Abstract
Many proximate factors determine a bird’s laying date, including environmental and social stimuli as well as individual responses to internal and external factors. However, the relative importance of these factors has not been experimentally demonstrated. Here we show that (i) large differences in the onset of first clutches between different populations result from variation in different responses to photoperiod and not from variation in responses to any other proximate factors and (ii) the same response mechanism causes maladaptive laying dates in habitats modified by humans. We present, to our knowledge, the first experimental demonstration that a single response mechanism is responsible for evolutionary adaptive intraspecific variation in a vertebrate life history trait.
A bird’s onset of egg laying is proximately determined by photoperiod, local climate, food to form eggs, territory quality, nest site, the quality and behavior of the mate, characteristics of the individual (e.g., age, body condition), and responses to external and internal factors (1–3). The relative importance of these proximate factors and response mechanisms in the determination of intraspecific variation in a bird’s onset of egg laying has not been experimentally established. The proximate determination of population differentiation of vertebrate life history traits rarely has been studied in combination with long term comparative field and laboratory studies that (i) established a genetic basis for intraspecific variation in life history traits and (ii) determined and quantified selection pressures of life history traits in relation to fitness components in natural populations (3). The identification of the proximate determination of variation of life history traits in natural populations is crucial for the understanding of how organisms can cope with habitat heterogeneity or human-induced habitat changes.
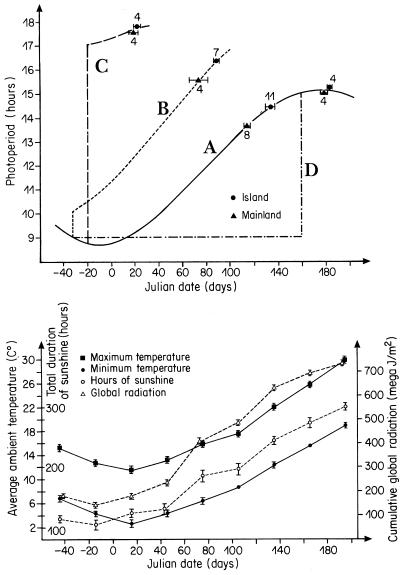
In different areas of the Mediterranean, blue tits start their breeding seasons at different dates. A 20-year study (1202 nests) showed that, after controlling for latitude, altitude, and habitat type, mainland blue tits of southern France (Parus caeruleus caeruleus) lay first clutches on average 3 weeks earlier than blue tits in isolated evergreen forests on the island of Corsica (P. c. ogliastrae) (4). A 10-year study showed that these population differences remain if captive blue tit pairs are kept in large outdoor aviaries in southern France (treatment A, Fig. 1), whatever the age at which the birds are taken into captivity (hatched in captivity, nestling, yearling, or adult) (5, 6). These results suggest that the large population differences in the onset of first clutches have a genetic basis (5, 6) and that the differences proximately do not result from regional differences in habitats. We showed that mainland and island blue tits advance onset of egg laying when captive pairs are exposed to a sudden increase in photoperiod and that mainland blue tits lay during shorter day lengths than Corsican blue tits (7). This experiment did not exclude the possibility that the large mainland–island differences in laying date are influenced by variation in responses to proximate factors other than photoperiod.
Figure 1.
Onset of first clutches (average ± SE in Julian dates; January 1 = 1, February 1 = 32) of captive mainland and island blue tits of the Mediterranean region breeding in outdoor aviaries at Montpellier, southern France presented with one to four different changes in photoperiod (Upper) and the monthly change in local climate (average ± SE, 1985–1995) to which the birds were exposed (Lower).
The aim of this study was to determine whether more than one proximate factor or response mechanism is responsible for the differences in average laying date between the mainland and Corsican blue tits. We conducted experiments with captive blue tit pairs in the course of the long term field studies of adaptive variation in the timing of blue tit egg laying in the Mediterranean region.
METHODS
We trapped individuals in or near the sites of the long term studies of natural blue tit populations in the Mediterranean region (4–6). Mainland and Corsican blue tit pairs were distributed randomly over different large outdoor aviaries at the Centre National de la Recherche Scientifique campus in Montpellier, southern France. Each aviary contained one blue tit pair, one or two evergreen oak trees (Quercus ilex), two nest boxes, and artificial food provided ad libitum. The wild and captive populations were at the same latitudes and were exposed to similar natural photoperiod conditions (7). The survival rates of the captive blue tits were at least as high as those observed in the wild (unpublished data).
To test the relative importance of proximate factors and response mechanisms in the determination of the large mainland–island differences in onset of first clutches, we presented the captive pairs of both populations with at least one of four different light treatments (A, B, C, or D) using the same outdoor aviaries and artificial food for each treatment but with each of the four treatments starting at a different time of the year (see Fig. 1). Treatment A consisted of natural daylight throughout the year and was used as a control treatment (photoperiodic conditions as for the wild birds). Data of treatment A were gathered during a 10-year period between 1986 and 1996 (see above). In treatments B and C, blue tit pairs were presented with artificial photoperiods from short natural day lengths in November-December onward before the gonads developed. The artificial photoperiods consisted of the natural day plus an additional period with artificial light during the morning only (light intensity per aviary: one lamp of 500 W at 4 m from the ground and four neons of 36 W at 3 m from the ground). In treatment B, we exposed the pairs to an artificial 1-hour increase from November 28, 1993 onward followed by an increase of the artificial light period by 15 minutes per week during a 6-month period (light-dark = 10L-14D until 16:30L-7:30D). To increase the sample size, treatment B was repeated between November 1994 and May 1995. In treatment C, the photoperiod was increased to a long day of 17L-7D or more from December 14, 1992 onward, followed by a 2-month light treatment. In treatment D, pairs were kept indoors without air conditioning at a light-day cycle = 9L-15D from November 28, 1995 until June 5, 1996 and then placed in the outdoor aviaries with natural photoperiodic conditions. In treatments A and B, some females were followed for more than 1 year. Because the repeatability of the onset of first clutches in individual captive females is high, whatever the mate (see ref. 5), we calculated an average laying date per light treatment for females that bred in more than 1 year.
We tested the effects of light treatments (A vs. B, A vs. C, and A vs. D), population (mainland vs. island), and their interactions using two-way ANOVAs, type III (8). This test is robust for nonnormality and heterogeneity of variances if results are statistically significant (9). Because the different treatments differed in light quality and because we assumed that light quality (e.g., intensity) influences gonad development (e.g., ref. 1), we limited our predictions to relative differences in onset of egg laying between the mainland and island pairs in response to the different treatments. Data for the local climate (1985–1995) to which the captive pairs were exposed were gathered by the National Institute of Agricultural Research-STEFCE (Fig. 1).
RESULTS
Fig. 1 shows that the start of first clutches differs between the different treatments. All of the main effects of population (mainland vs. island) and treatments (A vs. B, A vs. C, and A vs. D) on laying date were highly significant (all P < 0.001). If mainland and island blue tits are presented with a suitable artificial light treatment, they can lay eggs in outdoor aviaries at any time of the year.
In photosensitive species exposed to a stimulating day length, the onset of reproduction could be prevented or delayed because of responses to many additional external or internal factors. It is therefore possible that both blue tit populations are stimulated by exactly the same day length but that population differences in timing of egg laying are caused by population differences in response to additional factors. Therefore, if any proximate factor or response mechanism other than variation in responses to photoperiod is responsible for the observed population differences in average laying date, we predicted that the mainland pairs should lay well before the island ones, whatever the light treatment. This was not the case. Population differences in average laying date differed significantly when the laying dates in control treatment A were compared with those in treatments C (interaction population × treatment: F = 7.35; df = 1, 22; P = 0.0125) or D (population × treatment: F = 9.03; df = 1, 22; P = 0.0063). But the population differences did not differ when treatment A was compared with treatment B (population × treatment: F = 0.14; df = 1, 25; P = 0.71) (Fig. 1).
The local climate to which the pairs were exposed differed markedly among the four treatments (Fig. 1). If responses to climatic factors modify the responses to changes in day length in a different way in the two blue tit populations, as was found in other tit populations (10), differences in average laying date should not have been the same between treatments A and B or between treatments C and D. Contrary to these predictions, we found that, in treatments A and B, the mainland pairs laid eggs before the island ones and that, in treatments C and D, both populations started first clutches, on average, on the same date (see interactions above, Fig. 1). Furthermore, in treatments C and D, birds began laying 3–5 weeks after the start of the long day treatment, which is a shorter period than the 7- to 8-week period between the start of rapid gonad development and the start of first clutches reported in wild blue tits (11). Therefore, any constraints on laying cannot explain the same responses of both populations to treatments C and D. These results are consistent with the hypothesis that variation in response to photoperiod is the sole proximate mechanism explaining the large population differences in laying date. When pairs are exposed to changes in photoperiod, as in spring conditions, the prediction is that the mainland birds should lay well before the island ones, which was confirmed by the results of treatments A and B. If both populations are presented at once with a long day exceeding the day length at which they begin laying in the wild, the prediction is that the mainland and island birds should lay eggs on average at the same date, which was supported by the results of treatments C and D.
DISCUSSION
Successful reproduction is often only possible within a short time period of the year (12, 13). Long term studies have determined the optimal breeding time in tits. A mismatch between the nestling stage and a 2- to 3-week period of maximum caterpillar abundance, the main food of nestling tits, results in lower nestling condition, higher nestling mortality, or decreased local recruitment into the following generations (4, 12, 14–19). The timing of the peak date of caterpillars is therefore a major source of selection pressure on the timing of breeding in tits. In the Mediterranean, mainland blue tits that breed in broadleaved deciduous habitats and island blue tits that breed in isolated evergreen habitats at similar latitudes (between 42° and 44°) are exposed to similar photoperiodic conditions. But they preserved ultimately a 3- to 4-week difference in the onset of first clutches because of consistent year-to-year differences in the local timing of maximum caterpillar abundance (4–7). Because the onset of egg laying occurs before the time when the young are raised, the parents must use mechanisms to anticipate the most favorable period for raising the young. At higher latitudes, photoresponsiveness opens and closes a window during which reproduction is possible, but other factors can determine the date of laying (1, 20–22). Adaptive, intraspecific variation in the onset of reproduction across populations is often attributed to variation in responses to photoperiod if populations breed at different latitudes and is attributed to variations in other response mechanisms if populations breed at similar latitudes. Here we demonstrate that, after controlling for latitude, a large difference in the onset of first clutches between two populations results from a different response to photoperiod and not from other proximate factors or constraints. To our knowledge (see refs. 2, 3, 18), this is the first experimental demonstration that a single response mechanism causes intraspecific, adaptive (see ref. 23) variation in a vertebrate life history trait.
Although the large regional differences in blue tit laying date are adaptive in the natural habitats (see above), human-caused habitat changes can result in maladaptive clutches (24, 25). Controlling for latitude, the peak date for caterpillars is 3–4 weeks later in pine woods and evergreen oak woodlands than in broadleaved deciduous woods (4, 15, 19). On the European mainland, broadleaved deciduous oak woods are optimal for breeding blue tits (26), which explains why, across populations or years, the timing of the nestling stage is well synchronized with the peak date for caterpillars in these habitats (16). The synchronization between the nestling stage and the optimal breeding time in tits has been proximately attributed to plastic responses to changes in local climate or factors related to bud burst (4, 13–18). However, in new habitats that have been heavily modified by humans (e.g., pine plantations, extensive evergreen oak habitats in southern France; see ref. 27), the laying date is similar to that in broadleaved deciduous woods and is therefore at least 3 weeks before bud burst (4, 15, 19). Furthermore, the onset of first clutches is maladaptive because the nestling stage of the tits is too early in relation to optimal breeding time, and reproductive success is lower in these habitats (15, 19). These maladaptive laying dates are due to dispersal of tits adapted to broadleaved deciduous habitats into the evergreen habitats (4, 28, see also refs. 24, 25). We have shown that, with light treatment, blue tits can breed at any time of the year, whatever the local climate or changes in tree or food phenology (see above). Captive tits exposed to natural photoperiodic conditions have similar laying dates to those in evergreen woodlands in the wild. The birds breeding in habitats modified by humans lay at the same time as those in natural habitats because gene flow from the latter to the former prevents them from evolving a more suitable photoperiodic response that would enable them to lay at the best time for raising the young.
Acknowledgments
We are grateful to C. M. Perrins for valuable comments on an earlier draft and to an anonymous referee for useful suggestions. We also thank F. Sordillon and F. Loustalot for help in the aviaries, R. Ferris for drawings, and M. Methy for providing data of natural photoperiods and local climate. Birds were held with a license of the Ministère de l’Environnement, France. This research was partly supported by a grant from EGPN.
References
- 1.Murton R K, Westwood N J. Avian Breeding Cycles. Oxford: Clarendon; 1977. [Google Scholar]
- 2.Bronson F H. Biol Reprod. 1985;32:1–26. doi: 10.1095/biolreprod32.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Garland T, Jr, Adolph S C. Annu Rev Ecol Syst. 1991;22:193–228. [Google Scholar]
- 4.Blondel J, Dias P C, Maistre M, Perret P. Auk. 1993;110:511–520. [Google Scholar]
- 5.Blondel J, Perret P, Maistre M. J Evol Biol. 1990;3:469–475. [Google Scholar]
- 6.Lambrechts M M, Dias P C. Ibis. 1993;135:451–455. [Google Scholar]
- 7.Lambrechts M M, Perret P, Blondel J. Proc R Soc London B. 1996;263:19–22. [Google Scholar]
- 8.SAS Institute, Inc. SAS/STAT User’s Guide. Cary, NC: SAS Institute; 1989. [Google Scholar]
- 9.Ito P K. In: Handbook of Statistics. Krishnaiah P R, editor. Amsterdam: North–Holland; 1980. pp. 199–236. [Google Scholar]
- 10.Silverin B, Viebke P A. Horm Behav. 1994;28:199–206. doi: 10.1006/hbeh.1994.1017. [DOI] [PubMed] [Google Scholar]
- 11.Lebeurier E, Rapine J. Oiseau. 1944;14:5–31. [Google Scholar]
- 12.Lack D. Ibis. 1950;92:288–316. [Google Scholar]
- 13.Daan S, Dijkstra C, Drent R H, Meijer T. In: Proceedings of the XIX Ornithological Congress. Ouellet H, editor. Canada: Ottawa; 1989. pp. 392–407. [Google Scholar]
- 14.Gibb J A. Ibis. 1950;92:507–539. [Google Scholar]
- 15.Van Balen H. Ardea. 1973;61:1–59. [Google Scholar]
- 16.Banbura J, Blondel J, de Wilde-Lambrechts H, Galan M-J, Maistre M. Oecologia. 1994;100:413–420. doi: 10.1007/BF00317863. [DOI] [PubMed] [Google Scholar]
- 17.Nager R G, van Noordwijk A J. Am Nat. 1995;146:454–474. [Google Scholar]
- 18.van Noordwijk A J, McCleery R H, Perrins C M. J Anim Ecol. 1995;64:451–458. [Google Scholar]
- 19.Dias P C, Blondel J. Ibis. 1996;138:108–113. [Google Scholar]
- 20.Wingfield J C. In: Avian Endocrinology. Epple A, Stetson M H, editors. New York: Academic; 1980. pp. 367–390. [Google Scholar]
- 21.Gwinner E. Ibis. 1996;13:47–63. [Google Scholar]
- 22.Perrins C M. Ibis. 1996;138:2–15. [Google Scholar]
- 23.Reeve H K, Sherman P W. Q Rev Biol. 1993;68:1–32. [Google Scholar]
- 24.Perrins C M, Moss D. J Anim Ecol. 1975;44:695–706. [Google Scholar]
- 25.Dhondt A A, Adriaensen F, Matthysen E, Kempenaers B. Nature (London) 1990;348:723–725. [Google Scholar]
- 26.Partridge L. Nature (London) 1974;247:573–574. [Google Scholar]
- 27.Pons A. In: Mediterranean-Type Shrublands. Di Castri F, Goodall D W, Specht R L, editors. Amsterdam: Elsevier; 1981. pp. 131–138. [Google Scholar]
- 28.Dias P C, Verheyen G R, Raymond M. J Evol Biol. 1996;9:965–978. [Google Scholar]