Abstract
Innate immunity relies on effectors, which produce cytotoxic molecules that have not only the advantage of killing pathogens but also the disadvantage of harming host tissues and organs. Although the role of dietary antioxidants in invertebrate immunity is still unknown, it has been shown in vertebrates that carotenoids scavenge cytotoxic radicals generated during the immune response. Carotenoids may consequently decrease the self-harming cost of immunity. A positive relationship between the levels of innate immune defence and circulating carotenoid might therefore be expected. Consistent with this hypothesis, we show that the maintenance and use of the prophenoloxidase system strongly correlate with carotenoid concentration in haemolymph within and among natural populations of the crustacean Gammarus pulex.
Keywords: ecological immunology, immune costs, antioxidant
1. Introduction
Although immune defences are beneficial by fending off diseases, the activation and use of the immune system is costly and therefore cannot be sustained simultaneously with other demanding activities (Moret & Schmid-Hempel 2000). Furthermore, producing an immune response involves the release of cytotoxic chemicals that could be harmful to the host (Nappi & Ottaviani 2000; Sadd & Siva-Jothy 2006). This latter phenomenon referred to as self-harm, is likely to form part of the cost of immunity and may constrain the evolution of immune function. However, immune defence is essential and selection should act in a way that minimizes these costs.
A major component of the arthropod innate immune system is the prophenoloxidase (proPO) cascade, a general defence and non-self recognition system of arthropods that provides immunity against a large range of pathogens (Söderhäll & Cerenius 1998). The key enzyme phenoloxidase (PO) is present in the haemolymph as the inactive proenzyme proPO, which, when activated upon infection, catalyses the production of melanin (Söderhäll & Cerenius 1998). The activation of the proPO generates quinonoid intermediates and highly cytotoxic superoxide and hydroxyl radicals that participate in pathogen destruction (Nappi & Ottaviani 2000; Sugumaran et al. 2000). However, despite the presence of several mechanisms that should limit self-harm due to production in the haemocoel of these cytotoxic molecules by controlling PO activity (Sugumaran et al. 2000), the activation of the proPO system still results in damaged tissues and organs (Sadd & Siva-Jothy 2006).
Recently, carotenoid pigments have been suggested to mediate the honesty of signals through an allocation trade-off with other life-history traits such as health and reproduction (Blount 2004). In vertebrates, their roles as antioxidants and immunostimulants are well known (Møller et al. 2000). In particular, carotenoids scavenge free radicals and cytotoxic molecules produced during immune reactions (Chew & Park 2004) and consequently limit autoreactivity and self-damage (e.g. Walrand et al. 2005). In contrast, although carotenoids have been suggested to be involved in the invertebrate antioxidant system, evidence for this is still scarce (Felton & Summers 1995; Liñán-Cabello et al. 2002). By scavenging free radicals produced during the PO cascade, carotenoids may allow upregulation of immune defence, resulting in a positive covariation between carotenoid levels and immunity.
In this study, we examine the relationship between carotenoids and immunity in the amphipod crustacean Gammarus pulex, using correlative approaches in natural populations. We investigated variation in maintenance and use of the proPO system as well as variation in carotenoid concentration in haemolymph among and within populations through two successive studies. If carotenoids are involved in the control of self-harm induced by the immune response, we might expect that high levels of carotenoids in the haemolymph would allow higher investment in the immune function. We therefore predict a positive relationship between carotenoid levels and levels of immune activity at both the population and individual levels.
2. Material and methods
(a) Sampling and experimental design
Variation in immunity and carotenoid levels were first investigated among populations sampled from 12 localities near Dijon, eastern France (appendix 1 of the electronic supplementary material) in February 2006. We then complemented this study by investigating variation within and among 10 of these populations in August 2006. Gammarids were kept under standard laboratory conditions (Rigaud & Moret 2003).
On the day after field collection, haemolymph was collected into a sterile pre-chilled glass capillary after wounding gammarids between the seventh and eighth dorsal segments with fine scissors and flushed into a 0.5 ml microcentrifuge tube containing phosphate buffer saline (PBS: 8.74 g NaCl; 1.78 g Na2HPO4, 2H2O; 1000 ml of distilled water; pH 6.5). Body size was then measured (Rigaud & Moret 2003). For the first study, 20 individuals (10 males and 10 females) from each population were assigned to PO activity assays and 20 others to a carotenoid assay. For these samples, 2 μl of haemolymph were diluted in 18 μl of PBS. For the second study, haemolymph samples from approximately 25 males and 25 females were used for both PO and carotenoid assays. Each individual provided 3 μl of haemolymph that was diluted in 20 μl of PBS, vortexed and split into 10 μl for carotenoid and 13 μl for immunological assays. Samples were frozen in liquid nitrogen and stored at −80°C until later examination.
(b) Phenoloxidase activities
For each haemolymph extract, the activity of naturally activated PO only (PO activity) and the activity of the proenzymes (proPO) in addition to that of the PO (total activity) were measured using a spectrophotometric assay, following methods described in Rigaud & Moret (2003). The assay was performed using 5 μl of haemolymph extract added to a microplate well containing 20 μl of PBS and either 140 μl of distilled water to measure PO activity only or 140 μl of chemotrypsin solution (Sigma C-7762, 0.07 mg ml−1 of distilled water) to measure total activity.
(c) Carotenoid assay
Carotenoids were extracted from 10 or 20 μl haemolymph samples in PBS by the addition of the same volume of ethanol, homogenization and the addition of 200 μl of methyl-tert-butyl ether (MTBE). Samples were homogenized, centrifuged (4°C, 5 min, 5000 r.p.m.) and the organic phase containing carotenoids collected before a second wash with MTBE was performed. Pooled extracts were evaporated under nitrogen gas flow. For the colorimetric assay, extracts were dissolved in 120 μl of ethanol, 100 μl of which were used to measure absorbance at 470 nm in a microplate reader. The major carotenoids identified in G. pulex are astaxanthin and lutein (Gaillard et al. 2004). The concentration of carotenoids was therefore determined against a reference curve ranging from 0 to 50 ng μl−1 of a standard solution of astaxanthin and lutein (4 : 1) in ethanol (standards obtained from Extrasynthèse, Genay, France). Solvents used for extraction and colorimetry contained 0.01% of 2,6-di-tert-butyl-p-cresol (Fluka Chemika, Switzerland) as an antioxidant.
(d) Statistics
Data on PO and total activities were transformed using a Box–Cox procedure, carotenoid concentrations were natural log and square root transformed in the first and second data set, respectively.
At the interpopulation level, covariation among populations between immune parameters and haemolymph carotenoid concentration was investigated with Pearson's correlation tests on population means. Variations in immune parameters and carotenoid concentration in haemolymph were investigated using mixed linear models, with population as a random effect to estimate the covariance among individuals belonging to the same population, and sex as a fixed effect. In the second study, fixed effects also included carotenoid concentration and its interaction with sex (random intercept model). To test whether the slope of the relationship between immune parameters and carotenoid concentration differed among populations, a random slope and intercept model was tested with carotenoid concentration as a random effect. The null model likelihood ratio Χ2 test assessed whether the model including the random effect provided a significantly better fit than the same model without random effect. Models were compared by Akaike's information criterion. Body size differed significantly according to population and sex (males being bigger than females) in both sampling occasions. We initially included body size nested within sex effect in all models. On no occasion was it significant nor did it improve the model's fit, and was therefore not retained in the final models presented here. Mixed linear models were run using the MIXED procedure of the SAS software v. 8.2 (SAS Institute, Inc., 1999–2001, Cary, NC, USA).
3. Results
(a) Sources of variation in immunity and carotenoid levels
In the first study, total activity of the proPO system did not differ significantly among populations and between males and females (table 1). In contrast, individuals were more similar in PO activity and in carotenoid concentration within than among populations (the population covariance parameter explained 20 and 18% of variance, respectively), with females displaying higher PO activity and carotenoid levels than males (table 1).
Table 1.
Mixed linear model investigating variance in total and PO activity, and of carotenoids in haemolymph of G. pulex collected for the first study as a function of population and sex.
| source | covariance parameter estimates | type 3 tests of fixed effects | ||||||
|---|---|---|---|---|---|---|---|---|
| parameter | estimate ±s.e. | wald's Z | p | effect | estimate ±s.e. | Fd.f. | p | |
| total activity | population | 0.24±0.24 | 1.01 | 0.16 | sexa | −0.39±0.32b | 1.461,225 | 0.23 |
| residual | 6.23±0.59 | 10.58 | <0.0001 | |||||
| likelihood ratio test: Χ21df=2.03, p=0.15 | ||||||||
| PO activity | population | 0.44±0.23 | 1.93 | 0.03 | sexc | −0.48±0.17b | 7.561,222 | 0.006 |
| residual | 1.81±0.17 | 10.54 | <0.0001 | |||||
| likelihood ratio test: Χ21df=27.64, p<0.0001 | ||||||||
| carotenoids | population | 0.06±0.03 | 1.95 | 0.03 | sexd | −0.30±0.06b | 20.991,262 | <0.0001 |
| residual | 0.28±0.02 | 11.43 | <0.0001 | |||||
| likelihood ratio test: Χ21df=30.12, p<0.0001 | ||||||||
Mean±s.e. taken on raw data (measured as maximum change in optical density per minute), females, 2.83±0.24 milliunit min−1; males, 2.43±0.21 milliunit min−1.
Estimate of males relative to females taken as the reference (estimate=0).
Females, 2.12±0.22 milliunit min−1; males, 1.39±0.18 milliunit min−1.
Females, 531±30 ng μl−1; males, 377±19 ng μl−1.
In the second study, individuals significantly differed among populations for all three parameters, although population explained a relatively lower proportion of variance for PO activity (7%) as compared with total activity (21%) and carotenoid concentration (19%; table 2). Females maintained lower total activity but higher levels of PO activity than males, whereas carotenoid concentration was similar between males and females (table 2).
Table 2.
Mixed linear model investigating variance in total and PO activity as a function of population, sex and carotenoid concentration, and variance in carotenoid concentration as a function of population and sex, in samples of G. pulex collected for the second study.
| source | covariance parameter estimates | type 3 tests of fixed effects | ||||||
|---|---|---|---|---|---|---|---|---|
| parameter | estimate ±s.e. | wald's Z | p | effect | estimate ±s.e. | Fd.f. | p | |
| total activity | population | 1.16±0.60 | 1.93 | 0.03 | sexa | 0.52±0.21b | 6.381,413 | 0.01 |
| residual | 4.27±0.30 | 14.32 | <0.0001 | carotenoids | 0.17±0.03 | 36.481,419 | <0.0001 | |
| likelihood ratio test: Χ21df=65.09, p<0.0001 | ||||||||
| PO activity | population | 0.007±0.004 | 1.64 | 0.05 | sexc | −0.08±0.03b | 7.551,414 | 0.006 |
| residual | 0.084±0.006 | 14.29 | <0.0001 | carotenoids | 0.02±0.004 | 40.551,401 | <0.0001 | |
| likelihood ratio test: Χ21df=16.56, p<0.0001 | ||||||||
| carotenoids | population | 3.21±1.66 | 1.93 | 0.03 | sexd | 0.13±0.36b | 0.121,420 | 0.73 |
| residual | 13.14±0.91 | 14.44 | <0.0001 | |||||
| likelihood ratio test: Χ21df=63.52, p<0.0001 | ||||||||
Mean±s.e. taken on raw data (measured as maximum change in optical density per minute); females, 3.37±0.22 milliunit min−1; males, 4.03±0.22 milliunit min−1.
Estimate of males relative to females taken as the reference (estimate=0).
Females, 0.72±0.08 milliunit min−1; males, 0.50±0.08 milliunit min−1.
Females, 213±9 ng μl−1; males, 205±7 ng μl−1.
(b) Relationship between carotenoids and immunity among populations
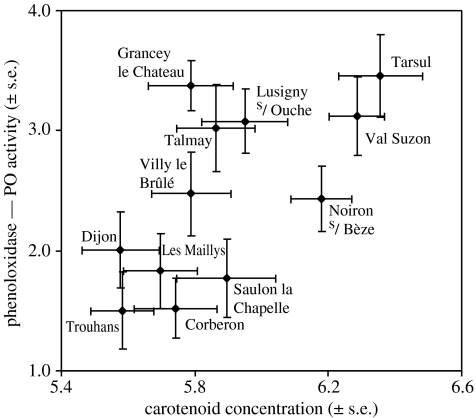
In the first study, no relationship was found between mean carotenoid concentration and mean total activity among populations (r=0.38, p=0.221, n=12), which was expected since all the populations exhibited similar total activity. Mean carotenoid concentration was, however, positively correlated to mean PO activity among populations (figure 1, r=0.65, p=0.023, n=12). In the second study, mean carotenoid concentration did not correlate to mean total activity (r=0.52, p=0.12, n=10) or to mean PO activity (r=0.41, p=0.24, n=10), but this last result may be due to the low level of differentiation among populations detected for PO activity.
Figure 1.
Relationship among G. pulex populations between haemolymph carotenoid concentration (natural log transformation) and PO activity (transformed by a Box–Cox procedure).
(c) Relationship between carotenoids and immunity within populations
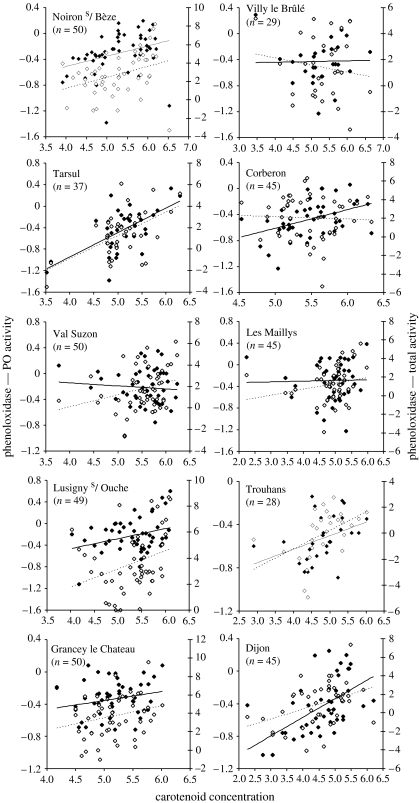
Total and PO activity were both strongly positively related to carotenoid concentration in haemolymph (table 2, figure 2). The slope of the relationship between total or PO activity and carotenoid concentration did not vary among populations.
Figure 2.
Relationship between haemolymph carotenoid concentration (square root transformation) and phenoloxidase activities (transformed by a Box–Cox procedure) for each population of G. pulex. Values of carotenoid concentration are given on the abscissa, PO activity (dark diamonds, full line) on the left ordinate and total activity (open diamonds, dashed line) on the right ordinate.
4. Discussion
The two independent sampling sessions revealed high variability in the relative importance of the sources of variance in immune parameters within and among populations, as the differences that could be detected among sexes and populations were not always constant across the two studies. Differences in carotenoid concentration between males and females were not detectable in the second study, although the relative proportion of the variance attributed to differences among populations appeared constant across the two studies.
Nevertheless, in both studies, we found positive relationships between carotenoid concentration in haemolymph and immune parameters. In the first study, we found that populations in which the immune system was the most activated also had higher concentrations of carotenoids circulating in the haemolymph. The second study provided evidence that, at the individual level within populations, carotenoid concentration covaried positively with both total and PO activity, a pattern that was consistently found across 10 populations.
Hence, a physiological relationship between investment in the proPO system and concentration of carotenoids in the haemolymph may exist. Our data do not provide information about the basis of such a relationship. Whether carotenoids play a role as an immunostimulant and/or antioxidant by scavenging free radicals produced during the PO cascade, thus allowing upregulation of the proPO system, remains unknown. Nevertheless, considering the capacity of crustaceans to store large amount of carotenoids in their tissues and the potential positive effects of these pigments on immunity, the evolution of immune defence in crustaceans may occur in a particular context. For instance, it has recently been shown that immune activation (especially the activity of the proPO system) in an insect induced self-harm through autoreactivity, which may contribute to the costs of immunity that constrains its evolution (Sadd & Siva-Jothy 2006). In crustaceans, carotenoids may help to reduce such a cost and therefore lift part of this constraint on the evolution of the immune function.
However, whether there is a direct and causal relationship between carotenoid levels and the maintenance and use of the immune system or whether there are environmental or physiological factors producing covariation between carotenoid levels and immune function need to be determined and now requires further experimental studies.
Acknowledgments
We thank M. J. F. Brown for critical reading and comments. Funding was provided by the CNRS to Y.M.
Supplementary Material
Localization of sampling sites
References
- Blount J.D. Carotenoids and life-history evolution in animals. Arch. Biochem. Biophys. 2004;430:10–15. doi: 10.1016/j.abb.2004.03.039. doi:10.1016/j.abb.2004.03.039 [DOI] [PubMed] [Google Scholar]
- Chew B.P, Park J.S. Carotenoid action on the immune response. J. Nutr. 2004;134:257S–261S. doi: 10.1093/jn/134.1.257S. [DOI] [PubMed] [Google Scholar]
- Felton G.W, Summers C.B. Antioxidant systems in insects. Arch. Insect Biochem. Physiol. 1995;29:187–197. doi: 10.1002/arch.940290208. doi:10.1002/arch.940290208 [DOI] [PubMed] [Google Scholar]
- Gaillard M, Juillet C, Cézilly F, Perrot-Minnot M.-J. Carotenoids of two freshwater amphipod species (Gammarus pulex and G. roeseli) and their common acanthocephalan parasite Polymorphus minutus. Comp. Biochem. Physiol. B. 2004;139:129–136. doi: 10.1016/j.cbpc.2004.07.001. doi:10.1016/j.cbpc.2004.07.001 [DOI] [PubMed] [Google Scholar]
- Liñán-Cabello M.A, Paniagua-Michel J, Hopkins P.M. Bioactive roles of carotenoids and retinoids in crustaceans. Aquacult. Nutr. 2002;8:299–309. doi:10.1046/j.1365-2095.2002.00221.x [Google Scholar]
- Møller A.P, Biard C, Blount J.D, Houston D.C, Ninni P, Saino N, Surai P.F. Carotenoid-dependent signals: indicators of foraging efficiency, immunocompetence or detoxification ability? Avian Poultry Biol. Rev. 2000;11:137–159. [Google Scholar]
- Moret Y, Schmid-Hempel P. Survival for immunity: the price of immune system activation for bumblebee workers. Science. 2000;290:1166–1168. doi: 10.1126/science.290.5494.1166. doi:10.1126/science.290.5494.1166 [DOI] [PubMed] [Google Scholar]
- Nappi A.J, Ottaviani E. Cytotoxicity and cytotoxic molecules in invertebrates. BioEssays. 2000;22:469–480. doi: 10.1002/(SICI)1521-1878(200005)22:5<469::AID-BIES9>3.0.CO;2-4. doi:10.1002/(SICI)1521-1878(200005)22:5<469::AID-BIES9>3.0.CO;2-4 [DOI] [PubMed] [Google Scholar]
- Rigaud T, Moret Y. Differential phenoloxidase activity between native and invasive gammarids infected by local acanthocephalans: differential immunosuppression? Parasitology. 2003;127:571–577. doi: 10.1017/s0031182003004050. doi:10.1017/S0031182003004050 [DOI] [PubMed] [Google Scholar]
- Sadd B.M, Siva-Jothy M.T. Self-harm caused by an insect's innate immunity. Proc. R. Soc. B. 2006;273:2571–2574. doi: 10.1098/rspb.2006.3574. doi:10.1098/rspb.2006.3574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderhäll K, Cerenius L. Role of the prophenoloxidase-activating system in invertebrate immunity. Curr. Opin. Immunol. 1998;10:23–28. doi: 10.1016/s0952-7915(98)80026-5. doi:10.1016/S0952-7915(98)80026-5 [DOI] [PubMed] [Google Scholar]
- Sugumaran M, Nellaiappan K, Valivittan K. A new mechanism for the control of phenoloxidase activity: inhibition and complex formation with quinone isomerase. Arch. Biochem. Biophys. 2000;379:252–260. doi: 10.1006/abbi.2000.1884. doi:10.1006/abbi.2000.1884 [DOI] [PubMed] [Google Scholar]
- Walrand S, et al. In vivo and in vitro evidences that carotenoids could modulate the neutrophil respiratory burst during dietary manipulation. Eur. J. Nutr. 2005;44:114–120. doi: 10.1007/s00394-004-0501-3. doi:10.1007/s00394-004-0501-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Localization of sampling sites