Abstract
The immunocompetence handicap hypothesis (ICHH) assumes that testosterone (T), required for the expression of sexual traits, can also incur a cost due to its immunosuppressive properties. However, T-dependent immunosuppression could also arise as an indirect consequence of energy reallocation from the immune system to other metabolic demands. Leptin is mostly produced in lipogenic tissues and its circulating level is positively correlated with the amount of lipid reserves. Leptin also has an important role as immunoenhancer and we suggest that this hormone could play a role as a mediator of the immunosuppressive effect of testosterone. In particular, we propose that only the individuals able to maintain large lipid reserves (with high leptin levels), while sustaining high testosterone levels, might be able to develop sexual displays without an impairment of their immune defences. Here, we tested one of the assumptions underlying this extension of the ICHH: leptin administration should attenuate testosterone-induced immunosuppression. T-implanted and control male zebra finches (Taeniopygia guttata) received daily injections of leptin or phosphate buffered saline. T-implants initially depressed the phytohaemagglutinin-induced immune response. However, T-birds injected with leptin enhanced their immune response to the level of control birds. These results open a new perspective on the study of the ICHH.
Keywords: immunocompetence handicap hypothesis, leptin, testosterone
1. Introduction
Folstad & Karter (1992) put forward the immunocompetence handicap hypothesis (ICHH) to explain the honesty of testosterone (T)-dependent sexual signals. This hypothesis assumes that high-circulating T levels, necessary for the full expression of sexual signals, can also incur a cost due to the immunosuppressive properties of T. Thus, only individuals with a performing immune system might afford the cost of sexual signalling.
Recent work has suggested that T-induced immunosuppression might arise as an indirect consequence of energy redistribution from the immune system to other metabolic demands (e.g. Owen-Ashley et al. 2004). The high levels of circulating testosterone are often associated with increased metabolic rate and locomotor activity (e.g. Casto et al. 2001), which could lead to a decrease in energy reserves, constraining the capacity to mount an immune response (e.g. Duckworth et al. 2001).
Several hormones involved in the regulation of metabolism, reproduction and immunity have been recently discovered (Meier & Gressner 2004). Leptin is one of them. This hormone is mostly produced in lipogenic tissues (Zhang et al. 1994) and its plasmatic levels are positively correlated with the amount of lipid reserves (Brann et al. 2002). Recently, it has been suggested that leptin could have evolved as a permissive factor informing the organism about the state of energetic reserves when facing an immune challenge (Demas 2004). Accordingly, it has been shown that leptin stimulates the cell-mediated immune response in mammals (e.g. Lord et al. 1998) and also in a bird species (Lohmus et al. 2004).
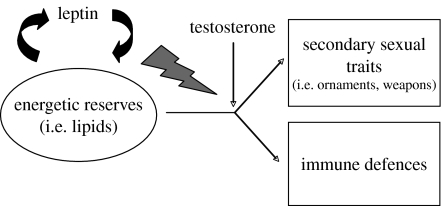
Based on the physiological properties of leptin, we propose here a refinement of the ICHH (figure 1). The expression of sexual displays and immune functioning depend on the amount of energetic reserves available for the organism. Therefore, individuals have to adopt the energy allocation rule that maximizes their fitness. This obviously depends on the amount of energy available and we might expect that endogenous physiological pathways provide reliable information to the organism on the individual-based optimal allocation rule. We suggest that testosterone and leptin might play such a role (figure 1). Testosterone drives resources to sexual signalling at the expenses of immune functioning. Leptin informs the organism on how much reserves it can allocate to the two functions. Depending on its energetic reserves, an individual might afford to develop exaggerated sexual displays without an excessive impairment of its immune defences. Females might gather reliable information on the phenotypic/genetic quality of a mate, because males with low energetic reserves (low levels of circulating leptin) would pay the cost of immunosuppression. This ICHH refinement provides some testable predictions, in addition to those envisaged by Folstad & Karter (1992): (i) levels of circulating leptin should correlate with immune functioning and the expression of sexual signals and (ii) exogenous administration of leptin should annul the immunosuppressive effect of T. In this article, we focus on this latter prediction.
Figure 1.
A refinement of the ICHH (see §1 for a full description).
2. Material and methods
Twenty-four male zebra finches (Taeniopygia guttata) were used. These birds had been used in a previous experiment (Alonso-Alvarez et al. 2007) where each individual received a subcutaneous implant filled with testosterone or left empty (n=12, each group; electronic supplementary material).
The leptin experiment started five weeks after birds were T-implanted. During five consecutive days, birds received daily intraperitoneal injections of recombinant murine leptin (L). The dose (electronic supplementary material) was adjusted following the previous work in birds (Lohmus et al. 2004), although no information on natural leptin levels is available for zebra finches. Control (C) birds were injected daily with 100 μl of phosphate buffered saline (PBS). The experimental groups were: T-implanted birds receiving leptin; T-implanted birds receiving PBS; empty-implant birds receiving leptin; and empty-implant birds receiving PBS (n=6 for each group).
Body mass and cell-mediated immune response were assessed the day prior to the start of leptin injections (day 35) and at the end of the experiment (day 40). The immune response was assessed after the injection of a mitogen (PHA) into the wing-web (electronic supplementary material).
T-birds showed a weaker immune response compared with C birds just prior to the start of leptin injections (means±s.e.: 0.89±0.06 mm and 1.22±0.05 mm, T- and C-males, respectively; F1,22=13.67, p=0.001). There were no initial differences in any other variable (electronic supplementary material).
The effect of T and leptin on immune response and body mass was analysed with repeated-measurement ANOVAs, including both treatments as fixed factors. We reported both the between- and the within-subject effects because the immune response of T-implanted males was already lower than that of C males at the start of leptin injections.
3. Results
Body mass decreased during the 5 days of the experiment, birds with empty implants lost more mass than T-birds (mean±s.e.: −0.74±0.84 g and −0.17±0.40 g, respectively). Leptin had no effect on body mass (table 1). The change in body mass (%) during the 5 days of leptin injections was not correlated with the immune response at day 35 (r=−0.116, p=0.588).
Table 1.
Effect of testosterone and leptin on body mass change in male zebra finches. (The table reports the between- and within-subject effects of a repeated-measurement ANOVA with the two treatments as fixed factors.)
| F | d.f. | p | |
|---|---|---|---|
| between-subject effects | |||
| leptin | 0.001 | 1,20 | 0.970 |
| testosterone | 1.33 | 1,20 | 0.262 |
| leptin×testosterone | 0.03 | 1,20 | 0.854 |
| within-subject effects | |||
| time | 10.82 | 1,20 | 0.004 |
| time×testosterone | 4.34 | 1,20 | 0.050 |
| time×leptin | 0.04 | 1,20 | 0.835 |
| time×testosterone×leptin | 0.66 | 1,20 | 0.425 |
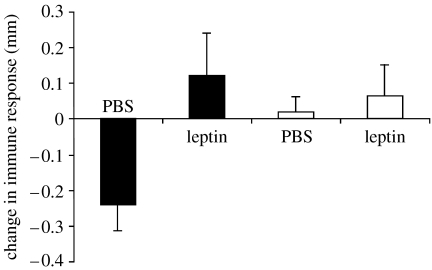
The immune response was affected by both T and leptin treatments (table 2). The between-subject effects showed a significant effect of testosterone, T-birds having a weaker immune response than C-birds (final value: mean±s.e., 0.83±0.09 mm and 1.26±0.08 mm for T- and C-males, respectively). The within-subject effects showed a significant interaction between testosterone and leptin treatments (table 2). T-birds treated with leptin increased their immune response compared with those injected with PBS (Tukey's test: p=0.034; figure 2). As a consequence, the immune response of T-males treated with leptin was statistically indistinguishable from the immune response of birds with empty implants (Tukey's tests: p>0.80). Removal change in body mass from the model only slightly affected the results with the p value of the testosterone by leptin treatment interaction moving from 0.05 to 0.07. T-birds injected with PBS showed a decrease in their immune response (F1,5=10.61, p=0.023; figure 2).
Table 2.
Effect of testosterone and leptin on the immune response in male zebra finches. (The table reports the between- and within-subject effects of a repeated-measurement ANOVA with both treatments as fixed factors and change in body mass as a covariate.)
| F | d.f. | p | |
|---|---|---|---|
| between-subject effects | |||
| body mass change | 0.11 | 1,19 | 0.748 |
| leptin | 0.83 | 1,19 | 0.374 |
| testosterone | 13.67 | 1,19 | 0.002 |
| leptin×testosterone | 0.30 | 1,19 | 0.591 |
| within-subject effects | |||
| time | 1.30 | 1,19 | 0.269 |
| time×body mass change | 1.28 | 1,19 | 0.272 |
| time×testosterone | 2.42 | 1,19 | 0.136 |
| time×leptin | 5.42 | 1,19 | 0.031 |
| time×testosterone×leptin | 4.36 | 1,19 | 0.050 |
Figure 2.
Change in immune response (final minus initial values) during the leptin treatment. Black bars represent testosterone-implanted males, whereas empty bars represent males with empty implants. Means+s.e.
4. Discussion
We propose a refinement of the ICHH based on leptin properties. Leptin might provide a reliable endogenous signal on the amount of energetic reserves available for the expression of competing functions, such as sexual signalling and immune functioning. Individuals with large reserves (high amount of leptin) might tolerate the cost of testosterone-based sexual signalling. This extension of the ICHH provides some testable predictions. Here, we tested one of them: exogenous administration of leptin should attenuate the immunosuppressive effect of T. In spite of a small sample size and low statistical power, the results of our experiment provide some support to the hypothesis. Male zebra finches implanted with T and receiving daily leptin injections resumed values of immune response comparable with those of birds with empty implants, whereas individuals with T implants and PBS injections exhibited the weakest immune response. Actually, the immune response of T-birds receiving PBS injections further decreased between days 35 and 40, reflecting that the immunosuppressive action of testosterone continued through time. These results call for a more comprehensive view of the regulatory mechanisms controlling the allocation of resources to conflicting functions.
During the experiment, all birds tended to lose body mass perhaps as a consequence of handling stress. T-implanted birds were those with the smallest decrease in body mass suggesting that T might have affected food intake. Testosterone affects food intake in male mammals. Gonadectomized male rats and mice have a decreased food intake and this effect is reversed by testosterone treatment (Asarian & Geary 2006). On the contrary, exogenous administration of leptin can suppress food intake, although the generality of such finding has been discussed (Asarian & Geary 2006). The observed decrease in body mass might also reflect the cost of mounting an immune response. Although we cannot fully discard this possibility, the lack of correlation between the immune response and the change in body mass makes this explanation unlikely.
The role of leptin in evolutionary biology has been overlooked, even though its properties make it a good candidate for a regulatory role in the allocation of resources to different functions. Leptin plays a key role in energy homeostasis through regulation of food intake and energy expenditure (Brann et al. 2002). Furthermore, leptin operates as a pivotal modulator of the hypothalamic–pituitary–gonadal axis (Brann et al. 2002). In mammals, leptin allows the production of gonadal steroids when a certain threshold of fatness is attained, acting as a permissive factor for reproduction (Brann et al. 2002; see also the electronic supplementary material for recent findings in birds).
The properties of leptin suggest that this hormone might, in interaction with other hormones such as testosterone, regulate the allocation of resources between sexual signals and immune functioning. Leptin and testosterone blood levels are negatively correlated in mammals (Kiess et al. 1999; Wauters et al. 2000), and experimental increases of plasma testosterone induce a decrease in leptin concentration (e.g. Kiess et al. 1999; Castrogiovanni et al. 2003) with potentially damaging side-effects on the ability to mount an immune response (review in Faggioni et al. 2001). However, if individuals are able to maintain a certain level of leptin during the period of sustained testosterone production, the immunosuppressive effect of T could be attenuated or cancelled out. This should only be possible when individuals have enough lipid reserves (leptin is mostly produced in the adipose tissue; Zhang et al. 1994). Ultimately, this hypothesis implies that T-dependent sexual signals might convey not only information on the performance of the immune system of their bearers, but also on the ability of individuals to manage their energy budget.
Here, we focused on only one prediction, namely that exogenous administration of leptin attenuates the T-induced immunosuppression. Although suggestive, the present results need to be replicated to draw a firm conclusion on the role of leptin. The other predictions of our model should also be tested, in particular, the effect of leptin on the expression of T-dependent sexual traits.
The verbal model presented in this article contributes to open a new perspective on the study of ICHH. An integrative approach including not only steroids but also other hormones related to energy homeostasis (i.e. Meier & Gressner 2004) is now required.
Acknowledgments
Thanks to S. J. Schoech and M. Tena-Sempere for their suggestions about the role of leptin. C.A.-A. was supported by the Ramón y Cajal fellowship (Spain).
Supplementary Material
Electronic supplementary material
References
- Alonso-Alvarez C, Bertrand S, Faivre B, Chastel O, Sorci G. Testosterone and oxidative stress: the oxidation handicap hypothesis. Proc. R. Soc. B. 2007;274:819–825. doi: 10.1098/rspb.2006.3764. doi:10.1098/rspb.2006.3764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Phil. Trans. R. Soc. B. 2006;361:1251–1263. doi: 10.1098/rstb.2006.1860. doi:10.1098/rstb.2006.1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brann D.W, Wade M.F, Dhandapani K.M, Mahesh V.B, Buchanan C.D. Leptin and reproduction. Steroids. 2002;67:95–104. doi: 10.1016/s0039-128x(01)00138-6. doi:10.1016/S0039-128X(01)00138-6 [DOI] [PubMed] [Google Scholar]
- Casto J.M, Nolan V, Jr, Ketterson E.D. Steroid hormones and immune function: experimental studies in wild and captive dark-eyed juncos (Junco hyemalis) Am. Nat. 2001;157:408–420. doi: 10.1086/319318. doi:10.1086/319318 [DOI] [PubMed] [Google Scholar]
- Castrogiovanni D, Perello M, Gaillard R.C, Spinedi E. Modulatory role of testosterone in plasma leptin turnover in rats. Endocrine. 2003;22:203–210. doi: 10.1385/ENDO:22:3:203. doi:10.1385/ENDO:22:3:203 [DOI] [PubMed] [Google Scholar]
- Demas G.E. The energetics of immunity: a neuroendocrine link between energy balance and immune function. Horm. Behav. 2004;45:173–180. doi: 10.1016/j.yhbeh.2003.11.002. doi:10.1016/j.yhbeh.2003.11.002 [DOI] [PubMed] [Google Scholar]
- Duckworth R.A, Mendonca M.T, Hill G.E. A condition dependent link between testosterone and disease resistance in the house finch. Proc. R. Soc. B. 2001;268:2467–2472. doi: 10.1098/rspb.2001.1827. doi:10.1098/rspb.2001.1827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faggioni R, Feingold K.R, Grunfeld C. Leptin regulation of the immune response and the immunodeficiency of malnutrition. FASEB J. 2001;15:2565–2571. doi: 10.1096/fj.01-0431rev. doi:10.1096/fj.01-0431rev [DOI] [PubMed] [Google Scholar]
- Folstad I, Karter A.K. Parasites, bright males and the immunocompetence handicap. Am. Nat. 1992;139:603–622. doi:10.1086/285346 [Google Scholar]
- Kiess W, Reich A, Meyer K, Glasow A, Deutscher J, Klammt J, Yang Y, Muller G, Kratzsch J. A role for leptin in sexual maturation and puberty? Horm. Res. 1999;51(Suppl. 3):55–63. doi: 10.1159/000053163. doi:10.1159/000053163 [DOI] [PubMed] [Google Scholar]
- Lohmus M, Olin M, Sundström L.F, Troedsson M.H.T, Molitor T.W, El Halawani M. Leptin increases T-cell immune response in birds. Gen. Comp. Endocrinol. 2004;139:245–250. doi: 10.1016/j.ygcen.2004.09.011. doi:10.1016/j.ygcen.2004.09.011 [DOI] [PubMed] [Google Scholar]
- Lord G.M, Matarese G, Howard J.K, Baker R.J, Bloom S.R, Lechler R.I. Leptin modulates the T-cell immune response and reverses starvation induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. doi:10.1038/29795 [DOI] [PubMed] [Google Scholar]
- Meier U, Gressner A.M. Endocrine regulation of energy metabolism: review of pathobiochemical and clinical chemical aspects of leptin, ghrelin, adiponectin, and resistin. Clin. Chem. 2004;50:1511–1525. doi: 10.1373/clinchem.2004.032482. doi:10.1373/clinchem.2004.032482 [DOI] [PubMed] [Google Scholar]
- Owen-Ashley N.T, Hasselquist D, Wingfield J.C. Androgens and the immunocompetence handicap hypothesis: unraveling direct and indirect pathways of immunosuppression in song sparrows. Am. Nat. 2004;164:490–505. doi: 10.1086/423714. doi:10.1086/423714 [DOI] [PubMed] [Google Scholar]
- Wauters M, Considine R.V, Van Gaal L.F. Human leptin: from an adipocyte hormone to an endocrine mediator. Eur. J. Endocrinol. 2000;143:293–311. doi: 10.1530/eje.0.1430293. doi:10.1530/eje.0.1430293 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman J.M. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. doi:10.1038/372425a0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Electronic supplementary material