Abstract
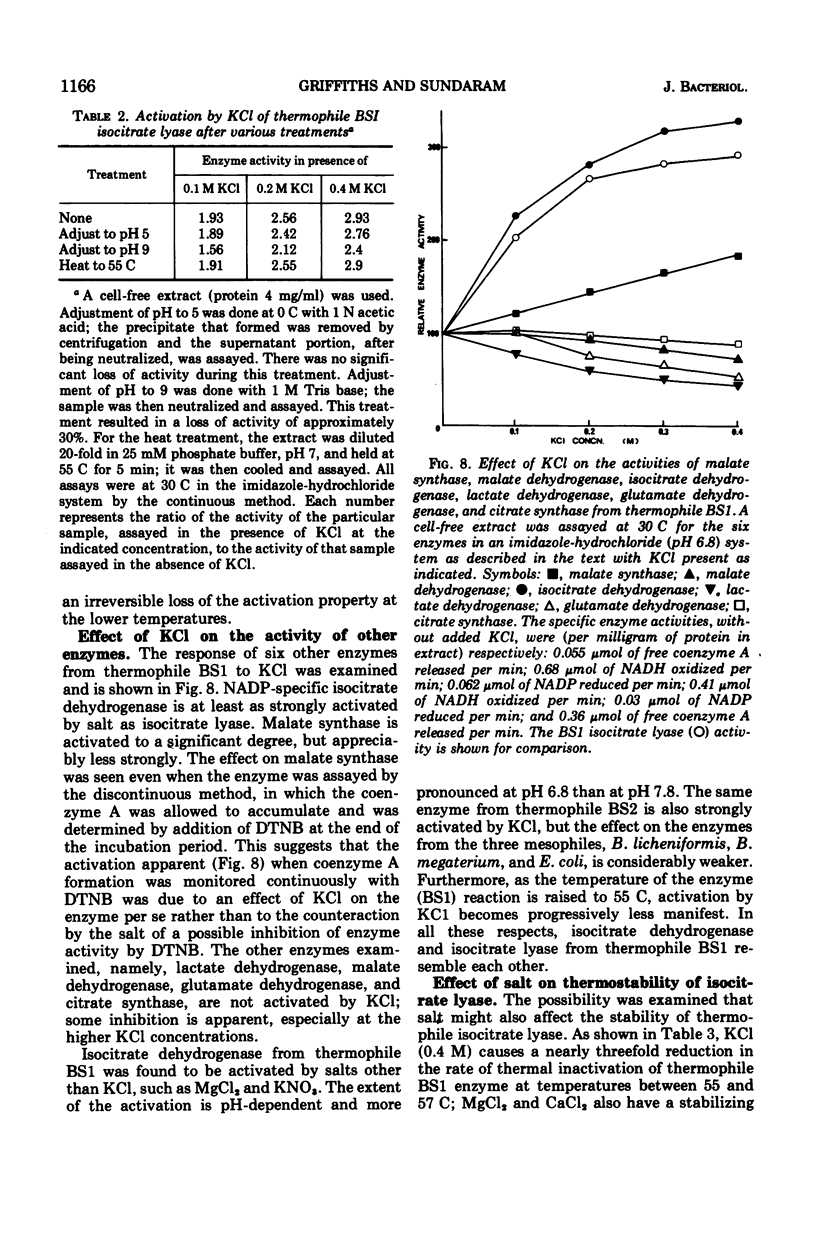
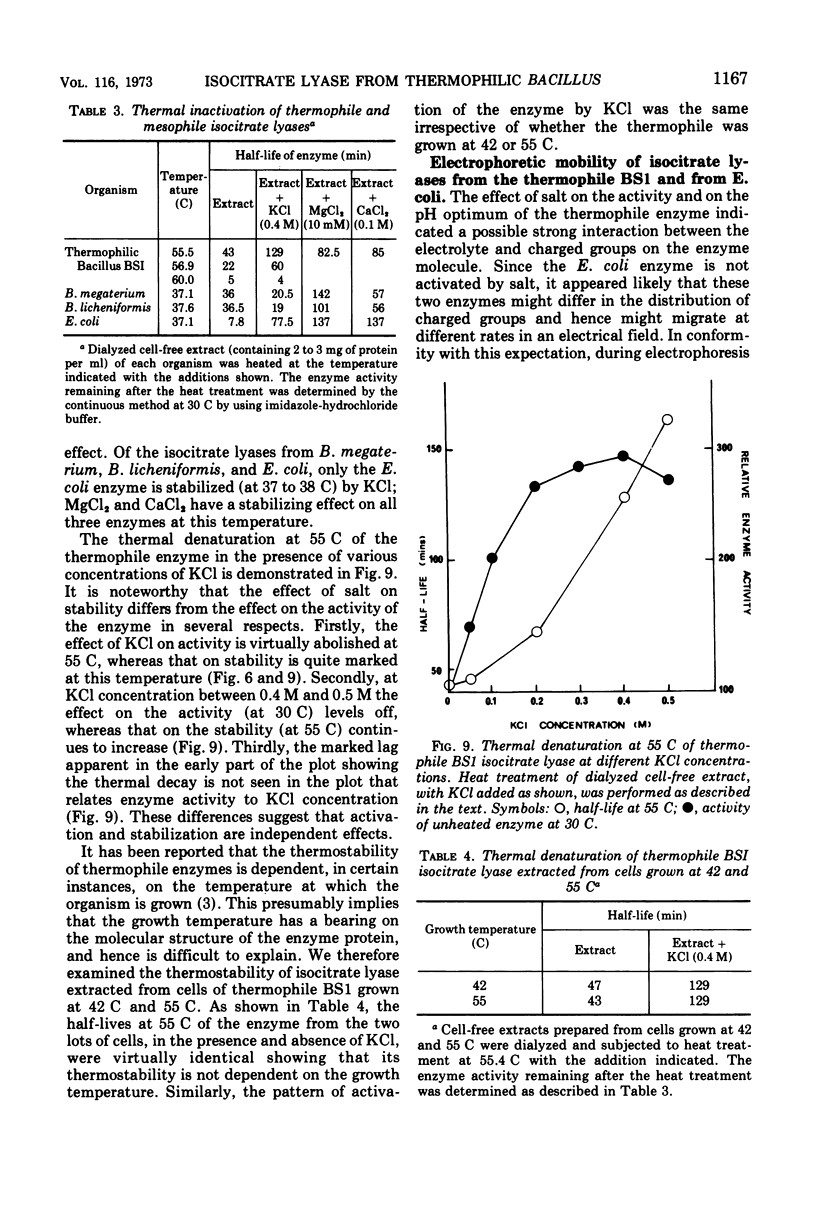
The isocitrate lyase from a thermophilic Bacillus is activated about threefold by a variety of salts. Such strong stimulation of activity is not seen with isocitrate lyase from the mesophiles, Bacillus licheniformis, Bacillus megaterium, Escherichia coli, and Aspergillus nidulans. The salt activation is markedly pH-dependent. At pH values above 8.6, salt (KCl) indeed inhibits the enzyme activity. Potassium chloride also causes a significant shift of the pH optimum of the enzyme towards the acid side. As the temperature of the enzyme reaction is raised, activation becomes progressively weaker. Potassium chloride also affords considerable protection against enzyme denaturation at 55 C. The activation and the stabilization, however, appear to be independent effects. Of six other enzymes in the thermophile that were examined, isocitrate dehydrogenase was equally strongly activated by KCl and malate synthase was less strongly, but significantly, activated; citrate synthase, malate dehydrogenase, glutamate dehydrogenase, and lactate dehydrogenase were unaffected or slightly inhibited by KCl. The property of being strongly activated by salt appears to be a peculiar characteristic of the thermophile isocitrate lyase and possibly evolved concomitantly with its thermostability.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armitt S., Roberts C. F., Kornberg H. L. The role of isocitrate lyase in Aspergillus Nidulans. FEBS Lett. 1970 Apr 16;7(3):231–234. doi: 10.1016/0014-5793(70)80168-5. [DOI] [PubMed] [Google Scholar]
- Brock T. D. Life at high temperatures. Evolutionary, ecological, and biochemical significance of organisms living in hot springs is discussed. Science. 1967 Nov;158(3804):1012–1019. doi: 10.1126/science.158.3804.1012. [DOI] [PubMed] [Google Scholar]
- Campbell L. L., Pace B. Physiology of growth at high temperatures. J Appl Bacteriol. 1968 Mar;31(1):24–35. doi: 10.1111/j.1365-2672.1968.tb00338.x. [DOI] [PubMed] [Google Scholar]
- Cazzulo J. J., Sundaram T. K., Kornberg H. L. Properties and regulation of pyruvate carboxylase from Bacillus stearothermophilus. Proc R Soc Lond B Biol Sci. 1970 Oct 13;176(1042):1–19. doi: 10.1098/rspb.1970.0030. [DOI] [PubMed] [Google Scholar]
- Daron H. H. Occurrence of isocitrate lyase in a thermophilic bacillus species. J Bacteriol. 1967 Feb;93(2):703–710. doi: 10.1128/jb.93.2.703-710.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson E. S., Sundaram T. K. Chromosomal location of a gene defining nicotinamide deamidase in Escherichia coli. J Bacteriol. 1970 Mar;101(3):1090–1091. doi: 10.1128/jb.101.3.1090-1091.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EL HAWARY M. F. S., THOMPSON R. H. S. Separation and estimation of blood keto acids by paper chromatography. Biochem J. 1953 Feb;53(3):340–347. doi: 10.1042/bj0530340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein I., Grossowicz N. Prototrophic thermophilic bacillus: isolation, properties, and kinetics of growth. J Bacteriol. 1969 Aug;99(2):414–417. doi: 10.1128/jb.99.2.414-417.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachimori A., Muramatsu N., Noso Y. Studies on an ATPase of thermophilic bacteria. I. Purification and properties. Biochim Biophys Acta. 1970 Jun 10;206(3):426–437. doi: 10.1016/0005-2744(70)90158-0. [DOI] [PubMed] [Google Scholar]
- Howard R. L., Becker R. R. Isolation and some properties of the triphosphopyridine nucleotide isocitrate dehydrogenase from Bacillus stearothermophilus. J Biol Chem. 1970 Jun;245(12):3186–3194. [PubMed] [Google Scholar]
- Humbert R. D., DeGuzman A., Fields M. L. Studies on variants of Bacillus stearothermophilus strain NCA 1518. Appl Microbiol. 1972 Apr;23(4):693–698. doi: 10.1128/am.23.4.693-698.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARGOLIN P. Genetic fine structure of the leucine operon in Salmonella. Genetics. 1963 Mar;48:441–457. doi: 10.1093/genetics/48.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta Y., Ogura Y., Wada A. Thermostable protease from thermophilic bacteria. I. Thermostability, physiocochemical properties, and amino acid composition. J Biol Chem. 1966 Dec 25;241(24):5919–5925. [PubMed] [Google Scholar]
- Ohta Y. Thermostable protease from thermophilic bacteria. II. Studies on the stability of the protease. J Biol Chem. 1967 Feb 10;242(3):509–515. [PubMed] [Google Scholar]
- Roncari G., Zuber H. Thermophilic aminopeptidases from Bacillus stearothermophilus. I. Isolation, specificity, and general properties of the thermostable aminopeptidase I. Int J Protein Res. 1969;1(1):45–61. doi: 10.1111/j.1399-3011.1969.tb01625.x. [DOI] [PubMed] [Google Scholar]
- SMITHIES O. Zone electrophoresis in starch gels: group variations in the serum proteins of normal human adults. Biochem J. 1955 Dec;61(4):629–641. doi: 10.1042/bj0610629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton R., Jr, Kimmel J. R., Amelunxen R. E. The amino acid composition and other properties of thermostable glyceraldehyde 3-phosphate dehydrogenase from Bacillus stearothermophilus. J Biol Chem. 1969 Mar 25;244(6):1623–1630. [PubMed] [Google Scholar]
- Sugimoto S., Noso Y. Thermal properties of fructose-I,6-diphosphate aldolase from thermophilic bacteria. Biochim Biophys Acta. 1971 Apr 14;235(1):210–221. doi: 10.1016/0005-2744(71)90049-0. [DOI] [PubMed] [Google Scholar]
- Sundaram T. K., Cazzulo J. J., Kornberg H. L. Anaplerotic CO2 fixation in mesophilic and thermophilic bacilli. Biochim Biophys Acta. 1969 Nov 18;192(2):355–357. doi: 10.1016/0304-4165(69)90377-8. [DOI] [PubMed] [Google Scholar]
- Sundaram T. K. Physiological role of pyruvate carboxylase in a thermophilic bacillus. J Bacteriol. 1973 Feb;113(2):549–557. doi: 10.1128/jb.113.2.549-557.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerkamp A., Mac Elroy R. D. Lactae dehydrogenase from an extremely thermophilic Bacillus. Arch Mikrobiol. 1972;85(2):113–122. doi: 10.1007/BF00409292. [DOI] [PubMed] [Google Scholar]