The discrepancy between fossil- and molecular-based age estimates for the diversification of modern birds has persisted despite increasingly large datasets on both sides (Penny & Phillips 2004). For the purpose of addressing this discrepancy, Ericson et al. (2006) recently generated a significant neoavian dataset that is well represented by taxa (87 species comprising 75 traditional families), characters (five nuclear genes) and fossil calibrations (n=23). The divergence times reported in this study are by far the youngest yet reported from genetic data. These authors conclude that there is no reliable molecular support for extensive diversification of Neoaves in the Cretaceous. While an increased agreement with the fossil record is encouraging (and, indeed, sought after), we find a number of problems with their study that calls this conclusion into question.
Our first concern with this paper involves the particular fossils used to calibrate and constrain estimated divergence times. Fossils are of fundamental importance in estimating dates with molecular sequence data, and care should be taken that they are taxonomically and stratigraphically well identified. While the fossils used in Ericson et al. (2006) appear to fit these criteria, we nevertheless take issue with the particular fossils used. First, Ericson et al. (2006) use a stem group galliform fossil (53 Myr; Mayr & Weidig 2004) to date the divergence between Galliformes and Anseriformes, despite the fact that an older (66 Myr), and therefore more appropriate, fossil anseriform calibration exists (Clarke et al. 2005). Ericson et al.'s (2006) estimate of the age of the Galliformes–Anseriformes split is approximately 53 Myr, 13 Myr younger than the minimum age definitively known from the fossil record (Benton & Donoghue 2006). Second, for the (required) fixed calibration, they use a 47.5 Myr stem group representative of Trochilidae to mark the splitting of hummingbirds from other Apodiformes. No rationale is given explaining why this fossil was adopted, and we note that an older (62 Myr), more derived and hence more appropriate fossil is established from the stem of Sphenisciformes (Slack et al. 2006). Regardless, owing to the importance of the single fixed constraint, alternatives should have been considered. Third, the authors impose a maximum constraint of 95 Myr on the age of Neoaves, despite the fact that earlier dates have been published (e.g. van Tuinen & Hedges 2001; Pereira & Baker 2006). Finally, one of their fossil calibrations (stem Strigiformes) is uninformative for dating purposes, as it is superseded by an equally old (55 Myr) but more derived fossil (Coliidae).
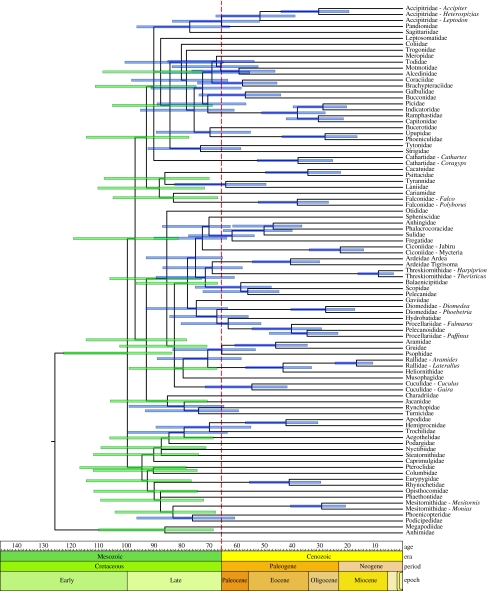
Our second concern involves the reliance on PATHd8 for estimating lineage ages. Ericson et al. (2006) also used the program r8s (Sanderson 2003), but dismissed these results simply because these dates are older than those generated by PATHd8 (although the older r8s dates are consistent with previous molecular-generated dates). The inferred dates from r8s directly contradict their claim of an absence of neoavian diversification in the Cretaceous. Agreement with the fossil record, while satisfying in terms of congruence, is not a sufficient criterion to arbitrate between sets of dates generated by different methods. Rather, arbitration should rely upon the performance of methods on both empirical and simulated data, and PATHd8 has yet to be tested in this way. To compare their PATHd8 results with those from a well-vetted program, we reanalysed the data of Ericson et al. (2006) using a Bayesian modelling of rate evolution (Thorne & Kishino 2002) and the revised calibrations outlined previously (see electronic supplementary information for methods). Contrary to their results, we find evidence for substantial diversification of Neoaves in the Cretaceous (figure 1).
Figure 1.
Chronogram for Neoaves estimated using a Bayesian modelling of rate evolution. The dashed vertical red line marks the K–T boundary. Error bars represent posterior probability (0.95) credible intervals (root node 104–154 Myr). An unambiguous ancient diversification of Neoaves is indicated by 24 credible intervals restricted to the Cretaceous (green bars).
Finally, and most importantly, nowhere do Ericson et al. (2006) mention any error intervals on their dating estimates. Given the proximity of many nodes to the K–T boundary, confidence intervals on age estimates would cross into the Cretaceous and render their conclusion untenable. Error estimates are easily generated using either non-parametric bootstrapping or considering a posterior distribution of trees. As error is inherent in each step of molecular dating (sequences, alignment, fossils, trees, etc.), the lack of error calculation is disturbing and undermines their ultimate assertion. When incorporating error intervals in our reanalysis, 24 basal neoavian divergences are restricted to the Cretaceous (figure 1, green bars). Of these, 15 lead directly to extant families. While the addition of further family representatives will undoubtedly break up some of these branches (forming crown clades), a Tertiary origin for much of Neoaves is clearly rejected.
Given the results of our reanalysis of the data of Ericson et al. (2006), the noteworthy problems attendant in their study and the plurality of genetic studies indicating a Cretaceous origin of modern birds, we respectfully disagree with their conclusion and find instead that there is no reliable molecular evidence against an extensive pre-Tertiary radiation of Neoaves.
Acknowledgments
We thank Ericson et al. for making their data freely available. J.W.B. thanks I. Pop, R. Asheton, S. Asheton and D. Alexander for their encouragement during this study.
Footnotes
The accompanying reply can be viewed at http://dx.doi.org/10.1098/rsbl.2007.0103.
Supplementary Material
Details of DNA alignment, fossil calibrations, and divergence time estimation procedures
References
- Benton M.J, Donoghue P.C.J. Paleontological evidence to date the tree of life. Mol. Biol. Evol. 2006;24:26–53. doi: 10.1093/molbev/msl150. doi:10.1093/molbev/msl150 [DOI] [PubMed] [Google Scholar]
- Clarke J.A, Tambussi C.P, Noriega J.I, Erikson G.M, Ketcham R.A. Definitive fossil evidence for the extant avian radiation in the Cretaceous. Nature. 2005;433:305–308. doi: 10.1038/nature03150. doi:10.1038/nature03150 [DOI] [PubMed] [Google Scholar]
- Ericson P.G.P, et al. Diversification of Neoaves: integration of molecular sequence data and fossils. Biol. Lett. 2006;4:543–547. doi: 10.1098/rsbl.2006.0523. doi:10.1098/rsbl.2006.0523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson, P. G. P., Anderson, C. L. & Mayr, G. 2007 Hangin' onto our rocks'n clocks: a reply to Brown et al.(doi:10.1098/rsbl.2007.0103)
- Mayr G, Weidig I. The Early Eocene bird Gallinuloides wyomingensis—a stem group representative of Galliformes. Acta Palaeontologica Polonica. 2004;49:211–217. [Google Scholar]
- Penny D, Phillips M.J. The rise of birds and mammals: are microevolutionary processes sufficient for macroevolution? Trends Ecol. Evol. 2004;19:516–522. doi: 10.1016/j.tree.2004.07.015. doi:10.1016/j.tree.2004.07.015 [DOI] [PubMed] [Google Scholar]
- Pereira S.L, Baker A.J. A mitogenomic timescale for birds detects variable phylogenetic rates of molecular evolution and refutes the standard molecular clock. Mol. Biol. Evol. 2006;23:1731–1740. doi: 10.1093/molbev/msl038. doi:10.1093/molbev/msl038 [DOI] [PubMed] [Google Scholar]
- Sanderson M.J. r8s: inferring absolute rates of molecular evolution and divergence times in the absence of a molecular clock. Bioinformatics. 2003;19:301–302. doi: 10.1093/bioinformatics/19.2.301. doi:10.1093/bioinformatics/19.2.301 [DOI] [PubMed] [Google Scholar]
- Slack K.E, Jones C.M, Ando T, Harrison G.L, Fordyce R.E, Penny D. Early penguin fossils, plus mitochondrial genomes, calibrate avian evolution. Mol. Biol. Evol. 2006;23:1144–1155. doi: 10.1093/molbev/msj124. doi:10.1093/molbev/msj124 [DOI] [PubMed] [Google Scholar]
- Thorne J.L, Kishino H. Divergence time and evolutionary rate estimation. Syst. Biol. 2002;51:689–702. doi: 10.1080/10635150290102456. doi:10.1080/10635150290102456 [DOI] [PubMed] [Google Scholar]
- van Tuinen M, Hedges S.B. Calibration of avian molecular clocks. Mol. Biol. Evol. 2001;18:206–213. doi: 10.1093/oxfordjournals.molbev.a003794. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Details of DNA alignment, fossil calibrations, and divergence time estimation procedures