Abstract
Salvia divinorum, a mint plant originally used by the Mazatecs of Oaxaca, Mexico in spiritual rituals has gained popularity, in smoked form, as a legal hallucinogen in the United States and Europe. Abuse results in rapid onset, short lasting effects that include visual hallucinations and motor-function impairment. Salvinorin A, the psychoactive component of S. divinorum, is a uniquely potent agonist at κ-opioid receptors, targets for new therapeutic drugs. We labeled salvinorin A with C-11 by acylation of salvinorin B with [11C]-acetylchloride to study whether its kinetic behavior in the brain parallels its uniquely fast, yet brief physiological effects. Positron emission tomography (PET) studies performed in 6 adult female baboons indicated extremely rapid brain uptake reaching a peak accounting for 3.3% of the total administered dose in 40 s and clearing with a half-life of 8 min. [11C]-Salvinorin A was distributed throughout the brain with the highest concentration in the cerebellum and a notable concentration in the visual cortex, perhaps accounting for its physiological effects when smoked. Naloxone administration did not reduce the overall concentration of [11C]-salvinorin A significantly nor did it change its regional distribution. Peripheral organ kinetics suggested at least two modes of metabolism and excretion occur: through the renal and biliary systems. Our findings have revealed that the exceptionally rapid uptake and brief duration of salvinorin A in the brain match the time-course of visual hallucinations for S. divinorum when smoked. The effects of salvinorin A may occur at <10 μg in the human brain, emphasizing its remarkable potency.
Introduction
Salvia divinorum has become increasingly popular as a recreational drug (Vortherms et. al., 2006). Although demographic data are lacking, adolescent abuse has clearly been facilitated by the internet where dried salvia leaves and extracts are inexpensive and easily obtained, ‘how to’ guides are abundant, and thousands of videos on YouTube document its hallucinogenic effects (Gonzalez et. al., 2006). When smoked, S. divinorum causes visual hallucinations and behavioral impairment within seconds that last only minutes.
In parallel to the rise in recreational use over the past decade, research using S. divinorum has advanced our understanding of the opioid receptor system. Salvinorin A, the active component of S. divinorum, is a neoclerodane diterpene structurally distinct from all other psychoactive drugs and acts as a potent and selective κ-opioid agonist (Roth et. al., 2002). The κ-opioid receptor may be an important therapeutic target for analgesia (Millan, 1990) and neuroprotection (Zeynalov et. al., 2006), among others. The dichotomy of S. divinorum as a target for abuse and therapy has both challenged and preserved its legal status in the United States where it is currently not listed under the Controlled Substances Act.1
As a result of both S. divinorum's abuse liability and its therapeutic potential, there is a growing interest in understanding the in vivo behavior and effects of salvinorin A, other naturally occurring salvinorin compounds, and semi-synthetic derivatives of the salvinorin diterpene core (Vortherm and Roth, 2006; recently reviewed by Grundmann et. al., 2007). Herein, we synthesized carbon-11 (t1/2 = 20.4 min) labeled salvinorin A and used positron emission tomography (PET) to measure its pharmacokinetics and distribution in the brains and peripheral organs of female baboons. Our results are consistent with the rapid onset, short-lasting pharmacological effect of salvinorin A, which we conclude is directly linked to its kinetics in the brain.
Methods
General
[11C]Carbon dioxide was generated by an EBCO (Advanced Cyclotron Systems Inc) cyclotron by the nuclear reaction, 14N(p,α)11C, using a nitrogen/oxygen (1000 ppm) target. Semipreparative high-performance liquid chromatography (HPLC) was performed using a Knauer HPLC system (Sonntek Inc., Woodcliff Lake, NJ, USA) with a model K-5000 pump, a model 87 variable wavelength monitor and NaI radioactivity detector. Specific activity was determined by measuring the radioactivity and the mass; the latter is derived from a standard curve at UV (208 nm) using different concentrations of the authentic reference compounds. Radiochemical purity was also determined by thin-layer chromatography (TLC) using and measuring radioactivity distribution on Macherey–Nagel polygram sil G/UV254 plastic-back TLC plate with Bioscan system 200 imaging scanner (Bioscan Inc., Washington, DC). 11C radioactivity was measured by a Packard MINAXI γ 5000 automated gamma counter (Packard Instrument, Meriden, CT). All measurements were decay corrected. All experiments with animals were approved by the Brookhaven Institutional Animal Care and Use Committee. All chemicals including naloxone·HCl were obtained from Sigma-Aldrich (USA) except methyl magnesium bromide, which was obtained from Acros Organics (USA).
Salvinorin A isolation
S. divinorum dried leaves obtained from Bouncing Bear Botanicals (USA) were processed in 250 g batches as follows: Dried plant matter powdered using a common blender was stirred with 2 L of acetone for 4 h. The mixture was passed through a series of four screen mesh sieves with decreasing pores from 0.25 cm2 to 0.02 mm2 to remove plant debris. The filtrate was concentrated under reduced pressure and the resulting residue was washed with 3 × 50 mL of 1:3 iPrOH:hexanes. (Recovered acetone could be used for subsequent preparations). The remaining solid was dissolved in dichloromethane and dry loaded onto silica gel. FLASH chromatography using a gradient from hexanes to 1:3 hexanes:EtOAc afforded fractions of semi-pure salvinorin A, which crystallized from solution upon standing overnight. (Analytical TLC, 1:1 EtOAc:hexanes, was performed on EM Reagent 0.25 mm silica Gel 60-F254 plates with visualization by potassium permanganate stain.) Crystals were collected by filtration, washed with hexanes, and then recrystallized from 10% ethyl acetate in hexanes affording 250 – 300 mg of white needles (mp = 240 °C, sharp) per 250 g batch. 1H- and 13C-NMR spectra were consistent with the literature (Giner et. al., 2007).
Precursor Synthesis: Salvinorin B (according toTidgewell et. al. 2004)
A mixture of salvinorin A (250 mg, 0.58 mmol) in methanol (6 mL) was treated in one portion with anhydrous sodium carbonate (246 mg, 2.32 mmol). The mixture was stirred vigorously at rt for 4 h and then concentrated under reduced pressure. The resulting residue was partitioned between dichloromethane (25 mL) and 1 N HCl (50 mL). The organic layer was removed, dried over sodium sulfate, and concentrated under reduced pressure affording a slightly off-white solid. The solid was washed with cold MeOH (10 mL × 3) affording 147 mg (65 %) of salvinorin B (mp 214 – 216 °C). 1H and 13C NMR were consistent with the literature (Giner et. al., 2007). Material used for carbon-11 labeling was subjected to at least one round of the following: Salvinorin B (100 mg) was dissolved in 20 mL dichloromethane and dry loaded on to silica gel for FLASH chromatography. A slow gradient elution using EtOAc:hexanes provided salvinorin B in 6-10 fractions (as determined by TLC). The middle 2 – 3 fractions were pooled and allowed to slowly evaporate causing crystallization. The crystals were collected by centrifugation, decanting the mother liquor, and then washed with cold ethanol (35 – 40 mg were recovered, mp 214 °C). Purity was determined by reversed-phase HPLC (55% MeCN, 45% 0.1 M NH4CHOOaq at 208 nm) and was greater than 99%.
Carbon-11 Labeling
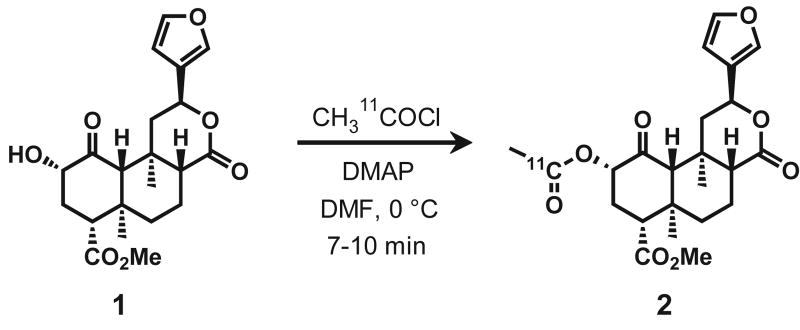
At the end of bombardment (EOB), [11C]-CO2 was trapped on crushed molecular sieves and then released in a slow stream of He (20 mL/min) at 370 °C. The released activity was trapped in a liquid nitrogen cooled copper coil. The coil was isolated in a closed system and was warmed to slowly recirculate the activity through a solution of methyl magnesium bromide in diethyl ether (300 μL, 1 M) using a diaphragm pump. After 3-4 min of recirculation, phthaloyl dichloride and 2,6-di-t-butylpyridine were added sequentially (200 μL each). After 2 min the reaction mixture was heated to 110 °C. It was critical to continue to bubble a stream of gas through the mixture during this process to prevent solidification that trapped evolving [11C]-acetyl chloride. The [11C]-acetyl chloride formed was swept with argon to a separate reaction vessel where it was bubbled through a solution of 1 (2.5 mg) and N,N-dimethylaminopyridine (5.0 mg) in 250 μL DMF at 0 °C (Fig.1). The reaction time was largely based on the distillation rate and was typically 7-10 min. The solution was allowed to warm toward room temperature for 2 min and then quenched by the addition of 1.0 mL HPLC buffer (∼ 20 min post EOB). Purification was accomplished by HPLC using 55% MeCN, 45% 0.1 M NH4CHOOaq at a flow rate of 5 ml/min on a semipreparative Gemini C18 (Phenomenex, 250×10 mm, 5 μm). The product was collected at the expected retention time, and the solvent was removed by azeotropic evaporation with acetonitrile. After dilution with ethanol (0.4 mL and water (3.6 ml) the solution was filtered through an Acrodisc 13-mm Syringe Filter with 0.2 μm HT Tuffryn Membrane (Pall Corporation, Ann Arbor, MI) into a sterile vial for delivery. For quality control, both analytical TLC and HPLC were performed. TLC was accomplished with 1:1 EtOAc:Hex giving the Rf value of 0.5 for carbon-11, which was coincident with an authentic standard of salvinorin A, cospotted with the sample and was detected by permanganate staining. A 20 μL sample of a known activity of formulated product was analyzed by HPLC at 208 nm to determine specific activity. An additional sample was spiked with salvinorin A and analyzed by HPLC to further confirm identity.
Fig 1.
Synthesis of [11C]-Salvinorin A (2) from Salvinorin B (1).
PET studies in baboon
Female Papio anubis baboons were anesthetized by an intramuscular injection of ketamine hydrochloride (10 mg/kg) and then maintained with oxygen (800 ml/min), nitrous oxide (1500 ml/min) and isoflurane (Forane, 1–4%) during scanning. [11C]-Salvinorin was injected through a catheter placed in a radial arm vein, and arterial blood was sampled through a catheter in the popliteal artery at the following time intervals: every 5 s for 2 min, then 2, 5, 10, 20, 30, 45, 60 min. Heart rate, respiration rate, pO2 and body temperature were checked during the PET scanning. Dynamic PET imaging was performed by Siemens HR+ (Siemen's high-resolution, whole-body PET scanner with 4.5×4.5×4.8 mm resolution at the center of field of view), for a total of 60 min with the following time frames in 3D mode: 12×5, 12×10, 6×20, 6×30, 8×60, 2×120, 4×300, 2×600 s. Either the brain or the torso was placed in the field of view, Table 1. For paired studies (i.e. test/retest and baseling/blocking), anesthesia was maintained after the completion of the first scan and a second injection of [11C]-salvinorin occurred 2 h after the first. At the end of one brain study (BEJ362dy2, 65 min post injection) a whole body image was collected (Figure 6) in three segments of emission/transmission scan pairs (10 min, 5 min) moving the animal 140 cm in the field of view between each segment. To determine torso kinetics, one scan study (i.e. one injection, BEJ364dy1) was performed using the following time frames: 12×5, 12×10, 6×20, 6×30, 8×60, 2×120, 2×450 s. Prior to each study, a transmission scan was obtained by rotating a 68Ge rod source to correct for attenuation.
Table 1. Summary of [11C]-Salvinorin A in vitro data (mean ± stdev).
| LogD (pH 7.4) | 2.34 ± 0.09 (n = 4) |
| LogP (unbuffered) | 2.29 ± 0.10 (n = 4) |
| Plasma Protein Binding | 16.1 ± 0.24 % unbound (n = 2) |
Fig 6.
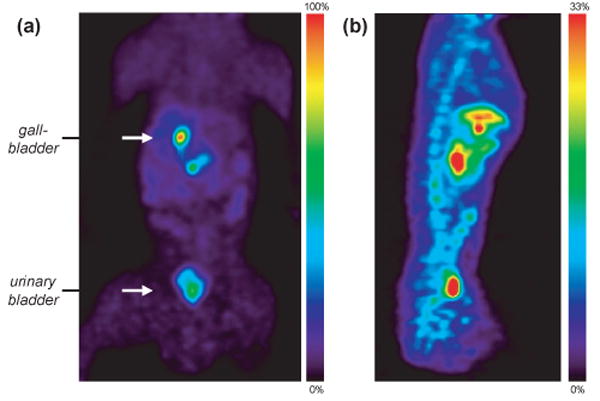
Whole body PET images collected from 65-110 min. (a) Coronal slice through the gallbladder and bladder (scaled to 100% of max pixel value). (b) Midline sagittal slice (scaled to 33% of max pixel value). The liver, gallbladder, urinary bladder, spine and intestinal tract can be seen.
Image analysis
All image data were reconstructed using filtered backprojection (FBP) and decay corrected to the time of injection. For image analysis, we constructed a region of interest (ROI) file with the published MR and H215O template images (Black et. al., 2001) using PMOD (PMOD Technologies, Ltd.). After ECAT7 files were converted to ANALYZE format and time frames 5-30 were summed for each file, they were coregistered H215O with template images manually and then normalized (12 nonlinear iterations) using PMOD. Time–activity curves were normalized with injected dose to yield percentage of injected dose per cubic centimeter (%ID/cc). Whole body images were analyzed using Amide Software Package (Loening and Ghambir, 2001) and manually drawn 3-D ROIs.
Results and Discussion
Salvinorin A was labeled with carbon-11, a positron emitting isotope, and studied using PET, a powerful noninvasive technique to image drug pharmacokinetics in the living brain at concentrations far below pharmacologically active doses (Fowler and Wolf, 1997). Salvinorin A was isolated from dried S. divinorum by a procedure similar to those previously described (Munro et. al., 2003). Precursors for radiolabeling were synthesized by known chemical degradation pathways. Two positions for carbon-11 incorporation were considered: the acetate at C(2) and the methyl-ester at C(18). The methyl ester was an obvious candidate for labeling with [11C]-methyl iodide, but the precursor synthesis and subsequent methylation was plagued by epimerization (Beguin et. al., 2003). Conversely, the acetate at the C(2) position was a superior labeling site candidate. The C(2) acetate is the most labile functional group of salvinorin A and is believed to be the primary site of metabolic degradation and inactivation (Schmidt, M.S. et. al., 2005). This is advantageous in imaging studies where metabolic cleavage at the C(2) position would not result in any radioactive diterpene metabolites.
Indeed, the C(2) acetate was removed cleanly using sodium bicarbonate in methanol and afforded salvinorin B (1) in good purity without chromatography as previously reported (Tidgewell, et. al., 2004). However, it was later determined that trace impurities left from this process limited radiochemical yields. Consequently, these impurities were removed by iterative chromatography and crystallization for precursor batches used for radiolabeling.
Carbon-11 radiolabeling began with the production [11C]-acetyl chloride from [11C]-carbon dioxide using phthaloyl dichloride, a method adapted from the literature (Le Bars et. al., 1987). The [11C]-acetyl chloride was carried in a slow stream of argon through a solution of 1 and N,N-dimethylaminopyridine in DMF at 0 °C, Fig. 1. The total reaction and purification time from end of bombardment averaged 40 min and radiochemical yields (based on 11CO2) were 3.5 – 10 % decay corrected to the end of bombardment. Radiochemically, [11C]-Salvinorin A (2) was greater than 98 % pure for all imaging experiments and had a specific activity of 0.20 – 0.75 Ci/μmol. The low specific activity was most likely the result of atmospheric CO2 introduced into the Grignard solution during storage and liquid transfers, but could not be improved by fresh preparation and immediate use. Even at this specific activity, the maximum injected dose of salvinorin A was less than 0.35 μg/kg, an order of magnitude lower than measured psychoactive doses of 3.0-7.5 μg/kg when smoked (Siebert, 1994).
In vitro characterization suggested that [11C]-salvinorin was suitable for BBB penetration consistent with its reported potency (Siebert, 1994). The log D of [11C]-Salvinorin A at pH = 7.4 was 2.3 and 16 % was found in the free fraction in plasma protein binding assays, Table 1.
To examine BBB penetration, kinetics and brain distribution, six adult female baboons were administered 1 - 4 mCi of 2, Table 2. Salvinorin A is most commonly abused by smoking fortified leaves or extracts of S. divinorum (Gonzalez et. al., 2006; Babu et. al., 2008). In this way, a large dose can reach the brain within seconds to initiate visual hallucinations that reach full effect in about 30 s (Siebert, 1994). Intravenous administration of 2 served as a convenient model for the rapid delivery of a smoked drug to the brain. (Intravenous administration has been validated in numerous studies as a substitute for smoking. See for example studies with nicotine by Lux et. al., 1987). Animals anesthetized with ketamine and maintained with isoflurane were positioned in a PET scanner and given 2 formulated in water with 10% ethanol in paired studies 2 h apart to measure reproducibility of repeated measures and the effects of naloxone. No physiological effects (i.e. changes in blood pressure, heart rate, pO2, and body temperature) were observed during any of these studies. Dynamic data were collected for 60 min after injection of 2. For the second scan, a repeat injection was given. In four studies, animals were treated with 1.0 mg/kg naloxone in saline, 15 min prior, to assess binding specificity. Naloxone is a potent antagonist at μ-opioid receptors with lower affinity at κ- and δ-opioid receptors. Although nonspecific, it has been used to block behavioral effects of κ-opioid drugs (Tang and Collins, 1985) and binding of κ-opioid radiotracers in primates (see for example Talbot, et. al., 2005).
Table 2. Summary of baboon PET studies with [11C]-Salvinorin A.
| Study no. | Baboon | Injected Dose | Brain/Torso |
|---|---|---|---|
| Test | |||
| BEJ359dy1 | Reily | 1.86 mCi | Brain |
| BEJ364dy1 | Missy | 1.18 | Torso |
| Test-Retest | |||
| BEJ360dy1 | Brooke | 2.18 | Brain |
| BEJ360dy2a | Brooke | 2.80 | Brain |
| Paired Blockingb | |||
| BEJ185dy1 | Kennedy | 4.01 | Brain |
| BEJ185dy2 | Kennedy | 2.90 | Brain |
| BEH186dy1 | Missy | 2.10 | Brain |
| BEH186dy2 | Missy | 1.18 | Brain |
| BEJ369dy1 | LuLu | 3.71 | Brain |
| BEJ369dy2 | LuLu | 2.42 | Brain |
| Blocking onlyc | |||
| BEJ362dy2a | Brain |
Whole body images collected after brain scan
Baseline = dy1, Pretreatment with 1.0 mg/kg naloxone 15 min prior to scan = dy2
Baseline data not useable (transmission scan could not be acquired).
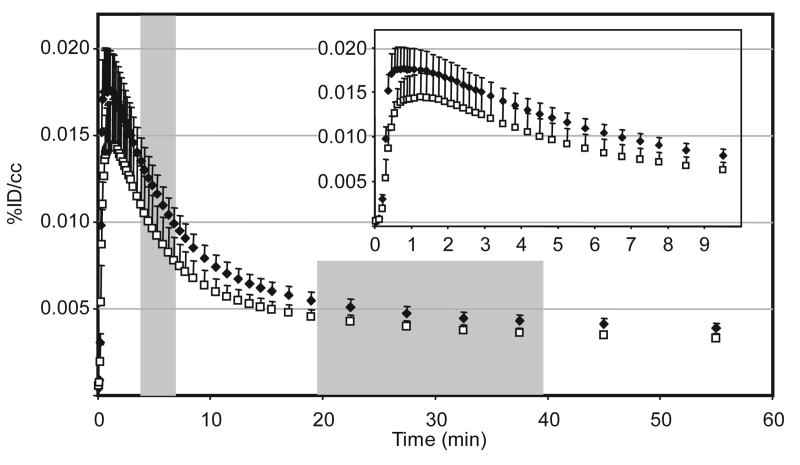
Using a whole brain region of interest, we determined that 2 rapidly entered the brain attaining a maximum concentration in approximately 40 s, Fig 2. This is significant given that the rate of a drug reaching its peak concentration in the brain has been linked directly to its reinforcing properties and is often as important as the dose (Balster and Schuster, 1973). For perspective, the input rate we observed was nearly an order of magnitude faster than the input of [11C]-cocaine, which peaks 5-6 min after intravenous administration (Volkow et. al., 1987). Clearance of C-11 from the brain was also astonishingly rapid with a half-life from peak of 8 min, reaching 25% of maximum in less than 30 min. The kinetics from our studies were consistent with the duration of action of salvinorin A, which is typically less than 10 min (Baggott et. al., 2004 and Siebert, 1994).
Fig 2.
[11C]-Salvinorin A in Papio anubis baboon brain. Time-activity curves for whole-brain VOIs, baseline (◆) n = 6, mean+sem; pretreated with 1 mg/kg naloxone (□), n = 4, mean+sem. Representative summed frames for individual studies that are given in Fig 2. Maximum activity was reached in ∼40 s and then cleared rapidly (t1/2 = 8 min).
The average maximum brain concentration of 2 was 0.0175 %ID/cc which corresponds to 3.3 % of the administered dose in the whole brain. Considering that smoked doses of 200 μg are effective in humans, we can estimate that less than 10 μg in the human brain account for the drug's psychoactive effects, further underscoring the extraordinary potency of salvinorin A. In paired pretreatment studies with naloxone, the average concentration of 2 in the brain was not significantly lower than the baseline scan. Test-retest variability examined in one study was determined to be 15%, with substantial inter-subject variability in the absolute brain concentration (standard error in Fig 2).
[11C]-Salvinorin A was widely distributed throughout the brain in both cortical and subcortical regions, (Fig 3 and Table 3). The most persistent 11C-concentration was seen in the spine and throughout the nasal and salivary tracts, Fig 3. Intriguingly, the highest concentration in the brain in all studies was in the cerebellum. This is particularly interesting given the behavioral effects of salvinorin A when abused and the role of the cerebellum in the integration of sensory perception and motor control. While κ-opioid receptors are distributed throughout the brain, including the cerebellum, the concentration of 2 did not correlate with κ-opioid density determined in previous studies (Talbot et. al., 2005 and references therein). This may indicate a high degree of non-specific binding or could indicate that other specific binding modes exist. Our studies with naloxone, a non-specific opioid antagonist, do not rule out either. Given its visual hallucinogenic properties, it is worth noting that there was also a significant amount of activity in the striate (visual) cortex.
Fig 3.
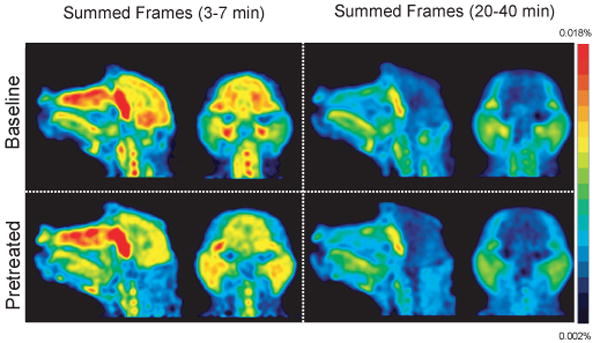
Whole head PET images of [11C]-Salvinorin A from a baseline and naloxone pretreated paired study (BEJ186) from 3-7 min (left) and 20-40 min (right). Images are normalized to injected dose (scale bar is %ID).
Table 3. Regional Distribution of [11C]-Salvinorin A in baboon braina.
| Region | 5 min | 30 min | ||
|---|---|---|---|---|
| Baseline | Pretreated | Baseline | Pretreated | |
| Cerebellumb | 0.016 ± 0.002 | 0.013 ± 0.002 | 0.005 ± 0.001 | 0.004 ± 0.002 |
| Striate Cortex | 0.014 ± 0.001 | 0.009 ± 0.002 | 0.005 ± 0.001 | 0.004 ± 0.001 |
| Amygdalac | 0.015 ± 0.001 | 0.011 ± 0.002 | 0.006 ± 0.001 | 0.005 ± 0.001 |
| Striatumc | 0.013 ± 0.001 | 0.012 ± 0.002 | 0.004 ± 0.001 | 0.003 ± 0.001 |
| Thalamusb | 0.015 ± 0.001 | 0.012 ± 0.003 | 0.003 ± 0.001 | 0.003 ± 0.002 |
| PFC | 0.013 ± 0.001 | 0.010 ± 0.002 | 0.004 ± 0.001 | 0.003 ± 0.001 |
| Cingulate | 0.013 ± 0.001 | 0.010 ± 0.002 | 0.004 ± 0.001 | 0.004 ± 0.002 |
Mean %ID/cc ± sem for n = 6 (baseline) and n = 4 (pretreated with 1 mg/kg naloxone)
Midline,
Average of left and right
Naloxone pretreatment (1 mg/kg i.v.) did not significantly change the concentration of [11C]-salvinorin A in the brain nor did it affect the magnitude of the area under the plasma time-activity curve (i.e. input). In order to examine this phenomenon more thoroughly and to determine if regional variations existed over time, we modeled the kinetic PET data using a variety of methods. We determined that due to the initial fast kinetics but persistent residual activity, a two-compartment model was most appropriate; we used a metabolite corrected plasma input to model time-activity curves. Modeling data were overwhelmed at late time points with noise from low counting statistics resulting in inconsistent and uninterpretable distribution volumes (hence data not shown). At later time points (i.e. 20 – 40 min), the small amount of C-11 remaining (less than 0.005% ID/cc) was still concentrated to the cerebellum. There was very little C-11 observed in white matter regions.
The absence of an effect by naloxone may indicate that [11C]-salvinorin A distribution was dominated by non-specific binding, that naloxone is not a sufficiently competitive binder at the κ-opioid agonist site, and/or that our specific activity was too low to observe molecular interactions. Taking conservative values for the Bmax (10 pmol/g), mass injected (10 μg), and dissociation constant (2.5 nM), we calculate that 47% occupancy is represented at our highest activity concentration in the brain i.e. 0.03 %ID/cc (Hume et. al., 1998). This occupancy, which we the feel is the absolute maximum for our experiments, was well above the 1-5% suggested for suitable tracers (Huang and Phelps, 1986). However, this level of occupancy does not change our interpretation of the PET imaging data, which we have used to probe salvinorin A pharmacokinetics. To evaluate [11C]-salvinorin A as a tracer for κ-opioid receptors, more studies would be required.
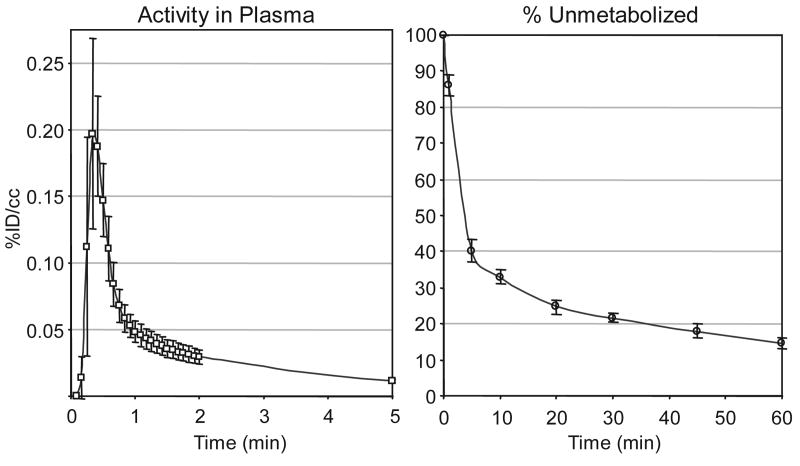
Blood samples collected during PET studies were used to determine the C-11 concentration in the plasma throughout the 60 min scan. The amount of unmetabolized 2 was determined assuming that radioactive metabolites would be more polar than salvinorin A (i.e. that the predominant metabolism would cleave the C(2) acetate), Fig 4. Indeed, solid phase extraction of plasma samples resulted in one polar and one non-polar fraction, each of which may represent multiple species. Control experiments demonstrated that [11C]-salvinorin A eluted in the non-polar fraction. The percent of C-11 in the non-polar fraction from the plasma (n = 7) is shown in Fig 4. The non-polar fraction of C-11 rapidly decreased to 40% by 5 min. Although we were not able to conclusively identify the number and identity of metabolic species by HPLC, the C-11 clears quickly from the blood indicating that transport to the brain is dominated by the first minute. Moreover, we must note that our kinetic analysis assumes that labeled metabolites had a small contribution to C-11 in the brain within the first ten minutes of imaging. The metabolism rate of 2 did not vary between individuals, nor was there significant variation introduced by naloxone pretreatment (n = 3). In all experiments, radioactivity in the plasma was below 0.01 %ID/cc within 10 min and an order of magnitude lower by 60 min. Our C-11 plasma data were highly consistent with the plasma pharmacokinetics of unlabeled salvinorin A determined in rhesus monkeys (Schmidt, M.D. et. al., 2005).
Fig 4.
Blood data. (left) Arterial blood was collected from the popliteal artery in 5 s intervals post injection and the activity in the plasma was counted. (right) Fraction of total radioactivity in the plasma attributed to 2 from 7 primates (mean ± stdev).
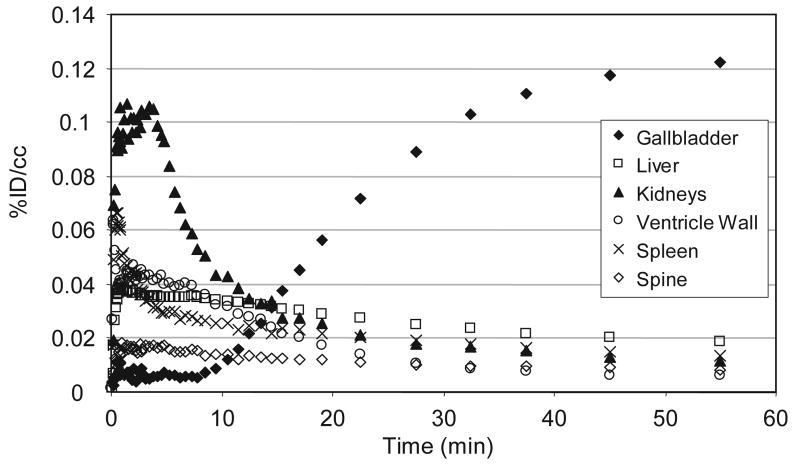
A further examination of the pharmacokinetics and distribution 2 and its labeled metabolites in peripheral organs was accomplished using PET, Fig 5. Initial uptake (concentration) was highest in the kidneys, however due to its size the total amount of C-11 was greatest in the liver. The liver uptake achieved 0.04 %ID/cc min and decreased gradually. Carbon-11 began accumulating in the gallbladder after 10 min, which at 60 min contained 0.12 %ID/cc, the highest of any organ. Our kinetic analysis suggests that at least two modes of metabolism and excretion occur; one through renal filtration (presumably hydrophilic metabolites) and the other through collection in the gall bladder (presumably lipophilic metabolites). Whole body images taken from 65 – 110 min indicate that approximately equal amounts of C-11 were in the gallbladder (and biliary tract) and urinary bladder, Fig 6. Carbon-11 in the spine endured well beyond the time in the brain and was easily observed two hours after injection. Naloxone did not appear to change the overall distribution of C-11 (based on images 65 – 110 min post injection), however kinetic differences, if any, were not determined.
Fig 5.
Time-activity curves for peripheral organs in the baboon torso (BEJ364).
Conclusion
By labeling salvinorin A with carbon-11, we were able to assess its pharmacokinetics using PET. Our main findings from PET studies in baboons were that (a) [11C]-salivorin A rapidly crosses the BBB reaching 3.3 % of the injected dose within 40 s and clearing to half of peak by 8 min; (b) [11C]-salvinorin A is distributed throughout the brain with a high concentration in the cerebellum and cortex; (c) [11C]-salvinorin is metabolized through at least two pathways. Our studies reveal an extraordinarily rapid uptake and short duration of salvinorin A in the brain. They are consistent with the distinctly rapid onset and recovery of hallucinations from smoked S. divinorum in humans and highlight the importance of salvinorin A pharmacokinetics in its behavioral effects and abuse liability. The high concentration of [11C]-salvinorin in the cerebellum and visual cortex may account for its behavioral and hallucinogenic effects when inhaled. Finally, the incredibly potency of salvinorin A can not be under emphasized. We estimate that less than 10 μg in the human brain can account for its psychoactive properties.
Acknowledgments
This work was carried out at Brookhaven National Laboratory under contract DE-AC02-98CH10886 with the U.S. Department of Energy and supported by its Office of Biological and Environmental Research. J.M.H. was supported by an NIH Postdoctoral Fellowship (1F32EB008320-01) and through the Goldhaber Distinguished Fellowship program at BNL. The authors are grateful to David Alexoff, Dr. Michael Schueller, Dr. Stephen Dewey, and Dr. Jean Logan for their helpful discussions and advice. We are grateful to Daniel Siebert for providing a reference sample of salvinorin A.
Footnotes
S. divinorum is listed as a Drug of Concern by the DEA. Illinois, Louisiana, Missouri, Tennessee, Oklahoma, Delaware, Maine, and North Dakota have passed legislation to control S. divinorum possession. Several other states will consider S. divinorum legislation in 2008.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Babu KM, McCurdy CR, Boyer EW. Opioid receptors and legal highs: Salvia divinorum and Kratom. Clinical Toxicology. 2008;46:146–152. doi: 10.1080/15563650701241795. [DOI] [PubMed] [Google Scholar]
- Baggott MJ, Erowid E, Erowid F, Mendelson JE. Use of salvia divinorum, an unscheduled hallucinogenic plant: A web-based survey of 500 users. Clinical Pharmacology & Therapeutics. 2004;75:P72. [Google Scholar]
- Balster RL, Schuster CR. Fixed-interval schedule of cocaine reinforcement: effect of dose and infusion duration. J Exp Anal Behav. 1973;20:119–129. doi: 10.1901/jeab.1973.20-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beguin C, Richards MR, Li JG, Wang Y, Xu W, Liu-Chen LY, Carlezon WA, Jr, Cohen BM. Synthesis and in vitro evaluation of salvinorin A analogues: effect of configuration at C(2) and substitution at C(18) Bioorg Med Chem Lett. 2006;16:4679–4685. doi: 10.1016/j.bmcl.2006.05.093. [DOI] [PubMed] [Google Scholar]
- Black KJ, Snyder AZ, Koller JM, Gado MH, Perlmutter JS. Template images for nonhuman primate neuroimaging: 1. Baboon. Neuroimage. 2001;14:736–743. doi: 10.1006/nimg.2001.0752. [DOI] [PubMed] [Google Scholar]
- Dischino DD, Welch MJ, Kilbourn MR, Raichle ME. Relationship between Lipophilicity and Brain Extraction of C-11-Labeled Radiopharmaceuticals. J Nucl Med. 1983;24:1030–1038. [PubMed] [Google Scholar]
- Fowler JS, Wolf AP. Working against Time: Rapid Radiotracer Synthesis and Imaging the Human Brain. Acc Chem Res. 1997;30:181–188. [Google Scholar]
- Giner JL, Kiemle DJ, Kutrzeba L, Zjawiony J. Unambiguous NMR spectral assignments of salvinorin A. Magn Reson Chem. 2007;45:351–354. doi: 10.1002/mrc.1972. [DOI] [PubMed] [Google Scholar]
- Gonzalez D, Riba J, Bouso JC, Gomez-Jarabo G, Barbanoj MJ. Pattern of use and subjective effects of Salvia divinorum among recreational users. Drug and Alcohol Dependence. 2006;85:157–162. doi: 10.1016/j.drugalcdep.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Grundmann O, Phipps SM, Zadezensky I, Butterweck V. Salvia divinorum and salvinorin A: an update on pharmacology and analytical methodology. Planta Med. 2007;73:1039–1046. doi: 10.1055/s-2007-981566. [DOI] [PubMed] [Google Scholar]
- Hume SP, Gunn RN, Jones T. Pharmacological constraints associated with positron emission tomographic scanning of small laboratory animals. Eur J Nucl Med. 1998;25:173–176. doi: 10.1007/s002590050211. [DOI] [PubMed] [Google Scholar]
- Huang SC, Phelps ME. Principles of tracer kinetic modeling in positron emission tomography and autoradiography. In: Phelps ME, Mazziotta JC, Schelbert HR, editors. Positron Emission Tomography and Autoradiography Principles and Applications for the Brain and Heart. New York, NY: Raven Press; 1986. pp. 287–346. [Google Scholar]
- Le Bars D, Luthra SK, Pike VW, Luu Duc C. The preparation of a carbon-11 labelled neurohormone--[11C]melatonin. Int J of Rad Appl and Instr Part A: Appl Rad Isotopes. 1987;38:1073–1077. doi: 10.1016/0883-2889(87)90073-6. [DOI] [PubMed] [Google Scholar]
- Loening AM, Gambhir SS. AMIDE: A completely free system for medical imaging data analysis. J of Nucl Med. 2001;42:192. [Google Scholar]
- Lux JE, Frecker RC. A Comparison of Nicotine Administered by Aerosol Inhalation and Intravenous-Injection. Clinical Pharmacology & Therapeutics. 1987;41:230–230. [Google Scholar]
- Millan MJ. Kappa-opioid receptors and analgesia. Trends Pharmacol Sci. 1990;11:70–76. doi: 10.1016/0165-6147(90)90321-x. [DOI] [PubMed] [Google Scholar]
- Munro TA, Rizzacasa MA. Salvinorins D-F, new neoclerodane diterpenoids from Salvia divinorum, and an improved method for the isolation of salvinorin A. J Nat Prod. 2003;66:703–705. doi: 10.1021/np0205699. [DOI] [PubMed] [Google Scholar]
- Roth BL, Baner K, Westkaemper R, Siebert D, Rice KC, Steinberg S, Ernsberger P, Rothman RB. Salvinorin A: a potent naturally occurring nonnitrogenous kappa opioid selective agonist. Proc Natl Acad Sci U S A. 2002;99:11934–11939. doi: 10.1073/pnas.182234399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MD, Schmidt MS, Butelman ER, Harding WW, Tidgewell K, Murry DJ, Kreek MJ, Prisinzano TE. Pharmacokinetics of the plant-derived kappa-opioid hallucinogen salvinorin A in nonhuman primates. Synapse. 2005;58:208–210. doi: 10.1002/syn.20191. [DOI] [PubMed] [Google Scholar]
- Schmidt MS, Prisinzano TE, Tidgewell K, Harding W, Butelman ER, Kreek MJ, Murry DJ. Determination of Salvinorin A in body fluids by high performance liquid chromatography-atmospheric pressure chemical ionization. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;818:221–225. doi: 10.1016/j.jchromb.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Siebert DJ. Salvia divinorum and salvinorin A: new pharmacologic findings. J Ethnopharmacol. 1994;43:53–56. doi: 10.1016/0378-8741(94)90116-3. [DOI] [PubMed] [Google Scholar]
- Talbot PS, Narendran R, Butelman ER, Huang YY, Ngo K, Slifstein M, Martinez D, Laruelle M, Hwang DR. C-11- GR103545, a radiotracer for imaging kappa-opioid receptors in vivo with PET: Synthesis and evaluation in baboons. J Nucl Med. 2005;46:484–494. [PubMed] [Google Scholar]
- Tang AH, Collins RJ. Behavioral effects of a novel kappa opioid analgesic, U-50488, in rats and rhesus monkeys. Psychopharmacology. 1985;85:309–314. doi: 10.1007/BF00428193. [DOI] [PubMed] [Google Scholar]
- Tidgewell K, Harding WW, Schmidt M, Holden KG, Murry DJ, Prisinzano TE. A facile method for the preparation of deuterium labeled salvinorin A: synthesis of [2,2,2-2H3]-salvinorin A. Bioorg Med Chem Lett. 2004;14:5099–5102. doi: 10.1016/j.bmcl.2004.07.081. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fischman MW, Foltin RW, Fowler JS, Abumrad NN, Vitkun S, Logan J, Gatley SJ, Pappas N, et al. Relationship between subjective effects of cocaine and dopamine transporter occupancy. Nature. 1997;386:827–830. doi: 10.1038/386827a0. [DOI] [PubMed] [Google Scholar]
- Vortherms TA, Roth BL. Salvinorin A: from natural product to human therapeutics. Mol Interv. 2006;6:257–265. doi: 10.1124/mi.6.5.7. [DOI] [PubMed] [Google Scholar]
- Zeynalov E, Nemoto M, Hurn PD, Koehler RC, Bhardwaj A. Neuroprotective effect of selective kappa opioid receptor agonist is gender specific and linked to reduced neuronal nitric oxide. J Cereb Blood Flow Metab. 2006;26:414–420. doi: 10.1038/sj.jcbfm.9600196. [DOI] [PubMed] [Google Scholar]