Abstract
Background
The choice of tetanus prophylaxis for patients with wounds depends on obtaining their vaccination history, which has been demonstrated to be unreliable. Use of a rapid immunoassay (Tétanos Quick Stick, the TQS), combined with knowledge of certain demographic characteristics, may improve the evaluation of tetanus immunity and thus help to avoid inadequate prophylactic measures and reduce costs.
Objectives
To evaluate the contribution of the TQS in the choice of tetanus prophylaxis and to perform a cost‐effectiveness analysis. The final aim was to define the place of the TQS in a modified algorithm for assessment of tetanus immunity in the emergency department.
Method
In this Belgian prospective, double‐blind, multicentre study, 611 adult patients with a wound were included; 498 (81.5%) records were valid. The TQS test was performed by a nurse before the vaccination history was taken and the choice of prophylaxis was made, using the official algorithm (Belgian Superior Health Council), by a doctor who was unaware of the TQS result.
Results
The prevalence of protective anti‐tetanus immunity was 74.1%. Immunity was lower in older patients and in female patients. The TQS was a cost‐effective tool for patients presenting with a tetanus‐prone wound and considered from the vaccination history to be unprotected. Use of the TQS would have improved management in 56.9% (95% CI 47.7% to 65.7%) of patients by avoiding unnecessary treatments, leading to a reduction in the mean cost per patient (€10.58/patient with the TQS versus €11.34/patient without). The benefits of the TQS use were significantly greater in patients <61 years old: unnecessary treatment would have been avoided in 76.9% (95% CI 65.8% to 85.4%) of cases and the mean cost per patient reduced to €8.31.
Conclusion
In selected patients, the TQS is a cost‐effective tool to evaluate tetanus immunity. An algorithm is proposed for ED assessment of tetanus immunity integrating age and the TQS result.
Keywords: tetanus prophylaxis, wound, immunologic test, cost‐effectiveness, algorithm
Despite the wide availability of an excellent vaccine, tetanus is still a prevalent disease. The estimated incidences in the World Health Organization European region and the USA in the 1990s were 0.8 and 0.16 per million inhabitants, respectively,1,2 but it is believed that there is significant underreporting.3,4 In industrialised countries, most cases of cases occur among non‐immunised patients after an acute wound,4,5,6 and approximately 30–40% of them are fatal.6,7 Tetanus prophylaxis is the cornerstone of disease prevention, and primary care providers have an important role to play. The decision whether or not to give prophylaxis currently depends on the characteristics of the wound (low or high risk) and on evaluation of the patient's immunity based on the vaccination history.8,9 However, vaccination history is unreliable in evaluation of tetanus immunity10,11,12 and thus prophylaxis may be suboptimum in relation to actual immunity. Although overimmunisation increases costs and risks of secondary effects unnecessarily, underimmunisation puts the patient at risk of contracting tetanus.
Recently, an immunochromatography‐based test (Tétanos Quick Stick; TQS) has been marketed, making bedside semiquantitative evaluation of anti‐tetanus immunity possible. We and others have shown that the TQS is a reliable and effective tool for the evaluation of immunity to tetanus in the emergency department (ED).10,13,14 To our knowledge, however, no cost–benefit analysis of TQS use has previously been performed. This multicentre study was designed to investigate the cost‐effectiveness of the TQS for the choice of tetanus prophylaxis for patients presenting in the ED with a wound. Because anti‐tetanus immunity has previously been shown to be related to age, sex, and birthplace,10,15,16 these demographic features were included in the analysis.
Finally, taking into account these results as well as those of our previous study,10 we were able to define the place of the TQS in a modified algorithm for management of patients presenting to the ED with a wound.
Methods
Survey design
This multicentre, prospective, double blind study was conducted in five EDs (three university‐affiliated hospitals and two local hospitals) located in urban or rural areas of the three regions of Belgium (Brussels, Flanders and Wallonia) (table 1).
Table 1 Description of investigative EDs and study group.
| Total | Emergency department identity number | p Value* | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||||||||||
| Investigative ED | ||||||||||||||
| Location (area, region) | Urban, Brussels | Urban, Flanders | Urban, Flanders | Rural, Wallonia | Rural, Wallonia | |||||||||
| University‐affiliated | Yes | Yes | No | Yes | No | |||||||||
| Study group | ||||||||||||||
| Patients with data suitable for analysis (n) | 498 | 100 | 101 | 98 | 106 | 93 | ||||||||
| Mean (SEM) age | 43.8 (0.78) | 44.1 (1.82) | 42.6 (1.73) | 47.5 (1.83) | 43.2 (1.70) | 41.6 (1.68) | NS | |||||||
| Male/female (%) | 69.9/30.1 | 74.0/26.0 | 74.2/25.8 | 63.3/36.7 | 67.9/32.1 | 69.9/30.1 | NS | |||||||
| Native/not native to Belgium (%) | 86.4/13.6 | 80.6/19.4 | 89.1/10.9 | 88.4/11.6 | 85.8/14.2 | 88.0/12.0 | NS | |||||||
| Prevalence of anti‐tetanus immunity (%) | 74.1 | 68.0 | 78.2 | 58.2 | 84.0 | 81.7 | <0.001* | |||||||
ED, emergency department; NS, non‐significant.
*Difference between groups.
The ethics committee of each participating centre approved the study, and the ethics committee of Erasme University Hospital (Brussels) was designated as the organising committee.
Selection of the participants and data collection
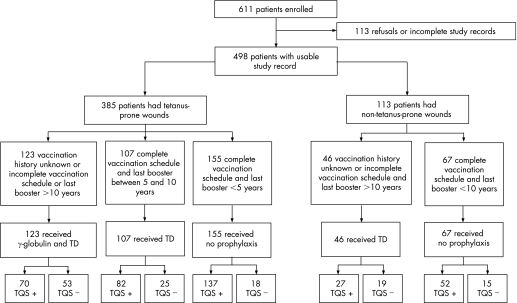
Consecutive adult patients were included if they attended the participating EDs with a wound or any other condition associated with the need to assess tetanus prophylaxis. Exclusion criteria were age <18 years, prior inclusion in the same study, and inability to provide a history (eg, psychiatric disease, dementia or confusion). In total, 611 adult patients who attended the five EDs between May and October 2006 were included; 498 (81.5%) records were suitable for analysis (fig 1). The TQS test was performed by a nurse before the vaccination history was taken by another nurse or a doctor, who were unaware of the TQS result. The choice of prophylaxis was made by the doctor (who again was unaware of the TQS result), using the official algorithm defined by the Belgian Superior Health Council.8 A patient was considered to be protected if they had undergone a complete vaccination programme (three doses of tetanus anatoxin) and their last booster had been administered within the previous 10 years. A wound that met at least one of the following criteria was considered at risk of tetanus: wound care delayed for >6 hours, depth >1 cm, burn, abrasive wound, chronic wound, wound contamination (bite, scratch, stain, foreign bodies, faeces), presence of necrotic tissue, or signs of infection. Presentation of a vaccination card or any other official document related to the patient's vaccination history was noted in the medical record. Sociodemographic characteristics (age, sex and place of birth) were systematically recorded.
Figure 1 Study design.
Evaluation of immunity by the Tétanos Quick Stick
Immunity against tetanus was evaluated by the TQS (Gamma, Angleur, Belgium). This semiquantitative test, designed for bedside use only, requires one drop of blood that can be taken by finger prick, and detects anti‐tetanus toxoid (anti‐TT) antibody using immunochromatography. There are no contraindications to this test. The principle and procedure have been described in detail elsewhere.13
The tests were performed on whole blood and were interpreted after 10 minutes in accordance with the manufacturer's recommendation. The detection threshold asserted by the company is 0.2 IU/mL on whole blood. Validation of the TQS test was performed in a previous recent study performed by our group.10 Compared with ELISA, which is a reference method for determination of anti‐TT antibody, the negative predictive value (NPV) of the TQS was 77.2%, which was significantly better than vaccination history (45.8%, p<0.0001) for determination of tetanus immunity, and the positive predictive value (PPV) was 92.1%, which differed only slightly from vaccination history (81.8%, p = 0.04) (see appendix 1).
A training course was organised for the ED nurse team before starting the study. Results (positive or negative) were recorded in the patient's medical records. All tests were carefully preserved. To avoid the variability of interpretation inherent in using different operators, a second interpretation of the tests was systematically performed by the most experienced main investigator (MS) who found that 22 tests (4.4%) interpreted as negative were actually positive.
Cost–benefit analysis
The cost of the tetanus prophylaxis based on the vaccination history was compared with the cost of prophylaxis based on the TQS result. Because the PPV of the TQS is as high as vaccination history (appendix 1) in the evaluation of immunity, this cost–benefit analysis was not performed in patients claiming to be protected against tetanus.
The unit prices of tetanus‐specific immunoglobulin (€7.46; Tetabulin, Baxter, Deerfield, Illinois, USA), tetanus vaccine (€3.88, tetanus combined with diphtheria anatoxin (TD); Tedivax, GlaxoSmithKline, Genval, Belgium) and TQS (€5.70; Gamma, Angleur, Belgium) used were those charged (including value added tax at 21%) for in‐hospital use in Belgium. In practice, the cost of prophylaxis is shared between the patient and the social insurance system, whereas the cost of the TQS is currently charged to the patient.
Statistical analysis
Data were analysed by descriptive statistics (mean (SEM), frequencies and 95% CI). One way analysis of variance or the χ2 test were performed to determine the differences between groups, and p<0.05 was considered significant. All statistical analyses were performed using SPSS V.12.0 (SPSS, Chicago, IL, USA)
Results
Prevalence of immunity against tetanus
The prevalence of protective immunity against tetanus was evaluated by calculating the percentage of patients with a positive TQS. Overall, 74.1% (95%CI 70.3% to 77.9%) of the patients were protected, but this proportion varied significantly among the different EDs (range 58.2% to 84.0%) (table 1). As we previously found that age, sex and birthplace are good predictive factors for the presence of tetanus immunity, we investigated whether differences in these demographic features could explain such variations in the seroprevalence rate. Interestingly, there were no significant differences among groups in mean age, sex ratio, or proportion of patients not native to Belgium (table 1).
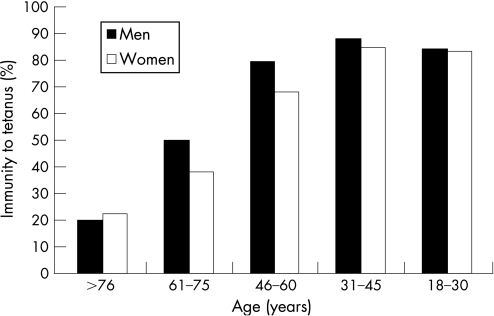
These results suggest that other factors influence the prevalence of tetanus immunity. For example, patients attending the ED of a hospital located in a rural area were generally better protected than those attending the EDs of urban hospitals (82.9% vs 68.2%, p<0.001). The presence of protective immunity was clearly related to age and sex but not to birthplace. The prevalence of immunity decreased sharply with increasing age, from 87.7% among patients aged 31–45 years to 21.2% in those aged >76 years (fig 2). Interestingly, the best seroprotection rate was observed in patients aged 31–45 years, rather than younger patients. When all groups of all ages were merged, women were significantly less protected than were men (63.3% vs 78.7%, p<0.001), but this sex difference in protection was not statistically significant within each age category.
Figure 2 Prevalence of immunity against tetanus according to age and sex.
Evaluation of tetanus immunity by vaccination history versus the TQS
Based on the vaccination history, the patients were separated into two groups, protected and unprotected (including “unknown”) and need for prophylaxis was determined accordingly.
Of 169 patients who were classified as unprotected, 97 (57.4%) in fact had a protective antibody level (fig 1). This proportion was similar for patients presenting with tetanus‐prone (70/123, 56.9%) and non‐tetanus‐prone (27/49, 58.7%) wounds. For these patients, evaluation of immunity by the TQS instead of by vaccination history would have changed the choice of prophylaxis in most cases. In contrast, 271 of the 329 patients (82.4%) considered as protected according to the vaccination history had a positive TQS result.
Only 10.2% of patients had a vaccination card. In most cases, therefore, the medical team had no objective evidence of vaccination status and had to select prophylaxis based only on the reported vaccination history.
Cost–benefit analysis of the TQS
We then investigated the effect of TQS use on the cost of the prophylactic treatment. As stated, only patients considered as unprotected (unknown vaccination history, incomplete vaccination programme, or last booster >10 years previously) would be able to benefit from the TQS. The analysis was thus limited to this category of patients. Because administration of tetanus‐specific immunoglobulin is indicated only for tetanus‐prone wounds, patients presenting with tetanus‐prone and non‐tetanus‐prone wounds were analysed separately. For tetanus‐prone wounds and combining all age groups, use of the TQS would have avoided unnecessary treatment in 56.9% (95% CI 47.7% to 65.7%) of cases (table 2), leading to a reduction in the mean cost per patient (€10.58/patient with the TSQ vs €11.34/patient without). Because patients aged >61 years were much better protected, the same analysis was performed in this selected group (table 2). Unnecessary treatment would have been avoided in 76.9% (95% CI 65.8% to 85.4%) of this group of patients. As expected, the financial gain with use of the TQS was significantly (p<0.001) higher in this group than in patients of all ages (€8.31/patient vs €10.58/patient respectively). For non‐tetanus‐prone wounds, despite allowing unnecessary treatment to be avoided in 58.7% (95% CI 44.4% to 72.9%) of patients, use of the TQS would not have been cost‐effective, whatever the patient's age (€7.30 with the TQS vs 3.88€/patient without; table 2). This finding was not surprising, considering the higher cost of specific immunoglobulin compared with the TT (and diphtheria) vaccine.
Table 2 Cost analysis of the TQS use among patients with unknown or unprotected vaccination history*.
| Determination of anti‐tetanus immunity | Proportion of patients eligible for treatment (γ‐globulin + Td) (%) | Proportion of saved treatments, % (95% CI) | Mean cost/ patient† | |||
|---|---|---|---|---|---|---|
| Patients with tetanus‐prone wound, all ages | ||||||
| Vaccination history | 100 | — | 11.34 | |||
| TQS | 43.1 | 56.9 (47.7 to 65.7) | 10.58 | |||
| Patients with tetanus‐prone wound aged <61 years | ||||||
| Vaccination history | 100 | — | 11.34 | |||
| TQS | 23.1 | 76.9 (65.8 to 85.4) | 8.31 | |||
| Patients with non‐tetanus‐prone wound, all ages | ||||||
| Vaccination history | 100 | — | 3.88 | |||
| TQS | 41.3 | 58.7 (44.4 to 72.9) | 7.30 |
*Incomplete vaccination programme or last booster >10 years previously.
†Price in euros, taxes included.
In conclusion, the use of the TQS has a cost benefit only for patients presenting with tetanus‐prone wounds and considered from the vaccination history to be unprotected (table 3).
Table 3 Benefit of TQS use.
| Tetanus‐prone wound | Vaccination history | ||
|---|---|---|---|
| Unknown or not protected | Partially protected*/fully protected† | ||
| Yes | Mean cost/patient reduced | Not useful because the positive predictive value of vaccination history is as high as the TQS for evaluation of tetanus immunity | |
| No | Mean cost/patient increased | ||
*Complete vaccination programme with last booster 5–10 years previously.
†Complete vaccination programme with last booster <5 or <10 years previously in presence of tetanus‐prone or non‐tetanus‐prone wound, respectively.
Discussion
Lack of tetanus immunity is the main cause of the persistence of tetanus, “the inexcusable disease”,17 in developed countries, and is common among patients presenting to the ED.11,18 According to the TQS results, 25.9% of patients with a tetanus‐risk wound had no protective antibody level. Because the spores of Clostridium tetanii are ubiquitous, exposure is frequent and difficult to prevent. Passive or active immunisation by immunoglobulin or vaccine, respectively, is the most efficient way to prevent the disease.
The increased number of cases of tetanus observed among geriatric patients4,9,19 is not surprising; this study, like previous studies,10,15,16,20 show that the level of protective antibodies decreases with age. There may be several reasons for this observation: lack of systematic vaccination before the late 1950s, increased life expectancy without administration of the recommended tetanus booster, and possibly a deficient immune response to vaccine associated with immunosenescence.21 Whatever the underlying reasons, age is an important variable to include in the assessment of tetanus immunity, as has already been suggested.11,12,13
The TQS represents a useful tool in the evaluation of tetanus immunity, but the economic benefit of such a tool needed to be investigated. Indeed, the cost of the TD vaccine is less than the price of the TQS; however, immunoglobulins are more expensive. In this double‐blind study, we compared the cost of prophylaxis chosen on the basis of vaccination history versus that based on the TQS result. Because the NPV (but not the PPV) of the TQS is higher than that for vaccination history, the cost analysis was performed only for patients believed to be unprotected. As expected, use of the TQS enabled the mean cost per patient to be decreased only for those requiring immunoglobulin, namely, those with a tetanus‐prone wound. The cost saving was significantly increased for patients aged <61 years, although the same low NPV of the vaccination history is observed in this age category, the proportion of immunised patients was higher. For tetanus‐prone wounds, 56.9% of patients claiming to be unprotected actually had a positive the TQS. Interestingly, this proportion is very similar to the 54.2% that we found in our previous study,10 suggesting that it is reproducible. According to the results, the treatment given (immunoglobulin and TD) was unnecessary in at least 47.7%, and potentially in 65.7%, of these patients. This proportion was increased to 76.9% in patients aged <61 years. Applying these results globally across Belgium, a crude calculation estimates the cost savings related to the TQS as between 300 000 and 400 000 euros per year (see appendix 3). Importantly, as well as the economic consequences, excessive administration of immunoglobulins and anatoxins increases the risk of adverse effects. Clinical features associated with overimmunisation induced by anatoxin range from local reaction at the injection site to serum disease.22,23 Immunoglobulins are biologically active blood byproducts, and their use may be associated with possible transmission of pathogenic agents.24
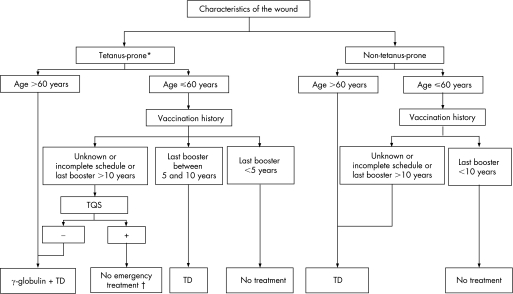
Our results clearly demonstrate that the TQS is a useful and cost‐effective tool in a group of patients defined by age and vaccination history. A conservative approach to the management of tetanus prophylaxis would be to increase treatment among older, less immunised patients, and a rational approach would be to better identify younger patients who are more likely to be immunised and who are eligible for treatment with immunoglobulin and TD. Following this logic, we propose a modified algorithm for management of tetanus prophylaxis based on the characteristics of the wound, the age, the vaccination history and the TQS result (fig 3). The cut‐off of 60 years was chosen on the basis of current epidemiological data but will need to be re‐evaluated in the future because of the difficulty in measuring the influence of extrinsic factors, such as the end of World War II and systematic vaccination, on the prevalence of immunity.
Figure 3 Proposed algorithm for management of patients presenting with wound in the ED. *Wounds with a risk of anaerobiosis such as, but not limited to, wounds contaminated with dirt, faeces, soil or saliva; puncture wounds; treatment delayed (>6 hours); avulsions; wounds resulting from burns or crushing; chronic wounds; wounds with signs of necrosis or infection. †Refer the patient to his general practitioner to check immunity and the need for Td booster.
Given these data, at least partial coverage of the cost of the test by the Belgian social insurance system should be considered. This algorithm needs to be tested in a future large‐scale prospective study, particularly to evaluate the feasibility of its use in clinical practice.
In conclusion, the choice of tetanus prophylaxis in patients with wounds is often not related to the true immunity of the patient. The practical and harmful consequences are that unnecessary and expensive treatments are given to young patients and poor anti‐tetanus immunisation is provided for older patients. The rationale for use of the TQS use is the cost benefit, and age is a key factor to include in the assessment of tetanus immunity. A modified algorithm is proposed that would improve the choice of tetanus prophylaxis and, at the same time, allow substantial economic savings.
Acknowledgements
The authors thank Professor Dramaix for help with the statistical analysis, the nurses and administrative teams of the emergency departments for their active and useful collaboration, and the international distributor of the Tétanos Quick Stick (Nephrotek Laboratory, Rungis, France) for providing the tests.
Abbreviations
ED - emergency department
ELISA - enzyme linked immunosorbent assay
NPV - negative predictive value
PPV - positive predictive value
TD - tetanus combined with diphtheria anatoxin vaccine
TQS - Tétanos Quick Stick
TT - tetanus toxoid
Appendix 1
Table A1 Comparison between vaccination history and the TQS for anti‐tetanus immunity evaluation .
| History | TQS | p Value | ||
|---|---|---|---|---|
| Concordance (kappa) | 0.27 | 0.71 | ||
| Sensitivity (%) | 60.3 | 85.3 | <0.0001 | |
| Specificity (%) | 73.3 | 87.2 | 0.065 | |
| Positive predictive value (%) | 81.8 | 92.1 | 0.039 | |
| Negative predictive value (%) | 45.8 | 77.2 | <0.0001 | |
Appendix 2
Results in a nutshell
Overall, 74.1% (95% CI 70.3% to 77.9%) of patients had protective anti‐tetanus immunity.
The prevalence of immunity differed significantly among the participating EDs despite lack of significant differences in the demographic characteristics of the study population.
The prevalence of immunity decreased with increasing age and was lower in women than in men.
Most patients who were unprotected according to their vaccination history in fact had a protective level of anti‐TT antibody by the TQS.
The TQS has cost benefits for patients presenting with a tetanus‐prone wound and with an “unknown” or “protected” vaccination history.
The economic gain is significantly increased for patients ⩽60 years.
Appendix 3
A projection: the case in Belgium
Based on consumption of 55 000 doses of γ‐globulin in 2006 for ∼ 106 inhabitants, use of the TQS would have saved a minimum of 47.7% (n = 26 235) and a maximum of 65.7% (n = 36 135) of treatments.
Considering the usual and recommended administration of TD (€3.88) in combination with γ‐globulin (€7.46), the estimated financial saving is €297 05 to €409 771.
Footnotes
An investigation group of the Belgian Society of Emergency and Disaster Medicine (BeSEDiM).
Competing interests: None.
References
- 1.Pascual F B, Mc Ginley E L, Zanardi L R.et al Tetanus surveillance, United States, 1998–2000. MMWR Surveill Summ 2003521–8. [PubMed] [Google Scholar]
- 2.World Health Organization Centralized information system for infectious diseases (CISID). http://data.euro.who.int/cisid (accessed 13 July 2007)
- 3.Rushdy A A, White J M, Ramsay M E.et al Tetanus in England and Wales, 1984–2000. Epidemiol Infect 200313071–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pedalino B, Cotter B, Ciofi degli Atti M.et al Epidemiology of tetanus in Italy in years 1971–2000. Euro Surveill 20027103–110. [DOI] [PubMed] [Google Scholar]
- 5.Peetermans W E, Schepens D. Tetanus – still a topic of present interest: a report of 27 cases from a Belgian referral hospital. J Int Medicine 1996239249–252. [DOI] [PubMed] [Google Scholar]
- 6.Hsu S. S, Groleau G. Tetanus in the emergency department: a current review, J Emerg Med 200020357–365. [DOI] [PubMed] [Google Scholar]
- 7.Sanford J P. Tetanus‐forgotten but not gone. N Engl J Med 1995332812–813. [DOI] [PubMed] [Google Scholar]
- 8.Health Council Advisory reports and recommendations, Federal Public Service, Health, Food Chain Safety and Environment. http://www.health.fgov.be/CSH_HGR/Francais/Brochures/fr2002_tetanos.pdf (accessed 13 July 2007)
- 9.Department of Health ( U K ) Immunisation against infectious disease – “The Green Book”. http://www.dh.gov.uk/en/Policyandguidance/Healthandsocialcaretopics/Greenbook/DH_4097254 (accessed 13 July 2007)
- 10.Stubbe M, Swinnen R, Crusiaux A.et al Seroprotection against tetanus in patients attending an emergency department in Belgium and evaluation of a bedside immunotest. Eur J Emerg Med 20071414–24. [DOI] [PubMed] [Google Scholar]
- 11.Talan D A, Abrahamian F M, Moran G J.et al Tetanus immunity and physician compliance with tetanus prophylaxis practices among emergency department patients presenting with wounds. Ann Emerg Med 200443305–314. [DOI] [PubMed] [Google Scholar]
- 12.Burton T, Crane S. Unnecessary tetanus boosters in the ED. Emerg Med J 200522609–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elkharrat D, Espinoza P, De la Coussaye J.et al Inclusion of a rapid test in the current Health Ministry Guidelines with the purpose of improving anti‐tetanus prophylaxis prescribed to wounded patients presenting at French Emergency Departments. Med Mal Infect 200535323–328. [DOI] [PubMed] [Google Scholar]
- 14.Ardelean‐Jaby D, Kaddari‐Himeur F, Nkana‐Tameze K.et al Evaluation of blood test the TQS (Tetanos Quick Stick) used in emergency units. Immuno‐analyse and Biologie Spécialisée 200217330–335. [Google Scholar]
- 15.McQuillan G M, Kruszon‐Moran D, Deforest A.et al Serologic immunity to diphtheria and tetanus in the United States. Ann Intern Med 2002136660–666. [DOI] [PubMed] [Google Scholar]
- 16.Gergen P J, McQuillan G M, Kiely M.et al A population‐based serologic survey of immunity tetanus in the United States. N Engl J Med 1995332761–766. [DOI] [PubMed] [Google Scholar]
- 17.Edsall G. Editorial: The inexcusable disease. JAMA 197623562–63. [DOI] [PubMed] [Google Scholar]
- 18.Brand D A, Acampora D, Gottlieb L D.et al Adequacy of antitetanus prophylaxis in six hospital emergency rooms. N Engl J Med 1983309636–640. [DOI] [PubMed] [Google Scholar]
- 19.Quinn E H, McIntyre B Peter Tetanus in elderly – An important preventable disease in Australia. Vaccine 2007251304–1309. [DOI] [PubMed] [Google Scholar]
- 20.Alagappan K, Rennie W, Kwiatkowski T.et al Seroprevalence of tetanus antibodies among adults older than 65 years. Ann Emerg Med 19962818–21. [DOI] [PubMed] [Google Scholar]
- 21.Hainz U, Jenewein B, Asch E.et al Insufficient protection for healthy elderly adults by tetanus and TBE vaccines. Vaccine 2005233232–3235. [DOI] [PubMed] [Google Scholar]
- 22.Edsall G, Elliott M W, Peebles T C.et al Excessive use of tetanus boosters. JAMA 1967202111–113. [DOI] [PubMed] [Google Scholar]
- 23.Levine L, Edsall G. Tetanus toxoid: what determine reaction proneness? J Infect Dis 1981144376. [DOI] [PubMed] [Google Scholar]
- 24.Creange A, Gray F, Cesaro P.et al Pooled plasma derivatives and Creutzfeldt‐Jakob disease. Lancet 1996347482. [DOI] [PubMed] [Google Scholar]