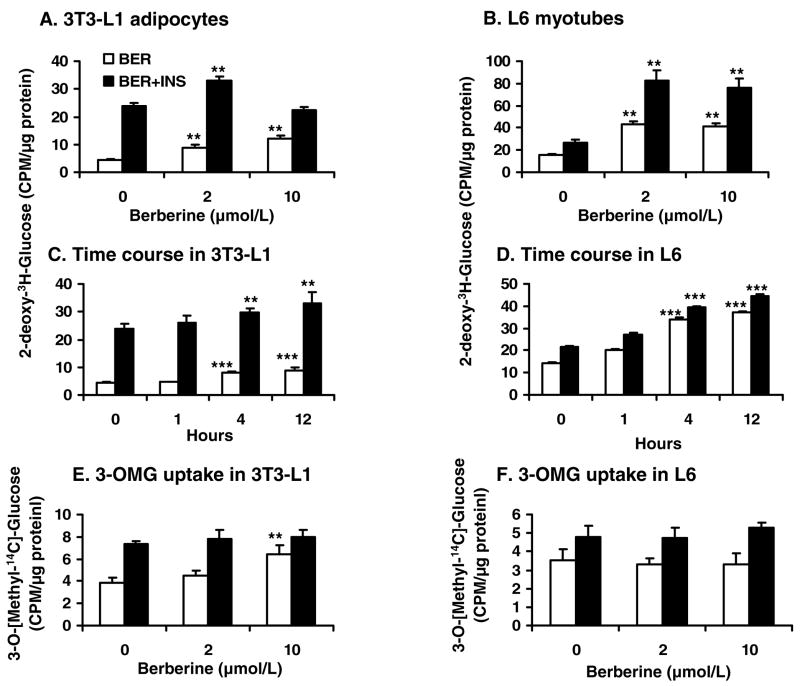
Glucose utilization (consumption) is regulated at several steps, such as glucose uptake, glucose oxidation, glycogen synthesis and gluconeogenesis. Glucose uptake was investigated for berberine in 3T3-L1 adipocytes and L6 myotubes with 2-deoxy-3H-D-glucose. In the absence of insulin, glucose uptake was elevated by 109.0% – 183.5% with berberine treatment in a dose dependent manner in 3T3-L1 adipocytes (Fig. 3A). In the presence of insulin, berberine (2 μmol/L) increased insulin activity by 38.9% (P<0.01). In L6 cells, berberine-induced glucose uptake was much stronger than that of insulin. At 2 μmol/L of berberine, glucose uptake was increased by 170% in the absence of insulin (P<.01; Fig. 3B). At 200 nmol/L of insulin, glucose uptake was increased only by 80%. These results suggest that berberine is able to stimulate glucose uptake in both myotubes and adipocytes independently of insulin. In the presence of insulin, berberine enhanced insulin activity. Time course results showed that berberine was able to increase glucose uptake after 4 h treatment in 3T3-L1 adipocytes and L6 myotubes (P<.001; Fig. 3, C and D).
Fig. 3.
Berberine (BER) increased 2-deoxy-glucose uptake in vitro. 3T3-L1 preadipocytes or L6 myoblasts were differentiated into mature adipocytes or myotubes in a 12-well plate. After serum-starvation in 0.25% BSA DMEM and berberine (2 μmol/L) treatment for 16 hours, the cells were treated with insulin (INS; final concentration 200 nmol/L) for 20 min at 37 C in PBS (1 ml/well). The glucose uptake was determined with 2-deoxy-3H-D-glucose or 3-O-[methyl-14C]-D-glucose. A: 2-deoxy-3H-D-glucose uptake in 3T3-L1 adipocytes. B: 2-deoxy-3H-D-glucose uptake in L6 myotubes. C: Time course of 2-deoxy-3H-D-glucose uptake was performed in 3T3-L1 adipocytes treated with 2 μmol/L berberine at different times. D: Time course of 2-deoxy-3H-D-glucose uptake was performed in L6 myotubes treated with 2 μmol/L berberine at different times. E. 3-O-[methyl-14C]-D-glucose (3-OMG) uptake in 3T3-L1 adipocytes. F: 3-O-[methyl-14C]-D-glucose uptake in L6 myotubes. Data was expressed as means ± SEM (n=3) of three experiments. Compared with control (0 μmol/L): **P<0.01, ***P<0.001
To study berberine effect on glucose transporter, 3-O-methyl-glucose (3-OMG) uptake was conducted. The results suggested that berberine had less effect on 3-OMG uptake compared with 2-deoxy-glucose uptake. Except 3-OMG uptake in 3T3-L1 adipocytes was enhanced by 52.8% with high dose (10 μmol/L) of berberine in the absence of insulin (P<0.01; Fig. 3E), little effect of berberine on 3-OMG uptake of L6 myotubes was observed (Fig. 3F).
Berberine-induced phosphorylation of AMPK
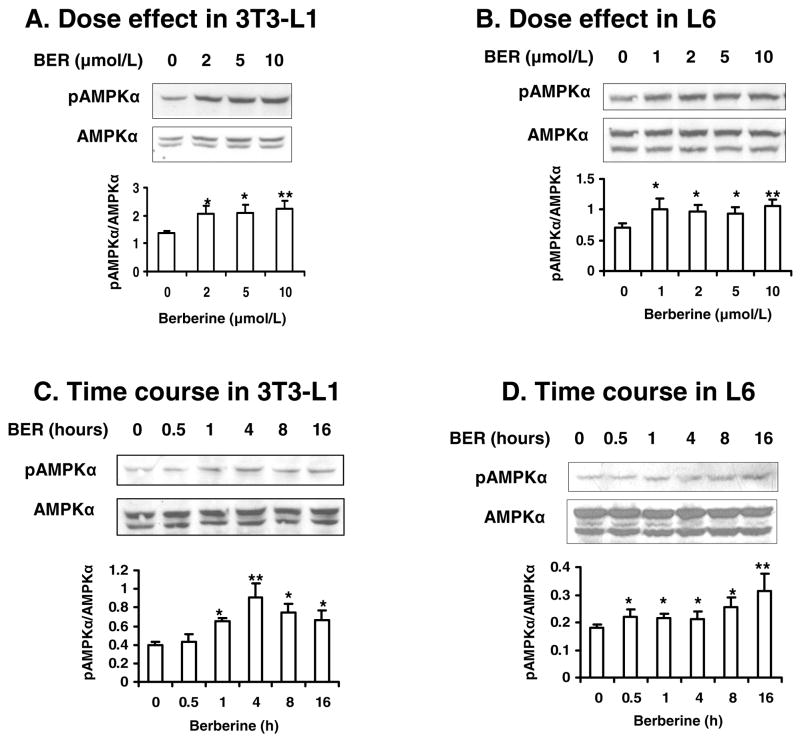
Effects of berberine on AMPK were detected in 3T3-L1 adipocytes and L6 myotubes (Fig. 4, A, B and E). Phosphorylation of AMPK (Thr172) was induced by 2 μmol/L berberine in 3T3-L1 cells. In contrast, AMPK phosphorylation was inhibited by insulin remarkably. This effect of insulin was blocked by berberine. Dose effect experiments showed that phosphorylation of AMPK was stimulated by berberine at either low dosage (1 μmol/L – 2 μmol/L; P<0.05) or high dosage (10 μmol/L; P<0.01; Fig. 5, A and B). Time course experiments showed that acute effect of berberine on phosphorylation of AMPK began at 0.5 h in L6 and 1 h in 3T3-L1 cells (P<0.05; Fig. 5, C and D). The AMPK phosphorylation lasted for at least 16 h. These data suggest that berberine is able to stimulate phosphorylation of AMPK rapidly and maintain the elevated phosphorylation for a long time in cells.
Fig. 4.
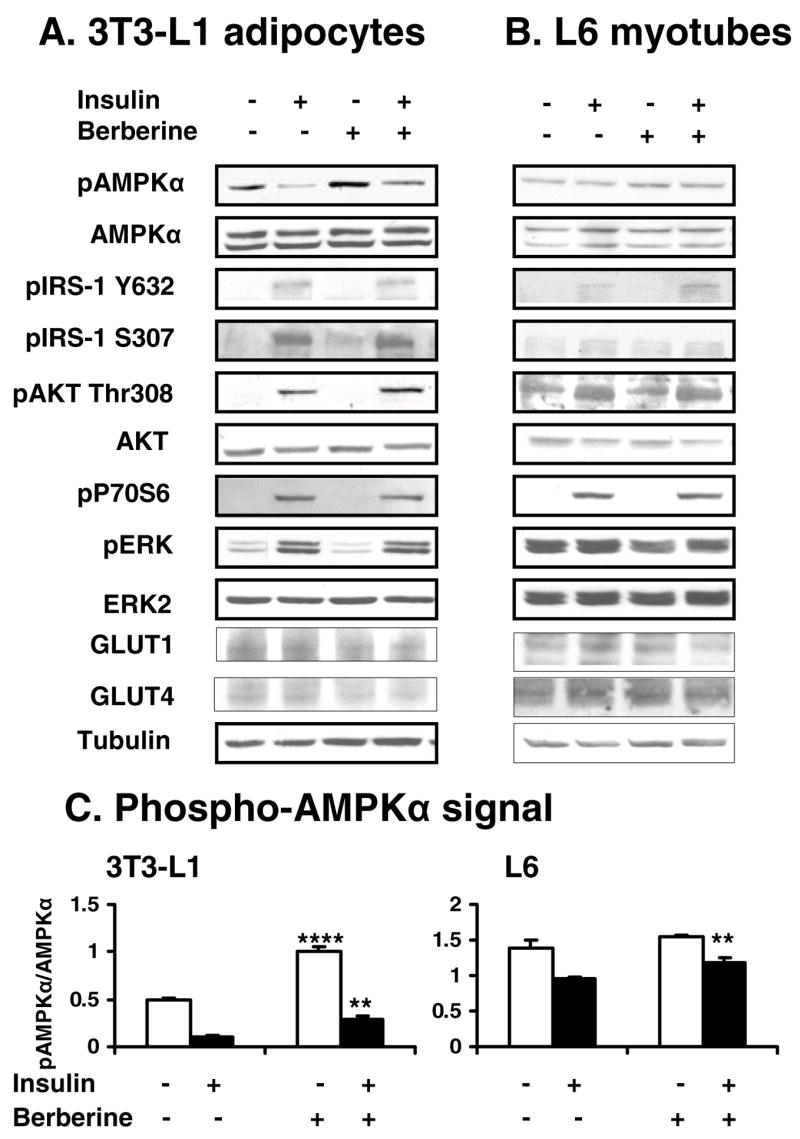
Effects of berberine on AMPK and insulin signaling pathway. Cells were treated with 2 μmol/L berberine for 16 hours after serum starvation in cell culture medium with 0.25% BSA. Twenty minutes prior to cell collection, insulin (200 nmol/L) was added to activate its signaling pathway. The whole cell lysate protein was examined in a western blot. Effects of berberine and insulin on AMPK and insulin signaling molecules were checked in 3T3-L1 adipocytes (A) and L6 myotubes (B). Similar results were obtained from triplicate experiments, and a representative experiment is shown. Ratio of pAMPK/AMPK was quantified in 3 independent experiments per condition (C). Data was expressed as means ± SEM (n=3). Compared with control (0 μmol/L): **P<0.01, ***P<0.001
Fig. 5.
Effects of berberine on phosphorylation of AMPK. A, B: 3T3-L1 adipocytes and L6 myotubes were treated with berberine for 16 hours after serum starvation in cell culture medium with 0.25% BSA. The whole cell lysate protein was examined in a western blot. C, D: The cells were treated with berberine for a variety of times after serum starvation in 0.25% BSA DMEM. Western blot was performed in the cells to check phosphorylation and total protein levels of AMPK. Similar results were obtained from triplicate experiments, and a representative experiment is shown. Ratio of pAMPK/AMPK was quantified in 3 independent experiments per condition. Data was expressed as means ± SEM (n=3). Compared with control (0 μmol/L): *P<0.05, **P<0.01
Insulin signaling pathway was examined in 3T3-L1 adipocytes and L6 myotubes in the Western blot (Fig. 4, A and B). In the positive control, insulin induced phosphorylation in IRS-1, AKT (Thr308), P70S6 (Thr389) and ERK. IRS-1 phosphorylation was increased in both tyrosine (Tyr632) and serine (Ser307) residues by insulin treatment for 20 minutes. Berberine exhibited little effect on these signaling molecules in the presence or absence of insulin. No significant change was observed in GLUT1 and GLUT4 in berberine-treated cells. These data suggest that berberine does not directly influence the metabolic pathway of insulin, and its insulin-like activity may be independent of insulin pathway.
Berberine had no cytotoxicity
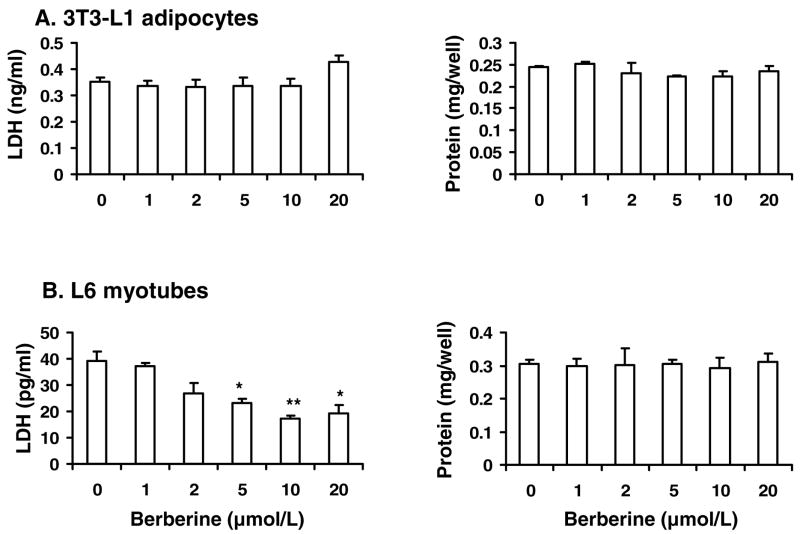
In this study, most of the cellular data were obtained with berberine treatment for 16 h or 24 h. It is necessary to know if berberine had toxicity in the cells. Thus, LDH-cytotoxicity assay and protein assay were conducted to address this issue. In 3T3-L1 adipocytes, berberine did not increase LDH release or reduce protein levels during the exposure time up to 24 h (Fig. 6 A). In L6 myotubes, berberine treatment decreased LDH activity in a dose-dependent manner without altering the protein levels (Fig. 6 B). These data suggest that berberine did not exhibit toxicity in our experiment system. It may protect plasma membrane of myotubes.
Fig. 6.
Berberine had no cytotoxicity. The cells were starved in serum-free DMEM supplemented with 0.25% BSA and treated with berberine for 24 hours. LDH concentrations in the medium and protein level of the cells were detected. A: Berberine treatment had no effects on the LDH release and protein level of 3T3-L1 adipocytes. B: Berberine decreased LDH release of L6 myotubes in a dose dependent manner. However, berberine had no effects on the protein level of L6 cells. Data was expressed as means ± SEM (n=3). Compared with control (0 μmol/L): *P<0.05, **P<0.01
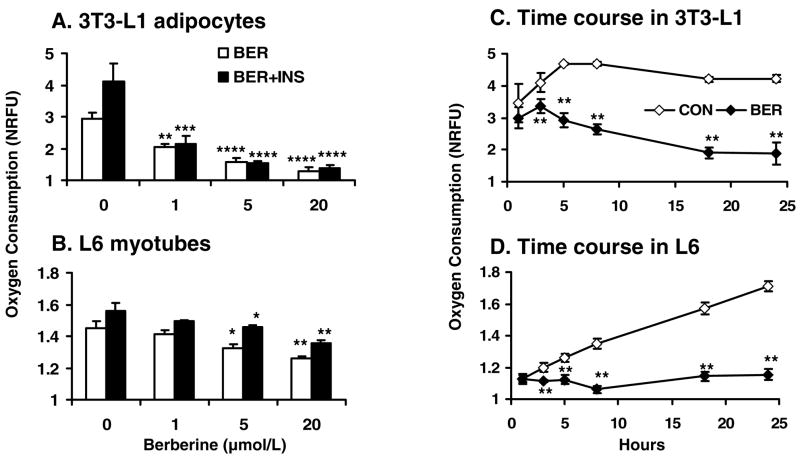
Berberine inhibited oxygen consumption in cells
Activation of AMPK is often a consequence of increase in AMP/ATP ratio in the cells. Since ATP production is dependent on oxygen in aerobic respiration in normal condition, we examined oxygen consumption in 3T3-L1 adipocytes and L6 myotubes. Oxygen consumption was significantly reduced by berberine in a dose-dependent manner. In 3T3-L1 adipocytes, the oxygen consumption was increased by insulin at 62.4% above the basal level. In the presence of berberine, the oxygen consumption was decreased by berberine even in the presence of insulin (Fig. 7A). The reduction was consistent in both types of cells. In 3T3-L1 adipocytes, the berberine activity was so strong that the basal oxygen consumption was reduced by 45.7% at 1 μmol/L of berberine in the absence of insulin (P<0.01). In the presence of insulin, the insulin-induced oxygen consumption was reduced by 63.5% (P<.001). In L6 myotubes, a similar activities were observed for berberine at concentrations of 5 – 20 μmol/L (P<0.05 - P<0.01; Fig. 7B). Time course study indicated the cells grew well after being transferred to the BD Oxygen Biosensor Systems since oxygen consumption of cells was increased in the control groups in a time-dependent manner, especially in L6 myotubes. On the contrary, oxygen consumption was not increased in the berberine groups (P<0.01) (Fig. 7, C and D), suggesting that oxygen consumption was inhibited by berberine. The difference between control and berberine groups was observed as early as 2–3 hours in the time course. The inhibition was enhanced with time of treatment in 3T3-L1 adipocytes. These data suggest that berberine may inhibit aerobic respiration in cells.
Fig. 7.
Berberine (BER) decreased oxygen consumption in cells. The cells were plated into a plate in DMEM culture medium supplemented with 10% FBS. The plate was embedded with oxygen-sensitive dye. After 6 hours, berberine was added to the medium with or without insulin (INS; final concentration 100 nmol/L). Fluorescence was read after 12 hour treatment and normalized over the blank wells to obtain normalized relative fluorescence units (NRFU). A: Oxygen consumption in 3T3-L1 adipocytes. B: Oxygen consumption in L6 myotubes. C: Time course of oxygen consumption in 3T3-L1 adipocytes. A similar condition was used in time course experiments in the absence of insulin. Fluorescence was determined at different times after berberine (5 μmol/L) treatment. D: Time course of oxygen consumption in L6 myotubes. Data was expressed as means ± SEM (n=4). The comparison was made between berberine-treated and untreated cells: *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001
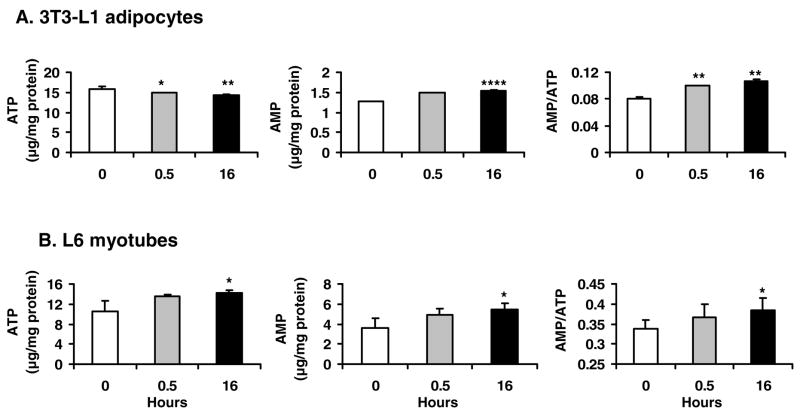
Effects of berberine on adenine nucleotide contents in vitro
Above data suggests that berberine may change adenine nucleotide contents in cells. To test this possibility, ATP and AMP contents were examined in 3T3-L1 adipocytes and L6 myotubes after berberine (5 μmol/L) treatment for 0.5 h and 16 h. In 3T3-L1 adipocytes, ATP was decreased and AMP was increased by berberine. Thus, the ratio of AMP/ATP was elevated by 24.2% at short term treatment and 33.3% at long term treatment (P<0.01; Fig. 8A). In L6 myotubes, AMP was increased significantly by berberine. Although ATP was also elevated, the ratio of AMP/ATP was increased by 13.3% at 16 h with berberine treatment (Fig. 8B). These data indicate that berberine increases the ratio of AMP/ATP in adipocytes and myotubes, which may stimulate activation of AMPK.
Fig. 8.
Adenine nucleotide contents in cells. The cells were treated with 5 μmol/L berberine (BER) in serum-free medium for 16 hours. Then the cells were lyzed and examined for ATP and AMP levels by HPLC analysis. A: AMP/ATP in 3T3-L1 adipocytes. Berberine decreased ATP contents and increased AMP contents. The ratio of AMP/ATP was increased in 3T3-L1 adipocytes. B: ATP, AMP contents and AMP/ATP ratio were all increased by berberine in L6 myotubes. Data was expressed as means ± SEM (n=3). Statistical analysis was conducted by comparison of berberine and control groups.
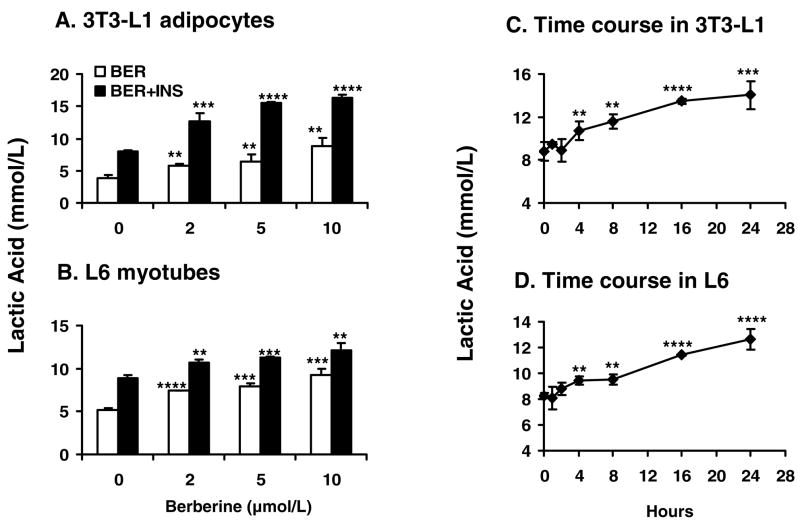
Berberine increased lactic acid release
Above data indicate that aerobic respiration was reduced by berberine without loss of ATP content in cells. This result suggests that anaerobic respiration might be increased to compensate the reduction in aerobic respiration. To test this hypothesis, release of lactic acid was measured in 3T3-L1 adipocytes and L6 myotubes. The lactic acid concentration was increased in the culture medium by berberine in a dose-dependent manner. In 3T3-L1 adipocytes, lactic acid release was increased with berberine by 47.4% – 163.8% in the absence of insulin, and by 57.1% – 106.7% in the presence of insulin (P<.01 - P<.0001; Fig. 9A). A similar change was observed in L6 cells. Lactic acid release was increased by 78.1% with 10 μmol/L berberine alone (P<.001; Fig. 9B). Time course results indicated that the berberine-induced lactic acid release was increased in a time-dependent manner from 4 h to 24 h after berberine treatment (P<0.01; Fig. 9, C and D). These data indicate that berberine is a potent stimulus for anaerobic respiration.
Fig. 9.
Lactic acid production by cells. A, B: 3T3-L1 adipocytes and L6 myotubes were treated with berberine in the presence or absence of 100 nmo/L insulin (INS) for 24 hours in serum-free medium. Berberine (BER) treatment significantly increased release of lactic acid into the supernatant. C, D: For time course study, berberine treatment was conducted in serum-free medium, and berberine was added at 24h, 16h, 8h, 4h, 2h and 1h before the samples were collected for lactic acid assay. The point of “0” represents a value of untreated sample that was collected at the same time as the treated. The final concentration of berberine was 5 μmol/L. Data was expressed as means ± SEM (n=3). Compared with untreated cells (0 μmol/L): **P<0.01, ***P<0.001, ****P<0.0001
DISCUSSION
We observed that berberine was able to induce activation of AMPK. This is consistent with observations made by several other labs (2, 22, 3, 9). Berberine was reported to activate AMPK pathway in HepG2 cells for inhibition of cholesterol and triglyceride synthesis (2). Activation of AMPK-P38 pathway by berberine was proposed to be responsible for induction of glucose uptake in muscle cells (3, 22, 9). However, the role of AMPK-P38 pathway is controversial for the metabolic activity of berberine. One study reported that berberine-induced glucose uptake was dependent on AMPK-P38 pathway in L6 cells (3). In contrast, another report suggested that p38 was not involved in berberine activity in 3T3-L1 adipocytes (22). The significance of AMPK pathway remains to be tested in the regulation of glucose metabolism by berberine.
Our study demonstrates for the first time that berberine inhibits oxygen-dependent glucose oxidation and enhances glycolysis in living cells. In cells, energy is transferred from glucose to ATP through aerobic respiration or anaerobic respiration (also called glycolysis). Aerobic respiration occurs in mitochondria and requires oxygen in the production of ATP from glucose. In contrast, glycolysis occurs outside mitochondria and produces ATP from glucose without consumption of oxygen. Glycolysis has a low efficiency in production of ATP, and takes more glucose than aerobic respiration in production of the same amount of ATP. In this study, we observed that the oxygen consumption was reduced in living cells by berberine in a dose dependent manner in cell culture. The reduction was observed in both adipocytes and myotubes. The reduction in oxygen consumption was observed at 2 h – 3 h of berberine treatment. These data suggest that berberine is able to reduce oxygen-dependent glucose oxidation in mitochondria. This observation is consistent with berberine activity in the purified mitochondria, in which berberine inhibited NAD-linked respiration (11, 12, 1). The reduction in oxygen consumption was associated with increase in glycolysis, suggesting that berberine stimulates ATP production through glycolysis. Since more glucose is required to produce ATP in glycolysis, anaerobic respiration may be the primary mechanism by which berberine increases glucose utilization in cells.
The time course study suggests that glycolysis may lead to glucose uptake in cells treated with berberine. Although the increase in lactic acid and glucose uptake was observed at the same time (4 h after berberine exposure), the stimulation of glycolysis may occur first. Lactic acid was determined in the cell culture medium in this study. The increase in lactic acid should occur earlier inside the cells since the secretion process delays the lactate increase in the culture medium. In this regard, the elevation in glucose uptake is likely a result of increased demand for glucose in glycolysis. Glycolysis may be a result of mitochondrial inhibition.
Inhibition of mitochondrial oxidation may contribute to AMPK phosphorylation induced by berberine. In the time course study, AMP/ATP ratio was increased as early as 0.5 h by berberine treatment. AMPK phosphorylation was observed in both 3T3-L1 adipocytes and L6 muscle cells, suggesting the role of AMP/ATP ratio in AMPK activation. In the published studies, the berberine activity was examined within 6 h for the AMPK activation. In the current study, the berberine effect was tested up to 16 h. Our data suggests that berberine-induced AMPK phosphorylation was detectable between 0.5 h –16 h. This activity of berberine is much stronger than that reported for adiponectin. The adiponectin-induced AMPK phosphorylation only lasts for 15 minutes (19). Activation of AMPK normally leads to reversion of AMP/ATP ratio by stimulation of ATP production in mitochondria. However, this did not happen in the berberine-treated cells. The AMP/ATP ratio remained at the high level for 16 h in the current study. The reason may be related to mitochondria inhibition by berberine, which is indicated by the persistent reduction in oxygen consumption in the cell culture. These data suggest that the long-lasting phosphorylation of AMPK in berberine-treated cells is a result of persistent mitochondria inhibition by berberine.
This study indicates that berberine has a weak effect on the glucose transporter as indicated by the 3-OMG data. Berberine is known to enhance glucose uptake (3, 7, 22). However, it is not clear if the increase is due to glucose utilization or glucose transportation cross the cell membrane. It was reported that GLUT4 translocation was increased by berberine (9, 7). However, this activity is controversial since it was not observed in another study (22). The difference might be related to methods for the glucose uptake assays. In these published studies, the glucose uptake was measured with 2-deoxy-glucose that can be phosphorylated by hexokinase. The 2-deoxy-glucose assay is not able to differentiate transportation from utilization when glucose uptake is increased. In the current study, 3-OMG, a non-phosphorylatable glucose analog (4), was used to test glucose transportation in cells. The 3-OMG uptake was not altered in the L6 myotubes by berberine at all of the dosages tested. In the 3T3-L1 adipocytes, the 3-OMG uptake was increased by berberine only at a higher concentration (10 μmol/L). This fact suggests that berberine may have a weak effect on the glucose transporter at the high concentration. Our data suggests that the GLUT4 translocation may contribute to the mechanism of berberine action, but it may not be the primary effect of berberine. Berberine also increased glucose consumption in hepatocytes. Hepatocytes do not express GLUT4 (20).
We did not observe a significant effect of berberine on the insulin-activated PI3K pathway. Our early study indicated that berberine had an insulin-independent effect on glucose metabolism in vitro (20), but the mechanism was not clear. Two studies demonstrated that berberine had no effects on the insulin receptor signaling pathway (22, 3). However, this conclusion was challenged by other reports in which berberine was shown to activate insulin signaling pathway (7, 9). In the current study, we examined protein level and phosphorylation status of major components (IRS-1, Akt, P70S6K, and ERK) in the insulin receptor signaling pathway. Insulin-induced phosphorylation of the signaling molecules was not changed by berberine.
The cytotoxicity assay suggests that berberine has no toxicity in adipocytes, and exhibits a protective effect in myotubes. One study suggested that berberine protected cardiac myocyte in the ischemia-reperfusion condition since LDH and methylenedioxyamphetamine (MDA) leakage was reduced by berberine (21). Our data supports that this effect of berberine is muscle-specific since it was not observed in adipocytes.
In summary, our data suggests that berberine improves glucose metabolism through induction of glycolysis. The increase in glycolysis is likely a consequence of inhibition of glucose oxidation in mitochondria. The elevated glycolysis may be a primary cause for up-regulation of glucose uptake in muscle and adipocytes by berberine. Inhibition of mitochondrial oxidation by berberine may contribute persistent elevation in AMP/ATP ratio and long-lasting AMPK phosphorylation. Our data does not support that berberine directly regulate signaling molecules in the insulin receptor signaling pathway. Berberine represents a novel class of drug candidates for treatment of type 2 diabetes.
Acknowledgments
We thank Dr. Qing He, Jinhua Yan and Ms. Wei Tseng for their excellent technical support. We are grateful to Dr. Mingdao Chen and Dr. Ying Yang for their help in the animal experiments.
GRANT
This study is supported by NIH grant 1P50AT002776-010002 to Ye J.
References
- 1.Barreto MC, Pinto RE, Arrabaca JD, Pavao ML. Inhibition of mouse liver respiration by Chelidonium majus isoquinoline alkaloids. Toxicol Lett. 2003;146:37–47. doi: 10.1016/j.toxlet.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Brusq JM, Ancellin N, Grondin P, Guillard R, Martin S, Saintillan Y, Issandou M. Inhibition of lipid synthesis through activation of AMP-kinase: An additional mechanism for the hypolipidemic effects of Berberine. J Lipid Res. 2006 doi: 10.1194/jlr.M600020-JLR200. [DOI] [PubMed] [Google Scholar]
- 3.Cheng Z, Pang T, Gu M, Gao AH, Xie CM, Li JY, Nan FJ, Li J. Berberine-stimulated glucose uptake in L6 myotubes involves both AMPK and p38 MAPK. Biochim Biophys Acta. 2006;1760:1682–1689. doi: 10.1016/j.bbagen.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Faik P, Morgan M, Naftalin RJ, Rist RJ. Transport and accumulation of 2-deoxy-D-glucose in wild-type and hexokinase-deficient cultured Chinese-hamster ovary (CHO) cells. Biochem J. 1989;260:153–155. doi: 10.1042/bj2600153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao Z, Chiao P, Zhang X, Zhang X, Lazar MA, Seto E, Young HA, Ye J. Coactivators and corepressors of NF-kappaB in IkappaB alpha gene promoter. J Biol Chem. 2005;280:21091–21098. doi: 10.1074/jbc.M500754200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao Z, Zhang X, Zuberi A, Hwang D, Quon MJ, Lefevre M, Ye J. Inhibition of insulin sensitivity by free fatty acids requires activation of multiple serine kinases in 3T3-L1 adipocytes. Mol Endocrinol. 2004;18:2024–2034. doi: 10.1210/me.2003-0383. [DOI] [PubMed] [Google Scholar]
- 7.Ko BS, Choi SB, Park SK, Jang JS, Kim YE, Park S. Insulin sensitizing and insulinotropic action of berberine from Cortidis rhizoma. Biol Pharm Bull. 2005;28:1431–1437. doi: 10.1248/bpb.28.1431. [DOI] [PubMed] [Google Scholar]
- 8.Lau CW, Yao XQ, Chen ZY, Ko WH, Huang Y. Cardiovascular actions of berberine. Cardiovasc Drug Rev. 2001;19:234–244. doi: 10.1111/j.1527-3466.2001.tb00068.x. [DOI] [PubMed] [Google Scholar]
- 9.Lee YS, Kim WS, Kim KH, Yoon MJ, Cho HJ, Shen Y, Ye JM, Lee CH, Oh WK, Kim CT, Hohnen-Behrens C, Gosby A, Kraegen EW, James DE, Kim JB. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes. 2006;55:2256–2264. doi: 10.2337/db06-0006. [DOI] [PubMed] [Google Scholar]
- 10.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 11.Mikes V, Dadak V. Berberine derivatives as cationic fluorescent probes for the investigation of the energized state of mitochondria. Biochim Biophys Acta. 1983;723:231–239. doi: 10.1016/0005-2728(83)90122-6. [DOI] [PubMed] [Google Scholar]
- 12.Mikes V, Yaguzhinskij LS. Interaction of fluorescent berberine alkyl derivatives with respiratory chain of rat liver mitochondria. J Bioenerg Biomembr. 1985;17:23–32. doi: 10.1007/BF00744986. [DOI] [PubMed] [Google Scholar]
- 13.Ni YX. Therapeutic effect of berberine on 60 patients with type II diabetes mellitus and experimental research. Zhong Xi Yi Jie He Za Zhi. 1988;8:711–713. 707. [PubMed] [Google Scholar]
- 14.Sack RB, Froehlich JL. Berberine inhibits intestinal secretory response of Vibrio cholerae and Escherichia coli enterotoxins. Infect Immun. 1982;35:471–475. doi: 10.1128/iai.35.2.471-475.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sinha S, Perdomo G, Brown NF, O’Doherty RM. Fatty acid-induced insulin resistance in L6 myotubes is prevented by inhibition of activation and nuclear localization of nuclear factor kappa B. J Biol Chem. 2004;279:41294–41301. doi: 10.1074/jbc.M406514200. [DOI] [PubMed] [Google Scholar]
- 16.Sultan KR, Henkel B, Terlou M, Haagsman HP. Quantification of hormone-induced atrophy of large myotubes from C2C12 and L6 cells: atrophy-inducible and atrophy-resistant C2C12 myotubes. Am J Physiol Cell Physiol. 2006;290:C650–659. doi: 10.1152/ajpcell.00163.2005. [DOI] [PubMed] [Google Scholar]
- 17.Wynants J, Van Belle H. Single-run high-performance liquid chromatography of nucleotides, nucleosides, and major purine bases and its application to different tissue extracts. Anal Biochem. 1985;144:258–266. doi: 10.1016/0003-2697(85)90114-9. [DOI] [PubMed] [Google Scholar]
- 18.Xiao CT, Cant JP. Relationship between glucose transport and metabolism in isolated bovine mammary epithelial cells. J Dairy Sci. 2005;88:2794–2805. doi: 10.3168/jds.S0022-0302(05)72959-3. [DOI] [PubMed] [Google Scholar]
- 19.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 20.Yin J, Hu R, Chen M, Tang J, Li F, Yang Y, Chen J. Effects of berberine on glucose metabolism in vitro. Metabolism. 2002;51:1439–1443. doi: 10.1053/meta.2002.34715. [DOI] [PubMed] [Google Scholar]
- 21.Zheng L, Zhou Z, Tao D, Lan T. Protective effect of berberine on cardiac myocyte injured by ischemia-reperfusion. Sichuan Da Xue Xue Bao Yi Xue Ban. 2003;34:452–454. [PubMed] [Google Scholar]
- 22.Zhou L, Yang Y, Wang X, Liu S, Shang W, Yuan G, Li F, Tang J, Chen M, Chen J. Berberine stimulates glucose transport through a mechanism distinct from insulin. Metabolism. 2007;56:405–412. doi: 10.1016/j.metabol.2006.10.025. [DOI] [PubMed] [Google Scholar]