Abstract
Biogeographic connections between Australia and other continents are still poorly understood although the plate tectonics of the Indo-Pacific region is now well described. Eupetes macrocerus is an enigmatic taxon distributed in a small area on the Malay Peninsula and on Sumatra and Borneo. It has generally been associated with Ptilorrhoa in New Guinea on the other side of Wallace's Line, but a relationship with the West African Picathartes has also been suggested. Using three nuclear markers, we demonstrate that Eupetes is the sister taxon of the South African genus Chaetops, and their sister taxon in turn being Picathartes, with a divergence in the Eocene. Thus, this clade is distributed in remote corners of Africa and Asia, which makes the biogeographic history of these birds very intriguing. The most parsimonious explanation would be that they represent a relictual basal group in the Passerida clade established after a long-distance dispersal from the Australo-Papuan region to Africa. Many earlier taxonomic arrangements may have been based on assumptions about relationships with similar-looking forms in the same, or adjacent, biogeographic regions, and revisions with molecular data may uncover such cases of neglect of ancient relictual patterns reflecting past connections between the continents.
Keywords: Eupetes, Oscine, biogeography, systematics, phylogeny
1. Introduction
The peculiar Eupetes macrocerus (Malaysian rail-babbler) is an uncommon bird of the lowland rainforests of the Malay Peninsula, Sumatra and Borneo. It belongs to the forest understorey, foraging on the forest floor among leaf litter and under fallen timber, using a walking gait with the neck extended forward and head jerking like a chicken. By appearance, it mostly resembles the Ptilorrhoa spp. (jewel-babblers) of New Guinea and early taxonomic treatments indeed placed them together in the genus Eupetes (Sharpe 1883; Temminck 1831). However, Peters (1940) noted some striking differences between E. macrocerus and the Ptilorrhoa spp. He pointed out several differences in the external morphology, but did not present evidence of a kind that would help placing these taxa in a phylogenetic context. Peters (1940) was the first to suggest that the genus Eupetes was monotypic and he proposed the genus name Ptilorrhoa (genus novum) for the Papuan birds. However, in his ambitious ‘Birds of the World’ (Peters 1964), he stuck with the name Ptilorrhoa to include the New Guinean species as well as the Malayan rail-babbler.
Serle (1952) noted a series of remarkable similarities between Eupetes and the West African Picathartes (rockfowl): similar proportions, position of nostrils (different from Ptilorrhoa), shape of forehead and tail but did not insist on a relationship. Sibley (1973) considered the resemblance between Picathartes and Eupetes to be the result of convergence, but Olson (1979) argued that there could well be a relationship between them. He mentioned a series of similarities: morphology, proportions, plumage pattern and behaviour, but failed to identify clear synapomorphies between them. Sibley & Ahlquist (1990) simply ignored the divergence of opinions as they stated that ‘The Cinclosomatinae includes the quail-thrushes (Cinclosoma), the three species of Papuan jewel-babblers (Ptilorrhoa), the Malaysian rail-babbler (Eupetes) and the whipbirds and wedgebills (Psophodes)’. Using molecular sequence data, Barker et al. (2004) and Beresford et al. (2005) associated Ptilorrhoa with Pachycephalidae (whistlers), which are placed above the Australian ‘false babblers’ (represented by the genera Pomatostomus and Orthonyx) in the Corvida phylogeny, but molecular data for Eupetes were lacking even in these studies.
It seems highly probable that earlier taxonomists simply looked for similar-looking forms within the same or adjacent geographical areas, thus a priori ruling out relationships between species living far apart. In order to scrutinize alternative possibilities, we used DNA sequence data to assess the systematic affinities of the Malaysian rail-babbler. The mere fact that E. macrocerus is distributed in Southeast Asia and that Ptilorrhoa is distributed only in New Guinea to the other side of Wallace's Line and Picathartes in West Africa makes the systematic status very interesting in relation to the current hypotheses about how songbirds (Oscines) dispersed out of the Australian region and colonized other parts of the world (Ericson et al. 2002; Barker et al. 2002, 2004; Jønsson & Fjeldså 2006b) and, for that sake, to similar cases in other taxonomic groups.
2. Material and methods
We compared the DNA sequences of Eupetes with data from 21 taxa representing a broad sampling of the passeriform radiation (as summarized by Jønsson & Fjeldså 2006a). The aligned dataset consists of 1742 bp obtained from three nuclear gene regions, myoglobin intron 2, ornithine decarboxylase (ODC) introns 6–7 and glyceraldehyde-3-phosphodehydrogenase (G3PDH) intron 11. See the electronic supplementary material for laboratory procedures, alignments, selection of models for nucleotide substitutions, parsimony analysis and Bayesian analyses of individual gene regions (table 1).
Table 1.
List of samples used in the study. (Acronyms are PFI, Percy FitzPatrick Institute, Cape Town; ZMUC, Zoological Museum of Copenhagen; AM, Australian Museum, Sydney; MV, Museum Victoria, Melbourne; NHMT, Natural History Museum, Tring; NRM, Swedish museum of Natural History.)
| species | family | source | G3P | ODC | Myo |
|---|---|---|---|---|---|
| Chaetops frenatus | Picathartidae | PFI uncat. | EF441212 | EF441234 | AY228289 |
| Colluricincla sanghirensis | Pachycephalidae | ZMUC123921 | EF441213 | EF441235 | EF441256 |
| Corcorax melanorhamphos | Corcoracidae | AM LAB 1059 | EF441214 | EF441236 | AY064737 |
| Cormobates placens | Climacteridae | MV E309 | EF441215 | EF441237 | AY064731 |
| Eopsaltria australis | Petroicidae | MV 1390 | EF441216 | EF441238 | AY064732 |
| Eupetes macrocerus | Cinclosomatidae | NHMT 1936.4.12.58 | EF441217 | EF441239 | EF441257 |
| Hirundo rustica | Hirundinidae | NRM 976238 | EF441218 | EF441240 | AY064258 |
| Malurus amabilis | Maluridae | MV C803 | EF441219 | EF441241 | AY064729 |
| Menura novaehollandiae | Menuridae | MV F722 | EF441220 | EF441242 | AY064744 |
| Oriolus flavocinctus | Oriolidae | MV1603 | EF441221 | EF441243 | EF441258 |
| Orthonyx temminckii | Orthonychidae | MV B831 | EF441222 | EF441244 | AY064728 |
| Pachycephala albiventris | Pachycephalidae | ZMUC117176 | EF441223 | EF441245 | EF441259 |
| Pachycephalopsis hattamensis | Petroicidae | NRM552153 | EF441224 | EF441246 | EF441260 |
| Picathartes gymnocephalus | Picathartidae | LSU B-19213 | EF441225 | EF441247 | AY228314 |
| Pomatostomus temporalis | Pomatostomidae | MV D257 | EF441226 | EF441248 | AY064730 |
| Prunella modularis | Prunellidae | NRM976138 | EF441227 | EF441249 | AY228318 |
| Ptilonorhynchus violaceus | Ptilonorhynchidae | MV B836 | EF441228 | EF441250 | AY064742 |
| Ptiloprora plumbea | Meliphagidae | MV C173 | EF441229 | EF441251 | AY064736 |
| Ptilorrhoa leucosticte | Cinclosomatidae | NRM 84405 | EF441233 | EF441255 | EF441261 |
| Saltator atricollis | Cardinalidae | NRM 966978 | EF441230 | EF441252 | AY228320 |
| Sturnus vulgaris | Sturnidae | NRM966615 | EF441231 | EF441253 | AY228322 |
| Sylvia atricapilla | Sylviidae | NRM 976380 | EF441232 | EF441254 | AY228323 |
We also investigated whether Eupetes or Pachycephalopsis possesses the insertion of one codon in exon 3 of the nuclear c-myc gene, which has been proposed to be diagnostic of Passerida (Ericson & Johansson 2003). Laboratory procedures are described in the electronic supplementary material.
An average rate of substitutions in myoglobin intron 2 in passerines has been calculated as 0.145% Myr ago−1 (Fjeldså et al. in press) and this rate was used to estimate the timing of the splits between Eupetes and its closest relatives (electronic supplementary material).
3. Results and discussion
We were able to sequence all the three gene regions almost completely for all included taxa (Prunella lacks 70 bp in the 3′ end in myoglobin, and in ODC all sequences obtained from study skins lack a 22 bp fragment in exon 7). With the missing base pairs taken into account, the sequences obtained varied in length between 708 and 729 bp for myoglobin intron 2, between 253 and 307 bp for G3PDH intron 11 and between 591 and 621 bp for the ODC region. The combined alignment consists of 1742 bp.
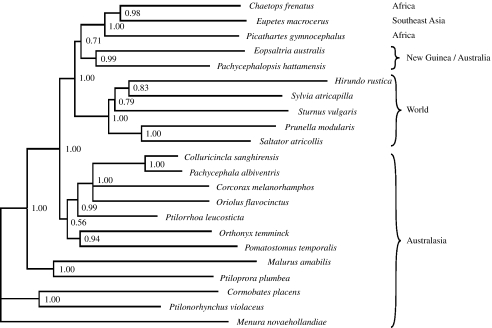
Analysis of three markers all provide strong support that E. macrocerus is nested within the Chaetops/Picathartes clade, its closest relatives being the rock-jumpers, which are endemic to the South African Cape region. A relationship between Chaetops and Picathartes was first suggested (with some hesitation) by Sibley & Ahlquist (1990) as a result of their DNA–DNA hybridizations, and it was later supported by several other studies using DNA sequence data (Barker et al. 2004; Beresford et al. 2005). It was unexpected as they are phenetically rather different: Chaetops resembling Australian grass wrens and inhabiting rocky places in open terrain, and Picathartes being larger and inhabiting rainforest understorey (Fry et al. 2000). Which of these specializations represent the ancestral condition is difficult to tell; on one hand southern Africa lost its forest cover far back in the Tertiary (Axelrod & Raven 1978), but it is also possible that range dynamics driven by climatic instability will gradually push species towards the more productive environments, leading to a general tendency for old lineages to persist in the major rainforest tracts (Storch et al. in press). According to the molecular clock model used (a discussion of the error margins for such clock estimates is trivial and we simply note that our estimates only represent a very rough idea of divergence times), the Picathartes/Chaetops/Eupetes clade diverged from the Australian Petroicidae (represented here by Eopsaltria and Pachycephalopsis) 48 Myr ago, in the Mid- Eocene. According to figure 1, the Picathartes/Chaetops/Eupetes clade is sister to the Australian Petroicidae and these in turn are sister to the Passerida (represented here by Hirundo, Sylvia, Sturnus, Prunella and Saltator). These nodes are poorly supported by our data, but the insertion of one codon in a conserved region of the c-myc gene in the Passerida and the Picathartes/Chaetops/Eupetes clade, as opposed to that in the Petroicidae and all Corvida groups, would seem to suggest that the Australian Petroicidae and Corvida groups are basal to the large Old World radiation of songbirds (Ericson & Johansson 2003).
Figure 1.
The 50% majority rule consensus tree obtained from the Bayesian analysis of the combined dataset (G3PDH intron 11, the myoglobin intron 2 and ODC introns 6–7) with geographical distributions. Posterior probability values are indicated to the right of the nodes.
Biogeographically, it is of great interest to find the Asian E. macrocerus nested together with two African taxa in a terminal position. Fuchs et al. (2006) and Jønsson & Fjeldså (2006b) proposed the idea that an Australian ancestor of the Passerida dispersed directly to Africa across stepping-stones in the southern Indian Ocean during the warm Eocene period (Kennett 1995), and that Passerida originated in Africa and from there radiated and dispersed to Eurasia and then to the rest of the world. It is of no doubt that the systematic position of Eupetes makes the diversification and dispersal patterns even more complex. Since several taxa representing deep branches within the Passerida are African (Beresford et al. 2005), and the Asiatic Eupetes and South African Chaetops are in a terminal position within their clade having diverged from Picathartes ca 44 Myr ago, the most parsimonious interpretation is still a dispersal of an Australian ancestor directly to Africa. The distribution of Picathartes, Chaetops and Eupetes would then be relictual, following a range expansion whereby this clade spread out of Africa. Eupetes retreated into the rainforests of the most southeastern part of mainland Asia and the closely associated islands of Sumatra and Borneo. At this time, the distance between Asia (with Greater Sundas) and terranes of Australian origin was too far apart for further range expansion. The other possible—though markedly less parsimonious—interpretation involves a dispersal event from Australia to Asia and then two independent dispersal events onwards to two different parts (and environments) of Africa.
A denser taxon sampling around the transition between Corvida and Passerida (e.g. Petroicidae) is a high priority for future work, as this might give a better idea about the ecological adaptations of the form that made the great leap out of Australia, whether that was via Asia or across the Indian Ocean. However, there is also a broad range of other odd relationships that, if correctly interpreted, would suggest ancient biogeographic connections between regions that are not connected today, such as the rainforests of Africa and the Australasian regions (for details see Olson 1973). Over the last decades, many such cases have been discussed in relation to plate tectonics, while earlier ideas about ancient land bridges were abandoned. However, the idea of trans-oceanic connections, including radiations within oceanic archipelagos, has recently received renewed attention (De Queiroz 2005; Filardi & Moyle 2005). For an understanding of the relative importance of vicariance driven by plate tectonics, or dispersal by past land bridges or island arcs, or sweepstake long-distance dispersal, it is important to revisit the suggested cases now with molecular data. Such data have revealed several previously neglected cases, where odd-looking species had been compared mainly with other taxa within the same part of the world, and thus were erroneously interpreted as aberrant members of such groups (e.g. Ericson et al. 2002; Fjeldså et al. 2003; Fuchs et al. 2006). A more systematic approach is now needed to compare a broad selection of potential cases of biotic links between ancient land masses.
Acknowledgments
We are grateful to the following people and institutions for granting access to toe pad, blood and tissue samples: Les Christidis at the Australian Museum, Sydney; Janette Norman at Museum Victoria, Melbourne; Mark Adams and Robert Prys-Jones at the Natural History Museum in Tring. We also acknowledge the support of a SYNTHESYS grant for K.A.J. (SE-TAF-1910) made available by the European Community, Research Infrastructure Action under the FP6 Structuring the European Research Area Programme. We also thank two anonymous reviewers who helped to improve the manuscript markedly.
Supplementary Material
Materials and methods
References
- Axelrod D.I, Raven P.H. Late Cretaceous and Tertiary vegetation history of Africa. In: Werger M.J.A, editor. Biogeography and ecology of southern Africa. Dr. W. Junk Publications; The Hague, The Netherlands: 1978. pp. 77–130. [Google Scholar]
- Barker F.K, Barrowclough G.F, Groth J.G. A phylogenetic hypothesis for passerine: birds: taxonomic and biogeographic implications of an analysis of nuclear DNA sequence data. Proc. R. Soc. B. 2002;269:295–308. doi: 10.1098/rspb.2001.1883. doi:10.1098/rspb.2001.1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker F.K, Cibois A, Schikler P, Feinstein J, Cracraft J. Phylogeny and diversification of the largest avian radiation. Proc. Natl Acad. Sci. USA. 2004;101:11 040–11 045. doi: 10.1073/pnas.0401892101. doi:10.1073/pnas.0401892101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beresford P, Barker F.K, Ryan P.G, Crowe T.M. African endemics span the tree of songbirds (Passeri): molecular systematics of several evolutionary ‘enigmas’. Proc. R. Soc. B. 2005;272:849–858. doi: 10.1098/rspb.2004.2997. doi:10.1098/rspb.2004.2997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson P.G.P, Johansson U.S. Phylogeny of Passerida (Aves: Passeriformes) based on nuclear and mitochondrial sequence data. Mol. Phylogenet. Evol. 2003;29:126–138. doi: 10.1016/s1055-7903(03)00067-8. doi:10.1016/S1055-7903(03)00067-8 [DOI] [PubMed] [Google Scholar]
- Ericson P.G.P, Christidis L, Cooper A, Irestedt M, Jackson J, Johansson U.S, Norman J.A. A Gondwanan origin of passerine birds supported by DNA sequences of the endemic New Zealand wrens. Proc. R. Soc. B. 2002;269:235–241. doi: 10.1098/rspb.2001.1877. doi:10.1098/rspb.2001.1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filardi C.E, Moyle R.G. Single origin of a pan-Pacific bird group and upstream colonization of Australasia. Nature. 2005;438:216–219. doi: 10.1038/nature04057. doi:10.1038/nature04057 [DOI] [PubMed] [Google Scholar]
- Fjeldså J, Zuccon D, Irestedt M, Johansson U.S, Ericson P.G.P. Sapayoa aenigma: a New World representative of ‘Old World suboscines’. Proc. R. Soc. B. 2003;270:S238–S241. doi: 10.1098/rsbl.2003.0075. doi:10.1098/rsbl.2003.0075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjeldså J, Irestedt M, Jønsson K.A, Ohlson J.I, Ericson P.G.P. Phylogeny of the ovenbird genus Upucerthia: a case of independent adaptations for terrestrial life. Zool. Scr. 2007;36:133–141. doi:10.1111/j.1463-6409.2006.00270.x [Google Scholar]
- Fry C.H, Keith S, Urban E.K. The birds of Africa. vol. VI. Academic Press; London, UK: 2000. [Google Scholar]
- Fuchs J, Fjeldså J, Bowie R.C.K, Voelker G, Pasquet E. The African warbler genus Hyliota as a long lost lineage in the oscine songbird tree: molecular support for an African origin of the Passerida. Mol. Phylogenet. Evol. 2006;39:186–197. doi: 10.1016/j.ympev.2005.07.020. doi:10.1016/j.ympev.2005.07.020 [DOI] [PubMed] [Google Scholar]
- Jønsson K.A, Fjeldså J. A phylogenetic supertree of oscine passerine birds (Aves: Passeri) Zool. Scr. 2006a;35:149–186. doi:10.1111/j.1463-6409.2006.00221.x [Google Scholar]
- Jønsson K.A, Fjeldså J. Determining biogeographic patterns of dispersal and diversification in oscine passerine birds in Australia, Southeast Asia and Africa. J. Biogeogr. 2006b;33:1155–1165. doi:10.1111/j.1365-2699.2006.01507.x [Google Scholar]
- Kennett J.P. A review of polar climatic evolution during the Neogene, based on the marine sediment record. In: Vrba E.S, Denton G.H, Partridge T.C, Burckle X, editors. Paleoclimate and evolution with emphasis on human origins. Yale University Press; New Haven, CT: 1995. pp. 49–64. [Google Scholar]
- Olson S.L. A classification of the rallidae. Wilson Bull. 1973;85:381–416. [Google Scholar]
- Olson S.L. Picathartes—another West African forest relict with probable Asian affinities. Bull. Br. Ornithol. Club. 1979;99:112–113. [Google Scholar]
- Peters J.L. A genus for Eupetes caerulescens Temminck. Auk. 1940;57:94. [Google Scholar]
- Peters J.L. Check-list of birds of the world. vol. X. The Heffernan Press, Inc; Worchester, MA: 1964. [Google Scholar]
- Queiroz A. The resurrection of oceanic dispersal in historical biogeography. Trends Ecol. Evol. 2005;20:68–73. doi: 10.1016/j.tree.2004.11.006. doi:10.1016/j.tree.2004.11.006 [DOI] [PubMed] [Google Scholar]
- Serle W. The affinities of the genus Picathartes lesson. Bull. Br. Ornithol. Club. 1952;72:2–6. [Google Scholar]
- Sharpe R.B. Catalogue of the birds in the British Museum. vol. 7. British Museum; London, UK: 1883. [Google Scholar]
- Sibley C.G. The relationships of Picathartes. Bull. Br. Ornithol. Club. 1973;93:23–25. [Google Scholar]
- Sibley C.G, Ahlquist J.E. Yale University Press; New Haven, CT: 1990. Phylogeny and classification of birds. A study in molecular evolution. [Google Scholar]
- Storch D, et al. Energy, range dynamics and global species richness patterns: reconciling mid-domain effects and environmental determinants of avian diversity. Ecol. Lett. 2006;9:1308–1320. doi: 10.1111/j.1461-0248.2006.00984.x. doi:10.1111/j.1461-0248.2006.00984.x [DOI] [PubMed] [Google Scholar]
- Temminck, C. J. 1831 Nouveau recueil de planches coloriées d'Oiseaux pour servir de suite et de complément aux planches enluminées de Buffon. Pl 516 In Dickinson, E. C. 2001 Systematic notes on Asian birds. 9. The "Nouveau recueil de planches coloriees" of Temminck & Laugier (1820–1839) Zool. Verh 335, 7–56. Levrault, Paris.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Materials and methods