Abstract
The intestinal flagellates of termites produce cellulases that contribute to cellulose digestion of their host termites. However, 75% of all termite species do not harbour the cellulolytic flagellates; the endogenous cellulase secreted from the midgut tissue has been considered a sole source of cellulases in these termites. Using the xylophagous flagellate-free termites Nasutitermes takasagoensis and Nasutitermes walkeri, we successfully solubilized cellulases present in the hindgut pellets. Zymograms showed that the hindguts of these termites possessed several cellulases and contained up to 59% cellulase activity against crystalline cellulose when compared with the midgut. Antibiotic treatment administered to N. takasagoensis significantly reduced cellulase activity in the hindgut, suggesting that these cellulases were produced by symbiotic bacteria.
Keywords: cellulase, termite, hindgut, bacteria, Termitidae, Nasutitermes
1. Introduction
Most animals cannot use cellulose because they do not have cellulolytic enzymes. By contrast, termites can efficiently hydrolyse cellulose with the aid of intestinal microbiota (Breznak & Brune 1994). Both the termites and their symbiotic flagellates can produce cellulases (Ohtoko et al. 2000; Watanabe & Tokuda 2001; Nakashima et al. 2002; Watanabe et al. 2002; Inoue et al. 2005). However, 75% of all termite species do not harbour cellulolytic flagellates in their alimentary canals. Despite many attempts to isolate cellulolytic bacteria from termites by the early 1980s, no convincing evidence of bacterial cellulose digestion in termites was provided (O'Brien & Slaytor 1982). Later, bacterial cellulase production in the flagellate-free termites Nasutitermes exitiosus and Nasutitermes walkeri was excluded by demonstrating that cellulase was released from the hindgut neither by sonication nor lytic treatment of bacteria (Hogan et al. 1988). Since these termites have strong cellulase activity in the midgut (Hogan et al. 1988), it is believed that they rely solely upon their own cellulases (Slaytor 1992, 2000). Indeed, recent molecular evidence has revealed that nasute termites secrete cellulase in the midgut (Tokuda et al. 1999, 2004), whereas symbiotic bacteria in termites are generally assumed not to participate in cellulose degradation (Brune & Stingl 2005).
However, we previously revealed that cellulase activities in the guts of flagellate-free termites were considerably lower than those of flagellate-harbouring termites (Tokuda et al. 2004, 2005). In addition, significant cellulase activity remained in the precipitate of the hindgut homogenate of Nasutitermes takasagoensis (Tokuda et al. 2005). Thus, it is possible that xylophagous flagellate-free termites depend not only on their own, but also on bacterial enzymes for cellulose digestion. In the present study, we show that the hindguts of xylophagous flagellate-free termites possess cellulases that are likely to be derived from the symbiotic bacteria.
2. Material and methods
The detailed descriptions are provided in the electronic supplementary material.
(a) Termites
Nasutitermes takasagoensis termites were collected and maintained as described previously (Tokuda et al. 1997). Nasutitermes walkeri termites were kindly provided by Dr Nathan Lo at the University of Sydney. Mature worker-caste termites were used unless otherwise indicated.
(b) Preparation of crude and pellet extracts
Five midguts or the enlarged part (P2–P5; Bignell 1994) of five hindguts (hereafter called ‘the hindgut’) were collected in 100 μl of proteinase inhibitor solution (Complete Mini EDTA-free; Roche). Each sample was sonicated and then centrifuged. Supernatants recovered are referred to as crude extracts. Each pellet was suspended with the same solution and centrifuged, which was repeated thrice. Then, the pellet was suspended with 100 μl of detergent reagent (CelLytic B; Sigma-Aldrich) and vortexed vigorously. The suspensions were incubated on ice for 10 min and centrifuged. The supernatants are referred to as pellet extracts.
(c) Detection of cellulase activity on SDS- and native-polyacrylamide gel electrophoresis gels
Zymograms were analysed as previously described (Tokuda et al. 1999) with slight modifications using SDS- and native-polyacrylamide gel electrophoresis (PAGE) gels. See electronic supplementary material for more details.
(d) Crystalline cellulose degrading activity
Crude or pellet extract was incubated with 200 μl of 2% microcrystalline cellulose (Sigmacell Type 20; Sigma–Aldrich) in McIlvaine's buffer (pH 5.8 and 6.5 for the midgut and the hindgut extracts, respectively) at 37°C for 1 h with intensive shaking (1200 oscillations per minute). Reducing sugars released into the supernatant were measured as previously described (Tokuda et al. 2005).
(e) Antibiotic treatment
Termites (six workers plus one soldier) were placed in a Petri dish containing a filter paper soaked in 1 ml of either 5.4 mM ampicillin or sterilized distilled water. Five replicates of each plate were prepared and the filter papers were replaced every 2 days. After one week, the termites were dissected and cellulase activity was measured as described earlier.
3. Results
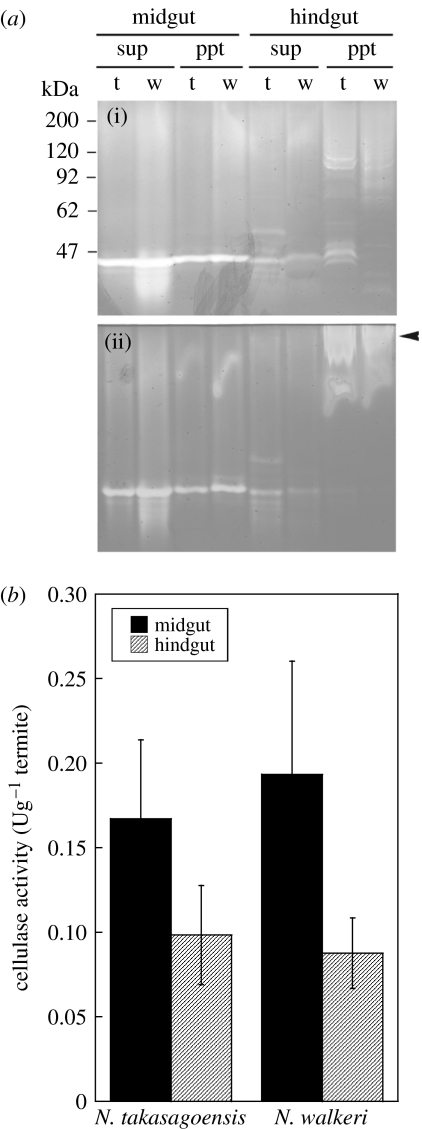
Although we have previously shown that the pellet of hindgut homogenates of N. takasagoensis contains cellulase activity (Tokuda et al. 2005), this activity was not solubilized by suspending the pellet with water or buffer. Thus, we used a detergent reagent, CelLytic B, which is often used for solubilization of recombinant proteins produced by bacteria without denaturing (Ni et al. 2005; Strauss et al. 2005). Figure 1a shows zymograms of crude and pellet extracts of the midgut and the hindgut in N. takasagoensis and N. walkeri. In both SDS- and native-PAGE, the midgut crude and pellet extracts revealed one major band of cellulase activity; the hindgut crude extract also showed one or two bands. In contrast, several bands of cellulase activity were observed in pellet extracts of the hindgut on zymograms of SDS-PAGE. These bands were diverse in size, which was different from the midgut cellulase. The mobility of these bands was changed in native-PAGE and the cellulase activities were detected nearer to the origin than the zymograms on SDS-PAGE (figure 1a).
Figure 1.
(a) Zymograms of cellulases present in the midguts and the hindguts of N. takasagoensis and N. walkeri. The same samples were analysed by (i) SDS-PAGE and (ii) native-PAGE. Winding lines observed in lanes of pellet extracts on the native gel are non-specific bands caused by the detergent reagent. Several cellulases detected in the pellet extracts of the hindguts on SDS-PAGE were changed in mobility on native-PAGE (arrow). sup, crude extract; ppt, pellet extract; t, N. takasagoensis; w, N. walkeri. (b) Cellulase activity against crystalline cellulose in the midgut and the hindgut of N. takasagoensis and N. walkeri. Bars are means of five determinations±s.d. See table S1 in the electronic supplementary material for more details.
Since the midgut of these termites abundantly produces a cellulase termed endo-β-1,4-glucanase (EC 3.2.1.4) (Hogan et al. 1988; Tokuda et al. 1997, 1999), which preferentially attacks amorphous regions of native cellulose (Lynd et al. 2002), it is likely that cellulosic food particles reaching the hindgut primarily comprise non-amorphous crystalline regions. Thus, we measured cellulase activity using crystalline cellulose as substrate. When compared with the midgut, the hindguts of N. takasagoensis and N. walkeri retained 59 and 45% of cellulase activities against crystalline cellulose, respectively (figure 1b; see table S1 in the electronic supplementary material for more details).
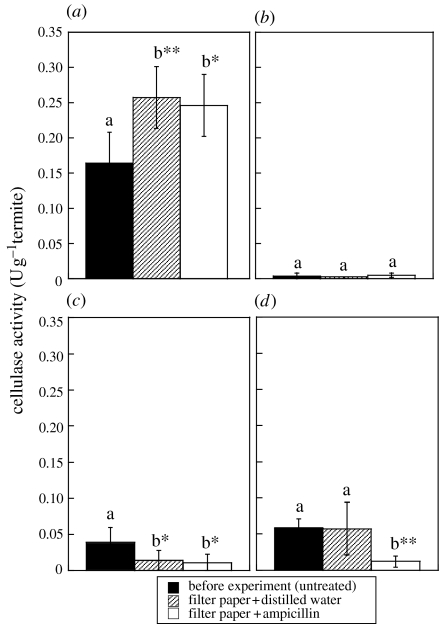
Since hindgut of these termites contains numerous prokaryotes (Tokuda et al. 2001), we tested how antibiotics affect cellulase activities against crystalline cellulose. The termites had fed on ampicillin- or water-soaked filter paper for one week. Unfortunately, most individuals of N. walkeri died before the end of the experiment. Cellulase activity in the midgut of N. takasagoensis was not decreased by the antibiotic treatment (figure 2a,b). When compared with the termites before starting the experiments, cellulase activity in the hindgut crude extract was significantly reduced in termites which fed on ampicillin (figure 2c). However, in this case, no significant difference was observed between ampicillin and distilled water treatments (figure 2c). In contrast, the experiments of the hindgut pellet extract resulted in the significant reduction in cellulase activity only in termites which fed on ampicillin (figure 2d).
Figure 2.
Effect of antibiotics on cellulase activities in N. takasagoensis. (a) Crude extract of the midgut. (b) Pellet extract of the midgut. (c) Crude extract of the hindgut. (d) Pellet extract of the hindgut. Bars are means of five determinations±s.d. In each gut extract, activities shown as ‘b’ are significantly different from those shown as ‘a’ (*p<0.05 or **p<0.01, ANOVA and Fisher's LSD).
4. Discussion
Based on the zymogram of the SDS-PAGE gel, the hindgut cellulolytic systems in both the species of termites comprise several cellulase components varying in size. The band patterns of the hindgut cellulases differed between N. takasagoensis and N. walkeri, suggesting that the origins of the enzymes were different or that cellulase components in the hindguts were variable in response to food available from their environments. Based on mobility of hindgut cellulases observed with the native-PAGE, these cellulases may form large clusters or enzymatic complexes when working in vivo. The current result suggests that, at least, the hindgut cellulolytic system is quite different from that of the midgut, where only one type of endo-β-1,4-glucanase plays a role in cellulose hydrolysis (Tokuda et al. 1997, 1999).
The hindgut contained up to 59% of cellulase activity when compared with the midgut. Although the midgut is considered as the primary site of cellulose digestion in these termites, the contribution of the hindgut cellulases to cellulose digestion is not negligible. Since the geographically isolated termites showed apparent cellulase activities in the hindgut, the contribution of the hindgut cellulases could be the common phenomenon among termites of the subfamily Nasutitermitinae.
Antibiotic treatment that reduced cellulase activity in the hindgut pellet extract of N. takasagoensis implies a bacterial origin of the hindgut cellulases. However, the significant reduction in cellulase activity in the hindgut crude extract observed in termites treated both with ampicillin and distilled water may be due to adsorption of the cellulases onto the filter paper that consisted of pure cellulose.
Our previous study proposed a possible involvement of cellulosomes in cellulose digestion of N. takasagoensis (Tokuda et al. 2005). Cellulosomes are cellulolytic enzyme complexes that adhere to the cell wall, mainly found in bacteria such as clostridia (Demain et al. 2005). The present study also supports this idea, although further investigations are needed to confirm the presence of such enzymatic structures.
The present study indicates that N. takasagoensis and N. walkeri have cellulases in the hindguts, which could be derived from symbiotic bacteria. Flagellate-free termites are diverse in feeding habits in response to their ecology (Waller & La Fage 1987). It is fascinating to apply our simple method for the solubilization of hindgut cellulases to explore not only cellulases, but also other enzymes hidden in the gut of such termites. Solubilization of precipitated proteins allows various analyses such as purification, characterization and determination of amino acid compositions of hidden digestive enzymes. Therefore, the present study is a vital stepping-stone to elucidate the real features of the diverse digestive systems in termites.
Acknowledgments
This study was conducted at NIAS under a visiting researcher program from University of the Ryukyus. The research was partially supported by the 21st COE programme and by the grant-in-aid for Scientific Research no. 17405025 and 16780037 from JSPS. We thank Dr A. Yamada at University of the Ryukyus for his critical comments.
Supplementary Material
Supplementary methods
Cellulase activity in the midgut and hindgut of N. takasagoensis and N. walkeri
Effect of pH on cellulase activity in pellet extracts of the hindguts of N. takasagoensis and N. walkeri
References
- Bignell D.E. Soil-feeding and gut morphology in higher termites. In: Hunt J.H, Nalepa C.A, editors. Nourishment and evolution in insect societies. Westview Press; Boulder, CO: 1994. pp. 131–158. [Google Scholar]
- Breznak J.A, Brune A. Role of microorganisms in the digestion of lignocellulose by termite. Annu. Rev. Entomol. 1994;39:453–487. doi:10.1146/annurev.en.39.010194.002321 [Google Scholar]
- Brune, A. & Stingl, U. 2005 Prokaryotic symbionts of termite gut flagellates: phylogenetic and metabolic implications of a tripartite symbiosis. In Molecular basis of symbiosis, vol. 41. Progress in molecular and subcellular biology (ed. J. Overmann), pp. 39–60. New York, NY: Berlin, Germany: Springer. [DOI] [PubMed]
- Demain A.L, Newcomb M, Wu J.H.D. Cellulase, clostridia, and ethanol. Microbiol. Mol. Biol. Rev. 2005;69:124–154. doi: 10.1128/MMBR.69.1.124-154.2005. doi:10.1128/MMBR.69.1.124-154.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan M, Veivers P.C, Slaytor M, Czolij R.T. The site of cellulose breakdown in higher termites (Nasutitermes walkeri and Nasutitermes exitiosus) J. Insect Physiol. 1988;34:891–899. doi:10.1016/0022-1910(88)90123-0 [Google Scholar]
- Inoue T, Moriya S, Ohkuma M, Kudo T. Molecular cloning and characterization of a cellulose gene from a symbiotic protist of the lower termite, Coptotermes formosanus. Gene. 2005;349:67–75. doi: 10.1016/j.gene.2004.11.048. doi:10.1016/j.gene.2004.11.048 [DOI] [PubMed] [Google Scholar]
- Lynd L.R, Weimer P.J, van Zyl W.H, Pretorius I.S. Microbial cellulose utilization: fundamentals and biotechnology. Microbiol. Mol. Biol. Rev. 2002;66:506–577. doi: 10.1128/MMBR.66.3.506-577.2002. doi:10.1128/MMBR.66.3.506-577.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima K, Watanabe H, Azuma J. Cellulase genes from the parabasalian symbiont Pseudotrichonympha grassii in the hindgut of the wood-feeding termite Coptotermes formosanus. Cell. Mol. Life. Sci. 2002;59:1554–1560. doi: 10.1007/s00018-002-8528-1. doi:10.1007/s00018-002-8528-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni J, Takehara M, Watanabe H. Heterologous overexpression of a mutant termite cellulose gene in Escherichia coli by DNA shuffling of four orthologous parental cDNAs. Biosci. Biotechnol. Biochem. 2005;69:1711–1720. doi: 10.1271/bbb.69.1711. doi:10.1271/bbb.69.1711 [DOI] [PubMed] [Google Scholar]
- O'Brien R.W, Slaytor M. Role of microorganisms in the metabolism of termites. Aust. J. Biol. Sci. 1982;35:239–262. [Google Scholar]
- Ohtoko K, Ohkuma M, Moriya S, Inoue T, Usami R, Kudo T. Diverse genes of cellulase homologues of glycosyl hydrolase family 45 from the symbiotic protists in the hindgut of the termite Reticulitermes speratus. Extremophiles. 2000;4:343–349. doi: 10.1007/s007920070003. doi:10.1007/s007920070003 [DOI] [PubMed] [Google Scholar]
- Slaytor M. Cellulose digestion in termites and cockroaches: what role do symbionts play? Comp. Biochem. Physiol. B. 1992;103:775–784. doi:10.1016/0305-0491(92)90194-V [Google Scholar]
- Slaytor M. Energy metabolism in the termite and its gut microbiota. In: Abe T, Bignell D.E, Higashi M, editors. Termites: evolution, sociality, symbioses, ecology. Kluwer Academic Publisher; Dordrecht, The Netherlands: 2000. pp. 307–332. [Google Scholar]
- Strauss B, Kelly K, Ekiert D. Cytochrome oxidase deficiency protects Escherichia coli from cell death but not from filamentation due to thymine deficiency or DNA polymerase inactivation. J. Bacteriol. 2005;187:2827–2835. doi: 10.1128/JB.187.8.2827-2835.2005. doi:10.1128/JB.187.8.2827-2835.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda G, Watanabe H, Matsumoto T, Noda H. Cellulose digestion in the wood-eating higher termite, Nasutitermes takasagoensis (Shiraki): distribution of cellulases and properties of endo-β-1,4-glucanase. Zool. Sci. 1997;14:83–93. doi: 10.2108/zsj.14.83. doi:10.2108/zsj.14.83 [DOI] [PubMed] [Google Scholar]
- Tokuda G, Lo N, Watanabe H, Slaytor M, Matsumoto T, Noda H. Metazoan cellulase genes from termites: intron/exon structures and sites of expression. Biochim. Biophys. Acta. 1999;1447:146–159. doi: 10.1016/s0167-4781(99)00169-4. doi:10.1016/S0167-4781(99)00169-4 [DOI] [PubMed] [Google Scholar]
- Tokuda G, Nakamura T, Murakami R, Yamaoka I. Morphology of the digestive system in the wood-feeding termite Nasutitermes takasagoensis (Shiraki) [Isoptera: Termitidae] Zool. Sci. 2001;18:869–877. doi:10.2108/zsj.18.869 [Google Scholar]
- Tokuda G, Lo N, Watanabe H, Arakawa G, Matsumoto T, Noda H. Major alteration of the expression site of endogenous cellulases in members of an apical termite lineage. Mol. Ecol. 2004;13:3219–3228. doi: 10.1111/j.1365-294X.2004.02276.x. doi:10.1111/j.1365-294X.2004.02276.x [DOI] [PubMed] [Google Scholar]
- Tokuda G, Lo N, Watanabe H. Marked variations in patterns of cellulose activity against crystalline- vs. carboxymethyl-cellulose in the digestive systems of diverse, wood-feeding termites. Physiol. Entomol. 2005;30:372–380. doi:10.1111/j.1365-3032.2005.00473.x [Google Scholar]
- Waller D.A, La Fage J.P. Nutritional ecology of termites. In: Slansky F Jr, Rodriguez J.G, editors. Nutritional ecology of insects, mites, spiders, and related invertebrates. Wiley-Interscience; New York, NY: 1987. pp. 487–532. [Google Scholar]
- Watanabe H, Tokuda G. Animal cellulases. Cell. Mol. Life Sci. 2001;58:1167–1178. doi: 10.1007/PL00000931. doi:10.1007/PL00000931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Nakashima K, Saito H, Slaytor M. New endo-β-1,4-glucanases from the parabasalian symbionts, Pseudotrichonympha grassii and Holomastigotoides mirabile of Coptotermes termites. Cell. Mol. Life Sci. 2002;59:1983–1992. doi: 10.1007/PL00012520. doi:10.1007/PL00012520 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary methods
Cellulase activity in the midgut and hindgut of N. takasagoensis and N. walkeri
Effect of pH on cellulase activity in pellet extracts of the hindguts of N. takasagoensis and N. walkeri