Abstract
Differentiation into castes and reproductive division of labour are a characteristics of eusocial insects. Caste determination occurs at an early stage of larval development in social bees and is achieved via differential nutrition irrespective of the genotype. Workers are usually subordinate to the queen and altruistically refrain from reproduction. Workers of the Cape honeybee (Apis mellifera capensis) do not necessarily refrain from reproduction. They have the unique ability to produce female offspring parthenogenetically (thelytoky) and can develop into ‘pseudoqueens’. Although these are morphologically workers, they develop a queen-like phenotype with respect to physiology and behaviour. Thelytoky is determined by a single gene (th) and we show that this gene also influences other traits related to the queen phenotype, including egg production and queen pheromone synthesis. Using 566 microsatellite markers, we mapped this gene to chromosome 13 and identified a candidate locus thelytoky, similar to grainy head (a transcription factor), which has been shown to be highly expressed in queens of eusocial insects. We therefore suggest that this gene is not only important for determining the pseudoqueen phenotype in A. m. capensis workers, but is also of general importance in regulating the gene cascades controlling reproduction and sterility in female social bees.
Keywords: thelytoky, pleiotropy, Apis mellifera
1. Introduction
In eusocial insects, one or a small number of reproductive females, termed queens, monopolize reproduction, whereas all other females are sterile workers (Wilson 1971). Differentiation into castes typically occurs at an early larval stage. It is usually achieved via differential larval feeding and genetic mechanisms underlying caste determination are rare (Helms Cahan & Keller 2003). Inclusive fitness theory (Hamilton 1964) has been used to explain the phenomenon of sterile altruistic workers aiding their mother queen in rearing her female offspring (the workers sisters). Additionally, potential intracolonial conflict over male production among workers is a phenomenon in insect societies, and worker policing (removal of worker laid eggs) is proposed as a mechanism that may resolve this conflict (Ratnieks 1988).
Worker policing fails in colonies with thelytokous worker reproduction (parthenogenetic production of female offspring; Moritz et al. 1999). Kin selection theory predicts selection for selfish individuals if workers produce female offspring (Hamilton 1964) parthenogenetically, because the relatedness benefits of altruistically rearing offspring disappear. The relatedness between a laying worker and her offspring is much higher (r∼1) than that among supersisters (r=0.75). Selection is driven by the potentially higher reproductive value of offspring developing into queens or pseudoqueens, when compared with worker produced males (Greeff 1996). A particularly suitable subject for studying the impact of thelytoky on social evolution is the Cape honeybee, Apis mellifera capensis. As predicted by theory (Hamilton 1964), selfish workers have evolved to become social parasites in thelytokously reproducing Cape honeybee workers (Neumann & Moritz 2002; Härtel et al. 2006). These parasitic workers enter colonies of the adjacent subspecies A. m. scutellata, kill the resident queen (Moritz et al. 2003), release queen substance (9-oxo-2-decenoic acid, 9-ODA) (Crewe & Velthuis 1980), suppress queen rearing and ovary development in the host workers and lay female eggs within a few days (Moritz et al. 2004).
The mode of parthenogenesis of selfish workers has been shown to be controlled by a single gene (th) segregating in a Mendelian fashion with th as a recessive allele (Lattorff et al. 2005). The ‘wild-type’ allele (+) is dominant and both th/+ and +/+ workers lay unfertilized eggs that produce drones parthenogenetically (arrhenotoky). In a simple breeding experiment, we tested whether this gene also controls selfish behaviour in the workers.
2. Material and methods
(a) Test cross and onset of oviposition
Heterozygous th/+ queens were backcrossed to single th drones using instrumental insemination. Sealed worker brood of these queens was kept in an incubator (60° r.h., 35°C) and freshly emerged workers (th/th or th/+) were individually labelled. Single workers were kept in cages with 50 young nurse bees (A. m. carnica), a piece of comb and honey and pollen ad libitum. The combs were monitored daily for the presence of eggs to determine the onset of oviposition. The experiment was terminated after 10 days. Workers and eggs were stored at −20°C until further analysis.
(b) Type of parthenogenesis
The type of parthenogenesis was determined by the ploidy of the eggs laid by workers (arrhenotoky=haploid, thelytoky=diploid). Five polymorphic microsatellite markers (A29, A35, A88, A113, IM) (Solignac et al. 2004), heterozygous in the laying workers, were used to genotype the eggs. Genotyping was done using a DNA sequencer (ABI 310).
(c) Queen substance
Heads of the workers were removed and fatty acids were extracted in dichloromethane. Extracts were evaporated to dryness using a flow of nitrogen. The residue was redissolved in an internal standard containing octanoic acid and tetradecane (not occurring in the mandibular gland secretion) and derivatized using bis-trimethyl-fluoroacetamide. One microlitre of this solution was injected into an HP 5890 gas chromatograph fitted with a split–splitless inlet and a 25 m×0.32 mm methyl silicone-coated fused silica capillary column. Helium was used as a carrier gas at a flow rate of 1 ml min−1, and the oven temperature was as follows: 60°C for 1 min; then heated at 50°C min−1 to 110°C; then 3°C min−1 from 110 to 220°C; and held at 220°C for 10 min. Chromatograms were recorded and peak areas quantified using HP ChemStation software; compounds were identified according to their retention times. The amounts of the queen substance (9-ODA) were calculated using relative mass ratios (Gehrke & Leimer 1971).
The quantitative phenotypes were classified into two qualitative groups using discriminant analysis (Statistica software package). A Fisher's exact-test was used to test the association of the phenotype with the genotype using the algorithm of the RxC v. 2.2 software (http://engels.genetics.wisc.edu/pstat/index.html) with 1 000 000 replicates.
(d) Bulked segregant analysis
DNA of workers was pooled according to their parthenogenetic phenotype. Using protocols previously described, 546 microsatellite loci were used (Solignac et al. 2004). PCR products were radiolabelled using dATP for amplifications and run on polyacrylamide sequencing gels. Loci showing a bias in inheritance were screened in individual samples. For the purpose of fine mapping, 20 additional microsatellite markers were developed directly from the genome sequence.
Carthagene software (Schiex & Gaspin 1997) was used for mapping the th gene. The markers were already genetically mapped (Solignac et al. 2004), thus a framework map with already established genetic distances was used. The th locus was then tested for integration into the existing map (command nicemapl). The gene was placed on the map at a position showing the largest LOD score for two-point estimations and between markers showing the lowest values of recombinant individuals.
(e) Identification of candidate genes
Candidate genes within the target region were identified using the genome assembly version 4.0 (Honeybee Genome Sequencing Consortium 2006). The physical region flanked by the closest microsatellite markers was analysed.
3. Results
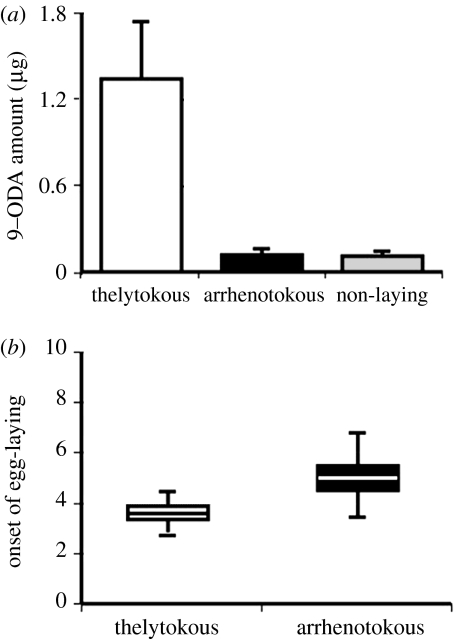
To analyse the effects of the th allele on worker reproduction, we tested offspring workers of a backcross th/+ queen and a single th drone (N=73 workers). Since the siring drone is haploid, all workers have identical paternal chromosomal sets and their relatedness is r=0.75. This ensured that we tested genotypically similar workers segregating for the alleles at the th locus. The type of parthenogenesis segregated in a Mendelian fashion with th/th workers producing female and th/+ workers producing male offspring (Lattorff et al. 2005). We determined reproductive dominance by analysing 9-ODA and the initiation of oviposition of the workers, as reliable indicators of reproductive dominance in honeybees (Crewe & Velthuis 1980). The amount of queen substance was about an order of magnitude higher in thelytokous (1.34±0.25 μg) than in arrhenotokous workers (0.12±0.001 μg; two-tailed t-test, t=2.8, d.f.=29, p<0.01; figure 1a). Thelytokous workers started oviposition more than one day earlier than arrhenotokous workers (3.61±0.14 days and 5.38±0.03 days, respectively, two-tailed t-test, t=3.718, d.f.=29, p<0.001; figure 1b). Thus, both indicators for reproductive success point towards the higher reproductive potential of the th/th workers.
Figure 1.
Reproductive traits of thelytokous (th/th, n=18) and arrhenotokous (th/+, n=13) workers. (a) The amount of 9-ODA produced by th/th workers was significantly higher than for th/+ workers. The latter were not significantly different from non-laying workers. Error bars, s.e. (b) The onset of oviposition was more than 1 day earlier in the th/th workers than in the th/+ workers. Boxes, s.e.; error bars, s.d.
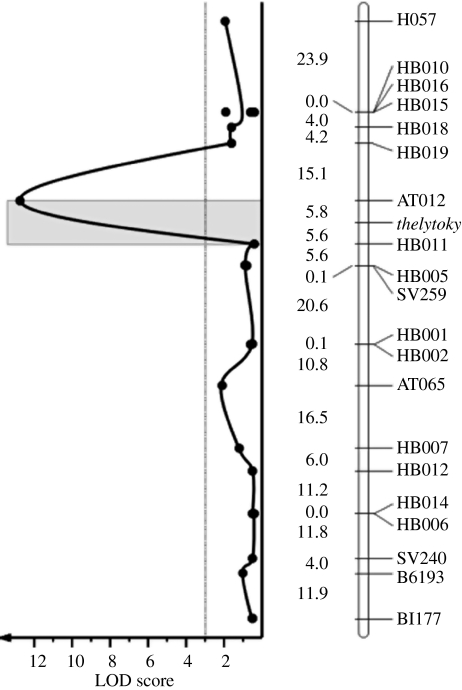
Bulked segregant analysis using 546 mapped microsatellite markers (Solignac et al. 2004) indicated that a single genomic region on chromosome 13 was associated with the type of parthenogenesis. Fine mapping using 20 newly developed markers decreased the target region to 11.4 cM, corresponding to 180 kb (figure 2). Analysis of the genome sequence (Honeybee Genome Sequencing Consortium 2006) showed 15 predicted genes within this region (table 2). The quantitative differences between thelytokous and arrhenotokous workers are so high that they can be transformed into a qualitative binary system of high and low pheromone production and early and late ovary activation (table 1) to analyse the data with a classical Mendelian segregation scheme. There is an almost complete co-segregation of the traits with the type of parthenogenesis suggesting that the thelytoky gene affects all three traits pleiotropically. The alternative explanation that several closely linked genes determine the type of parthenogenesis, pheromone production and ovary activation seems highly unlikely. Owing to the extremely high recombination rate of the honeybee genome 41 kb cM−1 (Solignac et al. 2004), even genes that are physically close are expected to lack linkage disequilibrium. The recombination frequency within that region is considerably higher (approx. 20 kb cM−1) than the genome average. A single gene influencing all three traits pleiotropically is the most parsimonious explanation of the results.
Figure 2.
Genetic map of the thelytoky region. Marker names are displayed on the right, genetic distances (cM) between markers are given on the left. The graph shows the LOD scores for two-point comparisons of the respective marker and the thelytoky gene.
Table 2.
Candidate genes of thelytoky gene region. (Nomenclature is used as in the database at NCBI for Apis mellifera build 4.0 (http://www.ncbi.nlm.nih.gov/genome/guide/bee/)).
| gene | species | conserved domain | function |
|---|---|---|---|
| Atf2 | M. mus. | — | transcription factor |
| similar to CG13921 | T. cas. | — | unknown |
| PTP | T. cas. | protein tyrosine phosphatase | phosphatase |
| similar to CG10927 | T. cas. | cytosine/adenosine deaminases | nucleotide metabolism |
| gemini | T. cas. | CP2 | transcription factor |
| similar to CG13210 | A. aeg. | — | unknown |
| dUTP pyrophosphatase | A. gam. | dUTPase | dUTP metabolism |
| similar to CG11333 | A. gam. | YcaC-related amidohydrolases | unknown |
| tungus | T. cas. | — | learning and memory |
| pawn | T. cas. | calcium-binding EGF-like domain | phototransduction |
| cyclin-dependent kinase 6 | T. cas. | serine/threonine protein kinases | cell cycle |
| similar to CG2862 | D. mel. | protein kinase C | nucleotide phosphatase activity |
| interacting protein-related | |||
| dihydrofolate reductase | A. gam. | dihydrofolate reductase | DNA metabolism |
| hypothetical protein LOC408407 | — | — | unknown |
| similar to CG9005 | T. cas. | — | unknown |
Table 1.
Pleiotropic effects of the th locus. (Fisher's exact-test revealed a highly significant deviation from a random association of phenotypic effects (p<10−6).
| th/th | +/th | |||
|---|---|---|---|---|
| type of parthenogenesis | thelytokous | arrhenotokous | thelytokous | arrhenotokous |
| amount of 9-ODA | 18 | 0 | 0 | 13 |
| high | low | high | low | |
| 18 | 0 | 0 | 13 | |
| onset of oviposition | early | late | early | late |
| 18 | 0 | 3 | 10 |
4. Discussion
Single genes determining a large suite of phenotypic characteristics are not rare in biological systems. For example, the mammalian sex determination system is strongly dominated by the presence of the transcription factor SRY, which influences all subsequent cascades implicated in sex determination (Brennan & Capel 2004). In general, transcription factors are known to interfere simultaneously in various different gene cascades (Levine & Tjian 2003). In the candidate gene list (table 2), we can identify two transcription factors as potential candidates, but also genes for signal transduction (PTP) or cell cycle regulation (CDK6) as putative candidate genes. Of the two transcription factors, one belongs to the CP2-family (Lee & Adler 2004), similar to the Drosophila homologues grainy head and gemini, and another one is similar to Atf2 identified in vertebrates. In an independent expression study, the gene grainy head has been shown to be exclusively expressed in queens of Melipona quadrifasciata (Judice et al. 2006), a stingless bee with a genetic caste determination system (Kerr 1950). Evans & Wheeler (2000) reported a CP2 transcription factor, which was overexpressed in honeybee queens. The current evidence therefore strongly supports the notion that thelytoky is homologous to grainy head and a gene which is of fundamental importance for determination of caste in social bees.
Why has the th- allele and selfish worker behaviour not become more widespread if it has such clear selective advantage for its bearer? Selection not only operates at the gene or individual level but also at the level of colonies in social insects (Moritz 1989). In the case of the honeybee, the frequency of parasitic workers in the colony is negatively correlated with colony reproductive success. Parasitized colonies eventually fail, because the parasitic workers do not engage in brood rearing (Hillesheim et al. 1989). Although selfish behaviour gives a fitness advantage for the individual worker in the colony, it is not an evolutionary stable strategy, because colonies of selfish workers do not produce queens or drones and can only maintain themselves by invading new host colonies. A similar mechanism may also maintain ‘royal alleles’ in non-thelytokous honeybees (Moritz et al. 2005). Individual fitness of female honeybees therefore has a stronger genetic variance than previously thought and will also depend on the effects of the th gene, which controls the reproductive potential of its bearer.
Acknowledgments
We thank B. Springer for artificial inseminations. The VW foundation (R.F.A.M., R.M.C.), D.F.G. (R.F.A.M.), NRF (R.M.C.) and EU-BABE network (R.F.A.M., M.S.) granted financial support.
Supplementary Material
Characteristics of newly developed microsatellite loci of Apis mellifera L.
References
- Brennan J, Capel B. One tissue, two fates: molecular genetic events that underlie testis versus ovary development. Nat. Rev. Genet. 2004;5:509–521. doi: 10.1038/nrg1381. doi:10.1038/nrg1381 [DOI] [PubMed] [Google Scholar]
- Crewe R.M, Velthuis H.H.W. False queens—a consequence of mandibular gland signals in worker honeybees. Naturwissenschaften. 1980;67:467–469. doi:10.1007/BF00405650 [Google Scholar]
- Evans J.D, Wheeler D.E. Expression profiles during honeybee caste determination. Genome Biol. 2000;2:1–6. doi: 10.1186/gb-2000-2-1-research0001. doi:10.1186/gb-2000-2-1-research0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrke C.W, Leimer K. Trimethylsilylation of amino acids derivatization and chromatography. J. Chrom. 1971;57:219–238. doi: 10.1016/0021-9673(71)80035-3. doi:10.1016/0021-9673(71)80035-3 [DOI] [PubMed] [Google Scholar]
- Greeff J.M. Effects of thelytokous worker reproduction on kin-selection and conflict in the Cape honeybee, Apis mellifera capensis. Phil. Trans. R. Soc. B. 1996;351:617–625. doi:10.1098/rstb.1996.0060 [Google Scholar]
- Hamilton W.D. The genetical evolution of social behaviour, II. J. Theor. Biol. 1964;7:17–52. doi: 10.1016/0022-5193(64)90039-6. doi:10.1016/0022-5193(64)90039-6 [DOI] [PubMed] [Google Scholar]
- Härtel S, Neumann P, Raassen F.S, Moritz R.F.A, Hepburn H.R. Social parasitism by Cape honeybee workers in colonies of their own subspecies (Apis mellifera capensis Esch.) Insect. Soc. 2006;53:183–193. doi:10.1007/s00040-005-0857-2 [Google Scholar]
- Helms Cahan S, Keller L. Complex hybrid origin of genetic caste determination in harvester ants. Nature. 2003;424:306–309. doi: 10.1038/nature01744. doi:10.1038/nature01744 [DOI] [PubMed] [Google Scholar]
- Hillesheim E, Koeniger N, Moritz R.F.A. Colony performance in honeybees (Apis mellifera capensis Esch.) depends on the proportion of subordinate and dominant workers. Behav. Ecol. Sociobiol. 1989;24:291–296. doi:10.1007/BF00290905 [Google Scholar]
- Honeybee Genome Sequencing Consortium. Insights into social insects from the genome of the honeybee Apis mellifera. Nature. 2006;443:931–949. doi: 10.1038/nature05260. doi:10.1038/nature05260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judice C.C, Carazzole M.F, Festa F, Sogayar M.C, Hartfelder K, Pereira G.A.G. Gene expression profiles underlying alternative caste phenotypes in a highly eusocial bee, Melipona quadrifasciata. Insect Mol. Biol. 2006;15:33–44. doi: 10.1111/j.1365-2583.2005.00605.x. doi:10.1111/j.1365-2583.2005.00605.x [DOI] [PubMed] [Google Scholar]
- Kerr W.E. Genetic determination of caste in the genus Melipona. Genetics. 1950;35:143–152. doi: 10.1093/genetics/35.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattorff H.M.G, Moritz R.F.A, Fuchs S. A single locus determines thelytokous parthenogenesis of laying honeybee workers (Apis mellifera capensis) Heredity. 2005;94:533–537. doi: 10.1038/sj.hdy.6800654. doi:10.1038/sj.hdy.6800654 [DOI] [PubMed] [Google Scholar]
- Lee H.Y, Adler P.N. The grainy head transcription factor is essential for the function of the frizzled pathway in the Drosphilla wing. Mech. Dev. 2004;121:37–49. doi: 10.1016/j.mod.2003.11.002. doi:10.1016/j.mod.2003.11.002 [DOI] [PubMed] [Google Scholar]
- Levine M, Tjian R. Transcription regulation and animal diversity. Nature. 2003;424:147–151. doi: 10.1038/nature01763. doi:10.1038/nature01763 [DOI] [PubMed] [Google Scholar]
- Moritz R.F.A. Colony level and within colony level selection in honeybees. Behav. Ecol. Sociobiol. 1989;25:437–444. doi:10.1007/BF00300190 [Google Scholar]
- Moritz R.F.A, Kryger P, Allsopp M.H. Lack of worker policing in the Cape honeybee (Apis mellifera capensis) Behaviour. 1999;136:1079–1092. doi:10.1163/156853999501766 [Google Scholar]
- Moritz R.F.A, Lattorff H.M.G, Crewe R.M. Honeybee workers (Apis mellifera capensis) compete for producing queen-like pheromone signals. Proc. R. Soc. B. 2004;271:S98–S100. doi: 10.1098/rsbl.2003.0113. doi:10.1098/rsbl.2003.0113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz R.F.A, Lattorff H.M.G, Neumann P, Kraus F.B, Radloff S.E, Hepburn H.R. Rare royal families in honeybees, Apis mellifera. Naturwissenschaften. 2005;92:488–491. doi: 10.1007/s00114-005-0025-6. doi:10.1007/s00114-005-0025-6 [DOI] [PubMed] [Google Scholar]
- Moritz R.F.A, Pflugfelder J, Crewe R.M. Lethal fighting between honeybee queens and parasitic workers (Apis mellifera) Naturwissenschaften. 2003;90:378–381. doi: 10.1007/s00114-003-0445-0. doi:10.1007/s00114-003-0445-0 [DOI] [PubMed] [Google Scholar]
- Neumann P, Moritz R.F.A. The Cape honeybee phenomenon: the sympatric evolution of a social parasite in real time? Behav. Ecol. Sociobiol. 2002;52:271–281. doi:10.1007/s00265-002-0518-7 [Google Scholar]
- Ratnieks F.L.W. Reproductive harmony via mutual policing by workers in eusocial Hymenoptera. Am. Nat. 1988;132:217–236. doi:10.1086/284846 [Google Scholar]
- Schiex T, Gaspin C. Carthagene: constructing and joining maximum likelihood genetic maps. Proc. Int. Conf. Intell. Syst. Mol. Biol. 1997;5:258–267. [PubMed] [Google Scholar]
- Solignac M, Vautrin D, Baudry E, Mougel F, Loiseau A, Cornuet J.M. A microsatellite-based linkage map of the honeybee, Apis mellifera L. Genetics. 2004;167:253–262. doi: 10.1534/genetics.167.1.253. doi:10.1534/genetics.167.1.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson E.O. Harvard University Press; Cambridge, MA: 1971. The insect societies. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristics of newly developed microsatellite loci of Apis mellifera L.