Abstract
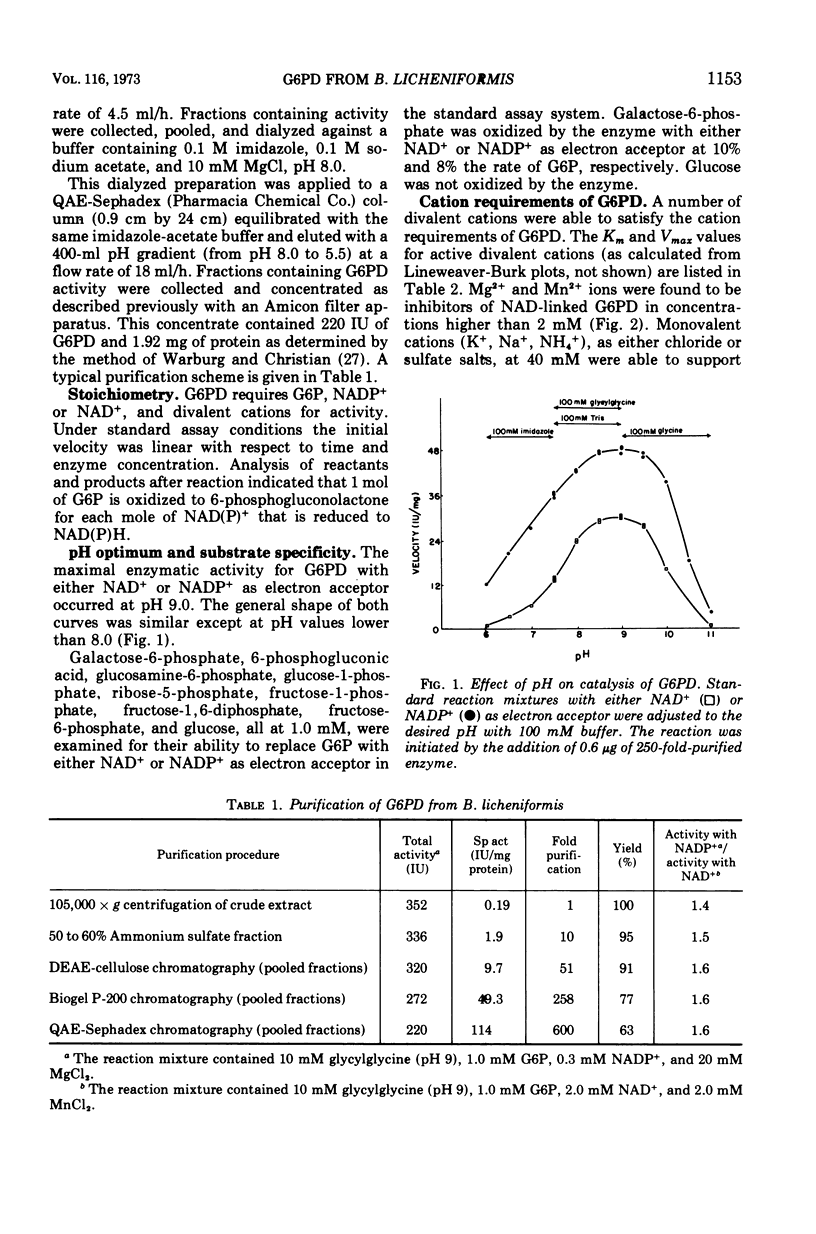
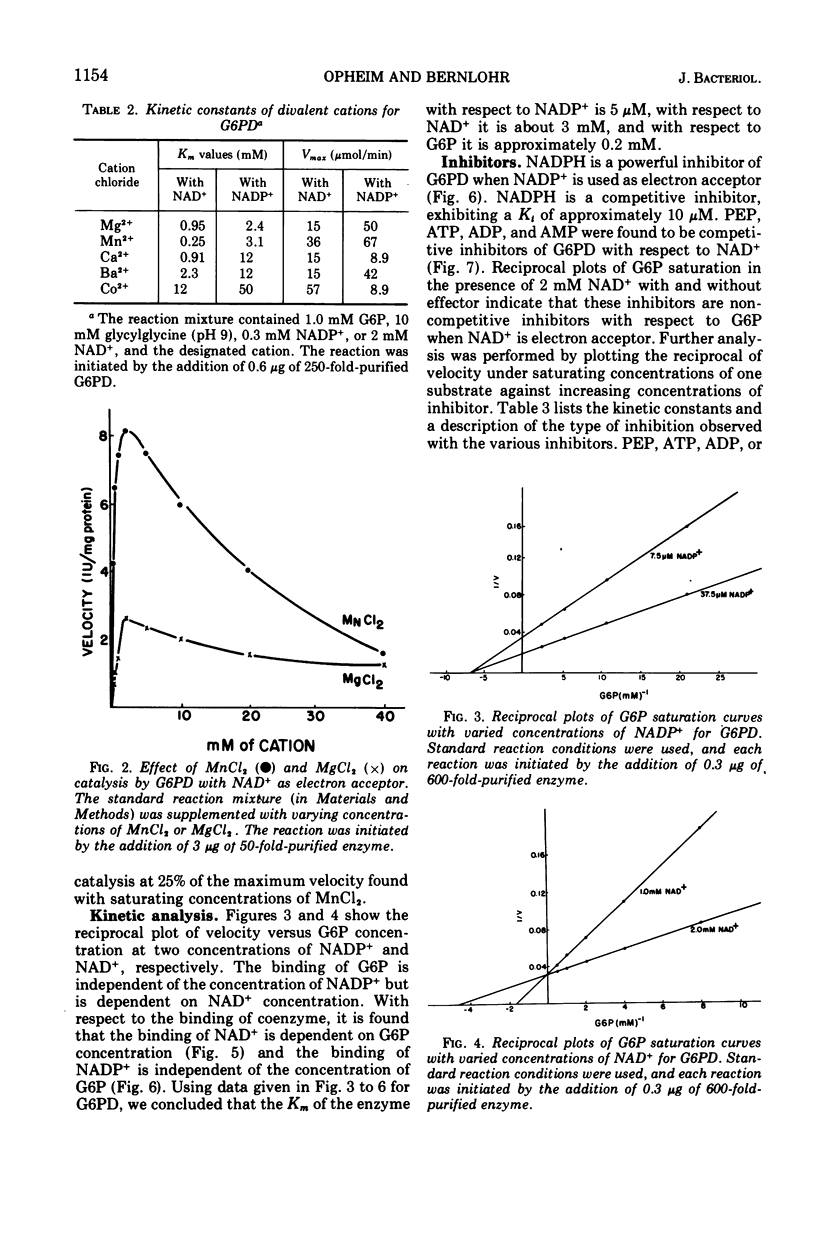
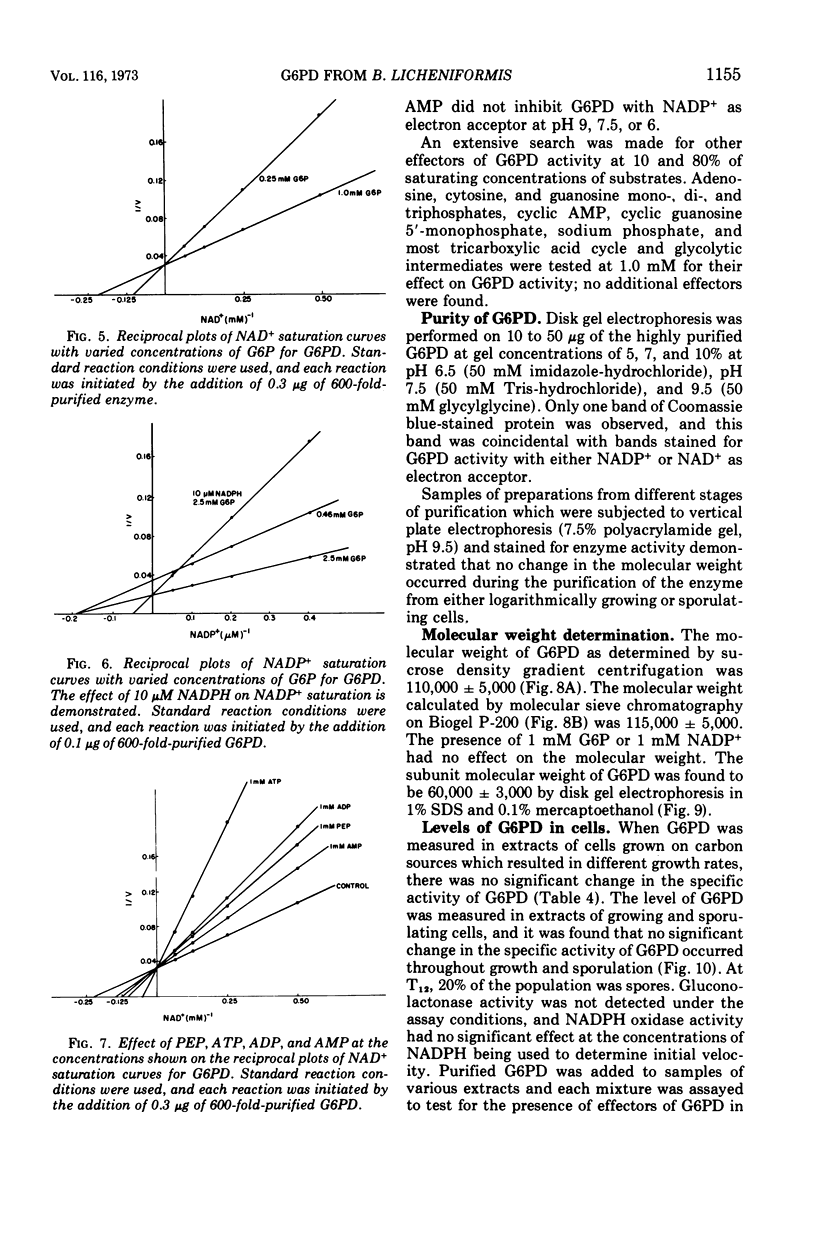
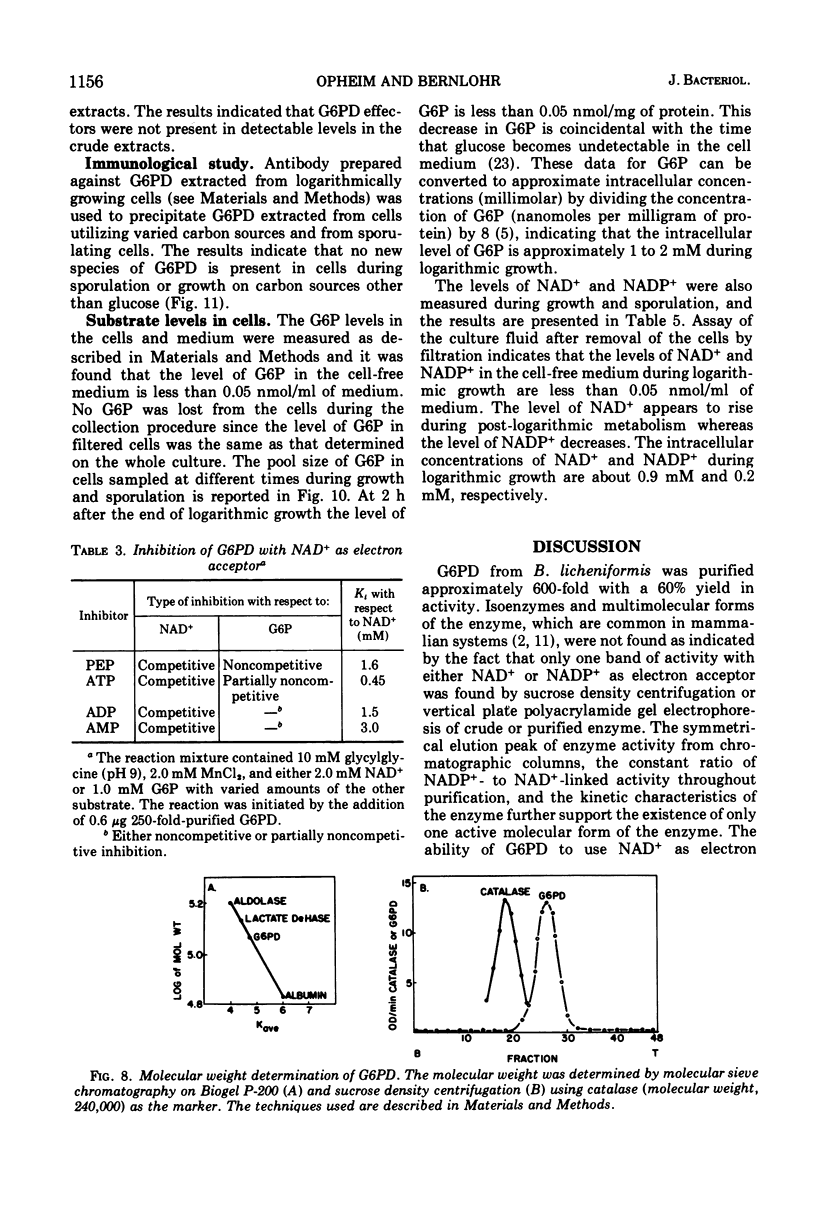
d-Glucose-6-phosphate nicotinamide adenine dinucleotide phosphate (NADP) oxidoreductase (EC 1.1.1.49) from Bacillus licheniformis has been purified approximately 600-fold. The enzyme appears to be constitutive and exhibits activity with either oxidized NAD (NAD+) or oxidized NADP (NADP+) as electron acceptor. The enzyme has a pH optimum of 9.0 and has an absolute requirement for cations, either monovalent or divalent. The enzyme exhibits a Km of approximately 5 μM for NADP+, 3 mM for NAD+, and 0.2 mM for glucose-6-phosphate. Reduced NADP (NADPH) is a competitive inhibitor with respect to NADP+ (Km = 10 μM). Phosphoenolpyruvate (Km = 1.6 mM), adenosine 5′-triphosphate (Km = 0.5 mM), adenosine diphosphate (Km = 1.5 mM), and adenosine 5′-monophosphate (Km = 3.0 mM) are competitive inhibitors with respect to NAD+. The molecular weight as estimated from sucrose density centrifugation and molecular sieve chromatography is 1.1 × 105. Sodium dodecyl sulfate gel electrophoresis indicates that the enzyme is composed of two similar subunits of approximately 6 × 104 molecular weight. The intracellular levels of glucose-6-phosphate, NAD+, and NADP+ were measured and found to be approximately 1 mM, 0.9 mM, and 0.2 mM, respectively, during logarithmic growth. From a consideration of the substrate pool sizes and types of inhibitors, we conclude that this single constitutive enzyme may function in two roles in the cell—NADH production for energetics and NADPH production for reductive biosynthesis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNLOHR R. W. POSTLOGARITHMIC PHASE METABOLISM OF SPORULATING MICROORGANISMS. I. PROTEASE OF BACILLUS LICHENIFORMIS. J Biol Chem. 1964 Feb;239:538–543. [PubMed] [Google Scholar]
- Beitner R., Naor Z. Isoenzymes of NADP + -and NAD + -glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase in rat adipose tissue. Biochim Biophys Acta. 1972 Aug 28;276(2):572–575. doi: 10.1016/0005-2744(72)91023-6. [DOI] [PubMed] [Google Scholar]
- Clark V. L., Peterson D. E., Bernlohr R. W. Changes in free amino acid production and intracellular amino acid pools of Bacillus licheniformis as a function of culture age and growth media. J Bacteriol. 1972 Nov;112(2):715–725. doi: 10.1128/jb.112.2.715-725.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOI R., HALVORSON H., CHURCH B. Intermediate metabolism of aerobic spores. III. The mechanism of glucose and hexose phosphate oxidation in extracts of Bacillus cereus spores. J Bacteriol. 1959 Jan;77(1):43–54. doi: 10.1128/jb.77.1.43-54.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraenkel D. G. Selection of Escherichia coli mutants lacking glucose-6-phosphate dehydrogenase or gluconate-6-phosphate dehydrogenase. J Bacteriol. 1968 Apr;95(4):1267–1271. doi: 10.1128/jb.95.4.1267-1271.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLASER L., BROWN D. H. Purification and properties of d-glucose-6-phosphate dehydrogenase. J Biol Chem. 1955 Sep;216(1):67–79. [PubMed] [Google Scholar]
- Gancedo J. M., Gancedo C. Fructose-1,6-diphosphatase, phosphofructokinase and glucose-6-phosphate dehydrogenase from fermenting and non fermenting yeasts. Arch Mikrobiol. 1971;76(2):132–138. doi: 10.1007/BF00411787. [DOI] [PubMed] [Google Scholar]
- Holmes P. K., Levinson H. S. Thermal stimulation of Bacillus megaterium glucose-6-phosphate dehydrogenase. Biochem Biophys Res Commun. 1970 Jan 6;38(1):143–148. doi: 10.1016/0006-291x(70)91096-x. [DOI] [PubMed] [Google Scholar]
- Holten D. Relationships among the multiple molecular forms of rat liver glucose 6-phosphate dehydrogenase. Biochim Biophys Acta. 1972 Apr 7;268(1):4–12. doi: 10.1016/0005-2744(72)90190-8. [DOI] [PubMed] [Google Scholar]
- Hylemon P. B., Phibbs P. V., Jr Independent regulation of hexose catabolizing enzymes and glucose transport activity in Pseudomonas aeruginosa. Biochem Biophys Res Commun. 1972 Sep 5;48(5):1041–1048. doi: 10.1016/0006-291x(72)90813-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lessie T., Neidhardt F. C. Adenosine triphosphate-linked control of Pseudomonas aeruginosa glucose-6-phosphate dehydrogenase. J Bacteriol. 1967 Apr;93(4):1337–1345. doi: 10.1128/jb.93.4.1337-1345.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London J., Knight M. Concentrations of nicotinamide nucleotide coenzymes in micro-organisms. J Gen Microbiol. 1966 Aug;44(2):241–254. doi: 10.1099/00221287-44-2-241. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Marschke C. K., Bernlohr R. W. Purification and characterization of phosphofructokinase of Bacillus licheniformis. Arch Biochem Biophys. 1973 May;156(1):1–16. doi: 10.1016/0003-9861(73)90335-4. [DOI] [PubMed] [Google Scholar]
- PEPPER R. E., COSTILOW R. N. GLUCOSE CATABOLISM BY BACILLUS POPILLIAE AND BACILLUS LENTIMORBUS. J Bacteriol. 1964 Feb;87:303–310. doi: 10.1128/jb.87.2.303-310.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudack D., Chisholm E. M., Holten D. Rat liver glucose 6-phosphate dehydrogenase. Regulation by carbohydrate diet and insulin. J Biol Chem. 1971 Mar 10;246(5):1249–1254. [PubMed] [Google Scholar]
- Sanwal B. D. Regulatory mechanisms involving nicotinamide adenine nucleotides as allosteric effectors. 3. Control of glucose 6-phosphate dehydrogenase. J Biol Chem. 1970 Apr 10;245(7):1626–1631. [PubMed] [Google Scholar]
- Setlow P., Kornberg A. Biochemical studies of bacterial sporulation and germination. XXII. Energy metabolism in early stages of germination of Bacillus megaterium spores. J Biol Chem. 1970 Jul 25;245(14):3637–3644. [PubMed] [Google Scholar]
- Tunail N., Schlegel H. G. Phosphoenolpyruvate, a new inhibitor of glucose-6-phosphate dehydrogenase. Biochem Biophys Res Commun. 1972 Dec 18;49(6):1554–1560. doi: 10.1016/0006-291x(72)90518-9. [DOI] [PubMed] [Google Scholar]
- Tuominen F. W., Bernlohr R. W. Energetics and motility in Bacillus licheniformis. J Bacteriol. 1967 May;93(5):1725–1726. doi: 10.1128/jb.93.5.1725-1726.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuominen F. W., Bernlohr R. W. Pyruvate kinase of the spore-forming bacterium, Bacillus licheniformis. I. Purification, stability, regulation of synthesis, and evidence for multiple molecular states. J Biol Chem. 1971 Mar 25;246(6):1733–1745. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wimpenny J. W., Firth A. Levels of nicotinamide adenine dinucleotide and reduced nicotinamide adenine dinucleotide in facultative bacteria and the effect of oxygen. J Bacteriol. 1972 Jul;111(1):24–32. doi: 10.1128/jb.111.1.24-32.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue R. H., Noltmann E. A., Kuby S. A. Glucose 6-phosphate dehydrogenase from brewers' yeast (Zwischenferment). 3. Studies on the subunit structure and on the molecular association phenomenon induced by triphosphopyridine nucleotide. J Biol Chem. 1969 Mar 10;244(5):1353–1364. [PubMed] [Google Scholar]



