Abstract
Phylogenetic relationships among termites, mantids and the five traditionally recognized cockroach families have been the subject of several studies during the last half-century. One cockroach lineage that has remained notably absent from such studies is the Nocticolidae. This group of small, elusive surface- and cave-dwelling species from the Old World Tropics has been proposed to represent an additional family. Using molecular sequences, we performed an initial phylogenetic examination of Nocticola spp. The hypothesis that they are phylogenetically divergent was confirmed from the analyses of three genes and a combined dataset. To supplement our phylogenetic analyses, we attempted to amplify 16S rRNA from the obligate mutualistic endosymbiont Blattabacterium cuenoti, present in all cockroaches studied to date. Unexpectedly, amplification was unsuccessful in all Nocticola spp. examined. This result was confirmed by microscopic examinations of fat body tissue. These Nocticola spp. are the first cockroaches found to be uninfected by B. cuenoti, which raise questions about when the bacterium first infected cockroaches.
Keywords: Nocticolidae, Blattaria, phylogeny, obligate symbiosis
1. Introduction
Cockroaches, among the most reviled of all insects, are members of the order Blattaria. Approximately 4000 extant species have been described within seven recognized families (Blattidae, Lamproblattidae, Tryonicidae, Polyphagidae, Cryptocercidae, Blattellidae, Blaberidae; McKittrick 1964; Roth 1991; Klass & Meier 2006). Phylogenetic relationships among most of these families, as well as between cockroaches, termites and mantids (which together form the superorder Dictyoptera), have been the subject of several studies during the last half-century (see Klass & Meier (2006) and references therein). One poorly recognized group of cockroaches has remained absent from phylogenetic studies to date. This group is the Nocticolidae, which contains 24 species within 8 genera, and has been proposed to represent an additional cockroach family (Princis 1966; Roth 1991). The Nocticolidae comprises small (less than approx. 1 cm), elusive surface and cave dwellers from the Old World Tropics (Roth 1988).
We set out to test the hypothesis that the Nocticolidae represents a phylogenetically divergent lineage of cockroaches, by determining gene sequences from representatives of the genus Nocticola. In addition to standard nuclear and mitochondrial sequences, we sought to determine sequences from the endosymbiont Blattabacterium cuenoti, which has been found in all cockroaches examined to date (Dasch et al. 1984), as well as in the termite Mastotermes darwiniensis (Jucci 1952). This bacterium resides within specialized cells (bacteriocytes) of the fat body tissue of both males and females, and is transmitted vertically to 100% of offspring, following migration to the oocytes during oogenesis (Sacchi et al. 1996). It appears to be an obligate mutualist, playing a role in host nitrogen metabolism (Guthrie & Tindall 1968; Cochran 1985).
The phylogenetic relationships among B. cuenoti from diverse cockroaches have been shown to be congruent with those of their hosts, from the interspecific to inter-family level (Bandi et al. 1994; Clark et al. 2001; Lo et al. 2003; Maekawa et al. 2006). Additionally, B. cuenoti from the most basal termite lineage M. darwiniensis (Thompson et al. 2000) is most closely related to those in cryptocercid roaches. This is in agreement with morphological and molecular data that suggest that termites are a family or subfamily within Blattaria, rather than a distinct order (Lo et al. 2000; Klass & Meier 2006). These results indicate that B. cuenoti infected the ancestor of all extant cockroaches and has been inherited vertically since that point, i.e. at least 130 Myr ago. Evidence exists for only one loss of the infection: in the ancestor of all termites other than M. darwiniensis. Praying mantids, widely regarded as the sister group of all extant cockroaches (and termites; Klass & Meier 2006), are reported to be free of infection by B. cuenoti (Buchner 1965).
The goals of this study were as follows: (i) to perform an initial phylogenetic examination of the genus Nocticola, in order to test the hypothesis that the Nocticolidae represents a diverse lineage and an eighth cockroach family and (ii) to screen Nocticola spp. for the presence of the endosymbiont B. cuenoti, thereby testing the hypothesis that all cockroaches harbour this symbiont. Studies of B. cuenoti in putatively divergent cockroach lineages such as the Nocticolidae are also of potential use in understanding the long-term evolution of this bacterium. Resolution of phylogenetic relationships among cockroach families and mantids was not a goal of this study.
2. Material and methods
Samples of Nocticola australiensis and Nocticola spp. were collected from caves in Chillagoe, Queensland, and Katherine, Northern Territory, Australia. Details are provided in the electronic supplementary material. Samples for molecular and microscopic analyses were stored respectively in 100% alcohol and 2.5% glutaraldehyde in phosphate buffered saline (pH 7.4).
DNA extraction, PCR and sequencing were performed using standard protocols (see electronic supplementary material for details). PCR was performed on the nuclear genes, 18S rRNA and histone 3, the mitochondrial (mt) gene, cytochrome oxidase II (COII), and the B. cuenoti gene, 16S rRNA. Sequences are available under GenBank accession numbers EF203092–EF203097.
Following BLAST nucleotide searches, homologous sequences from related insects were downloaded from GenBank for each gene. Sequences representing all cockroach and termite families, as well as a diverse cross-section of mantids (Svenson & Whiting 2004), were selected. Alignment and phylogenetic analysis were performed using standard methods (Bayesian inference, BI and maximum parsimony, MP; see electronic supplementary material).
For light and electron microscopy, fixed samples were washed in 0.1 M cacodylate buffer (pH 7.2) and postfixed in 1% OsO4 in the same buffer for 1.5 h at 4°C. Successively, all samples were dehydrated in ethanol and embedded in Epon 812. The semi-thin sections (1 μm), for light microscopy, were stained with 0.5% toluidine blue; thin sections (80 nm) were stained with uranyl acetate and lead citrate and examined under an EM900 transmission electron microscope (Zeiss, Germany).
3. Results and discussion
(a) Nocticola is a phylogenetically divergent genus
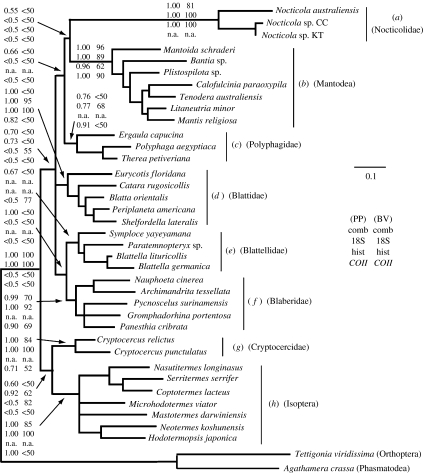
PCR of 18S rRNA, histone 3 and COII in Nocticola spp. enabled us to sequence fragments of approximately 1800, 350 and 650 bp, respectively. These were aligned with homologous sequences from cockroaches, termites and mantids, and subjected to phylogenetic analyses. Figure 1 shows the topology inferred from Bayesian analysis of the combined gene dataset, and provides a summary of results from the analysis of each of the four datasets (each individual gene plus combined genes). Eight monophyletic groups relevant to this study were recovered in most of the analyses, and are labelled (a)–(h) in figure 1. These clades consist of: (a) Nocticola spp. (b) mantids, (c)–(g) each of the five traditionally recognized cockroach families, and (h) termites. Nocticola spp. were found as a highly divergent lineage in all analyses, which is consistent with the idea that the Nocticolidae constitutes an additional family of cockroaches. It remains possible that the Nocticolidae has a close relationship with the recently erected families Lamproblattidae and Tryonicidae (Klass & Meier 2006), or other less well understood taxa not included in this study. The pattern of clustering of other taxa within each of the clades (b)–(h) was in agreement with familial and ordinal taxonomic designations.
Figure 1.
Phylogenetic relationships between Nocticola spp., mantids, termites and other cockroaches, inferred from a combined dataset of 18S rRNA, histone 3 and COII. The Bayesian inference (BI) topology was inferred using the program MrBayes, with the GTR+G model of sequence evolution for each gene. The tree was rooted with orthopteran and phasmid outgroups. Almost identical topologies and support values were found in two additional BI replicates. A maximum parsimony (MP) 50% majority-rule bootstrap analysis (1000 replicates) produced a tree that was similar to that shown, although less well resolved. BI and MP analyses were also performed on individual gene datasets. Support values at key nodes are given as posterior probabilities (PP) and bootstrap percentage values (BV) for each of the four analyses (comb, combined dataset; hist, histone 3). In the cases where a particular clade was represented only by a single terminal taxon in a given analysis, n.a. is entered. CC, Cutta Cutta cave; KT, Kintore cave. Scale bar, 0.1 substitutions per site.
Relationships among cockroach and mantid lineages were not well resolved. The sister group of Nocticola spp. was not consistent among the four datasets, and in no case was there strong support for it being closely related to a particular lineage. Thus, we were not able to hypothesize the sister group of the Nocticolidae. Notably, cockroaches did not form a monophyletic group to the exclusion of mantids in any of the analyses.
(b) Absence of B. cuenoti in the fat body tissues of Nocticola spp.
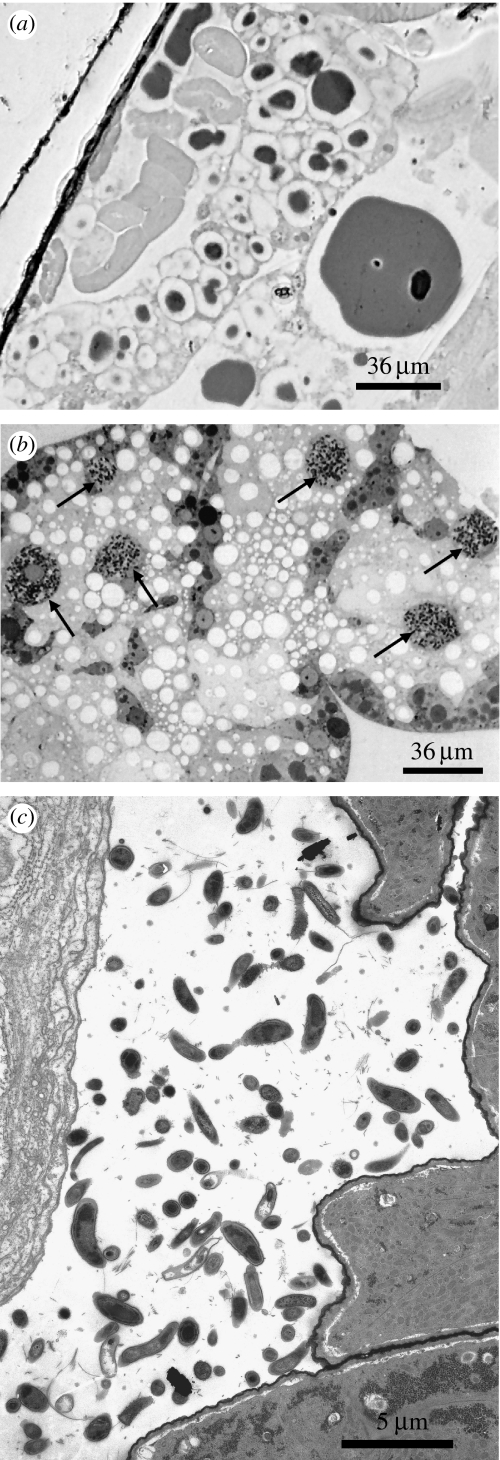
Using primers known to amplify a fragment of 16S rRNA from diverse strains of B. cuenoti, we were unable to obtain amplification from any of the Nocticola spp. specimens examined (10 individuals from 4 different caves). To confirm this observation, we examined fat body preparations from specimens collected in Donna and Royal Arch caves using light microscopy (figure 2a). In each case, no evidence of the characteristic bacteriocytes seen in the fat body of other cockroaches (figure 2b) was found. These results raise the possibility that the ancestor of the cockroaches present in the four caves was lacking B. cuenoti. An alternative explanation is that there have been multiple losses of the symbiont. Further sampling of other members of the Nocticolidae is required to test each of these scenarios.
Figure 2.
The absence of B. cuenoti in N. australiensis. (a) Light microscopy of a semithin section of fat body tissue of N. australiensis (Donna Cave, Chillagoe, Queensland). No bacteriocytes harbouring endosymbiotic bacteria are evident. Semithin sections from Nocticola sp. (Royal Arch Cave, Chillagoe, Queensland) also revealed the absence of bacteriocytes (data not shown). (b) Fat body section from Periplaneta americana. Arrows indicate prominent bacteriocytes harbouring B. cuenoti, typical of all cockroaches examined prior to this study. (c) Transmission electron microscopic image of the gut lumen of N. australiensis. Numerous prokaryotic forms are visible, suggestive of a diverse microflora.
To check for the presence of other microbes that might contribute to the metabolism of N. australiensis, transmission electron microscopy (TEM) was performed on cockroach midgut sections. A diverse bacterial fauna was found, consisting of rods and cocci showing characteristics of Gram-negative bacteria. In addition to digestive activities, this gut fauna may play a role in nitrogen recycling, as has been shown in termites (Breznak 2000).
(c) Evolution of B. cuenoti in cockroaches, mantids and termites
Previous phylogenetic analyses have shown that the ancestor of the five traditionally recognized cockroach families and termites was infected with B. cuenoti (Bandi et al. 1995; Lo et al. 2003). So far, evidence for only one loss of the bacterium has been found: in the ancestor of all termites other than the primitive lineage M. darwiniensis. Apart from mantids, Nocticola is the only other dictyopteran lineage reported to be uninfected by B. cuenoti. Owing to the uncertainty regarding the phylogenetic position of Nocticola in relation to other cockroaches and mantids, the reason for this absence remains unclear. If future studies indicate that Nocticola is clearly nested within the cockroach clade, this would suggest that B. cuenoti has been lost from this lineage secondarily, as in the case of termites other than M. darwiniensis. This could have occurred via the replacement of the role of B. cuenoti by gut microflora or perhaps via changes to a more nitrogen-rich diet. On the other hand, if both mantids and Nocticola are shown to be phylogenetically basal to all other cockroaches, this would suggest that the infection occurred in the ancestor of all cockroaches other than Nocticola, after the divergence of mantids and Nocticola from this lineage.
Acknowledgments
We thank Bob Hardy and Lana Little from Queensland Parks and Wildlife Service for their assistance with collection, and two reviewers for their useful suggestions. N.L. is supported by an Australian Research Council Postdoctoral Fellowship.
Supplementary Material
The supplementary material file contains details about collection of specimens, PCR and sequencing, Phylogenetic analyses, and a Maximum Parsimony Bootstrap tree not shown in the main text
References
- Bandi C, Damiani G, Magrassi L, Grigolo A, Fani R, Sacchi L. Flavobacteria as intracellular symbionts in cockroaches. Proc. R. Soc. B. 1994;257:43–48. doi: 10.1098/rspb.1994.0092. doi:10.1098/rspb.1994.0092 [DOI] [PubMed] [Google Scholar]
- Bandi C, Sironi M, Damiani G, Magrassi L, Nalepa C.A, Laudani U, Sacchi L. The establishment of intracellular symbiosis in an ancestor of cockroaches and termites. Proc. R. Soc. B. 1995;259:293–299. doi: 10.1098/rspb.1995.0043. doi:10.1098/rspb.1995.0043 [DOI] [PubMed] [Google Scholar]
- Breznak, J. A. 2000 Ecology of prokaryotic microbes in the guts of wood- and litter- feeding termites. In Termites: evolution, sociality, symbiosis, ecology (eds T. Abe, D. E. Bignell, & M. Higashi), pp. 209–232. Dordrecht, The Netherlands: Kluwer Academic Publishers.
- Buchner P. Wiley; New York, NY: 1965. Endosymbiosis of animals with plant microorganisms. [Google Scholar]
- Clark J.W, Hossain S, Burnside C.A, Kambhampati S. Coevolution between a cockroach and its bacterial symbiont: a biogeographical perspective. Proc. R. Soc. B. 2001;268:393–398. doi: 10.1098/rspb.2000.1390. doi:10.1098/rspb.2000.1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran D.G. Nitrogen excretion in cockroaches. Ann. Rev. Entomol. 1985;10:29–39. doi:10.1146/annurev.en.30.010185.000333 [Google Scholar]
- Dasch, G. A., Weiss, E. & Chang, K. P. 1984 Endosymbionts of insects. In Bergey's manual of systematic bacteriology, vol. 1 (eds N. R. Krieg & J. G. Holt), pp. 811–833. Baltimore, MD: Williams and Wilkins.
- Guthrie D.M, Tindall A.R. Edward Arnold Ltd; London, UK: 1968. The biology of the cockroach. [Google Scholar]
- Jucci C. Symbiosis and phylogenesis in the Isoptera. Nature. 1952;169:837. doi: 10.1038/169837a0. doi:10.1038/169837a0 [DOI] [PubMed] [Google Scholar]
- Klass K.D, Meier R. A phylogenetics analysis of Dictyoptera (Insecta) based on morphological characters. Entomol. Abh. 2006;63:3–50. [Google Scholar]
- Lo N, Tokuda G, Watanabe H, Rose H, Slaytor M, Maekawa K, Bandi C, Noda H. Evidence from multiple gene sequences indicates that termites evolved from wood-feeding cockroaches. Curr. Biol. 2000;10:801–804. doi: 10.1016/s0960-9822(00)00561-3. doi:10.1016/S0960-9822(00)00561-3 [DOI] [PubMed] [Google Scholar]
- Lo N, Bandi C, Watanabe H, Nalepa C.A, Beninati T. Evidence for co-cladogenesis between diverse dictyopteran lineages and their intracellular endosymbionts. Mol. Biol. Evol. 2003;20:907–913. doi: 10.1093/molbev/msg097. doi:10.1093/molbev/msg097 [DOI] [PubMed] [Google Scholar]
- Maekawa K, Masahiro K, Matsomoto T, Araya K, Lo N. Phylogenetic analyses of fat body endosymboints reveal differences in invasion times of blaberid wood-feeding cockroaches (Blaberidae: Panesthiinae) into the Japanese archipelago. Zool. Sci. 2006;22:1061–1067. doi: 10.2108/zsj.22.1061. doi:10.2108/zsj.22.1061 [DOI] [PubMed] [Google Scholar]
- McKittrick F.A. Evolutionary studies of cockroaches. Mem. Cornell Univ. Agric. Exp. Stat. 1964;389:1–197. [Google Scholar]
- Princis K. Blattariae: Subordo Blattoidea. Fam.: Nocticolidae. In: Beier M, editor. Orthopterum catalogus. Junk; Gravenhage, The Netherlands: 1966. pp. 602–614. [Google Scholar]
- Roth L. Some caverniculous and epigean cockroaches with six new species, and a discussion of the Nocticolidae (Dictyoptera: Blattaria) Rev. Suisse Zool. 1988;95:297–321. [Google Scholar]
- Roth, L. M. 1991 Blattodea. In The insects of Australia, vol. 1, pp. 320–329. Melbourne, Australia: Melbourne University Press.
- Sacchi L, Corona S, Grigolo A, Laudani U, Selmi M.G, Bigliardi E. The fate the endocytobionts of Blattella germanica L. (Blattaria: Blattellidae) and Periplaneta americana (Blattaria: Blattidae) during embryo development. Ital. J. Zool. 1996;63:1–11. [Google Scholar]
- Svenson G.J, Whiting M.F. Phylogeny of Mantodea based on molecular data: evolution of a charismatic predator. Syst. Entomol. 2004;29:359–370. doi:10.1111/j.0307-6970.2004.00240.x [Google Scholar]
- Thompson G.J, Kitade O, Lo N, Crozier R.H. Phylogenetic evidence for a single, ancestral origin of a ‘true’ worker caste in termites. J. Evol. Biol. 2000;13:869–881. doi:10.1046/j.1420-9101.2000.00237.x [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The supplementary material file contains details about collection of specimens, PCR and sequencing, Phylogenetic analyses, and a Maximum Parsimony Bootstrap tree not shown in the main text