Abstract
Many studies of social species have reported variation in the anti-predator vigilance behaviour of foraging individuals depending on the presence and relative position of other group members. However, little attention has focused on how foragers assess these variables. It is commonly assumed that they do so visually, but many social species produce frequent calls while foraging, and these ‘close’ calls might provide valuable spatial information. Here, we show that foraging pied babblers (Turdoides bicolor) are less vigilant when in larger groups, in the centre of a group and in closer proximity to another group member. We then show that foragers are less vigilant during playbacks of close calling by more individuals and individuals on either side of them when compared with calls of fewer individuals and calls on one side of them. These results suggest that foragers can use vocal cues to gain information on group size and their spatial position within a group. Future studies of anti-predator vigilance should consider the relative importance of both visual and vocal monitoring of group members.
Keywords: close calling, anti-predator vigilance, vocal communication, social foraging, social monitoring, vocal cues
1. Introduction
In socially foraging species, individuals tend to be less vulnerable to predation in larger groups, in the centre of a group and when closer to other group members (Krause & Ruxton 2002). Consequently, many studies have reported a reduction in vigilance by individuals foraging in these situations (see Krause & Ruxton 2002; Beauchamp 2003 for reviews; but Treves 2000 for exceptions). In contrast to the vast literature examining variation in the vigilance of group members, few studies have considered how these individuals assess the key variables to which they adjust their vigilance, specifically the size of their group and their spatial position and proximity to others within it (Beauchamp 2003).
It is commonly assumed that individuals gain information about the presence and position of other group members through visual scanning (e.g. Fernández-Juricic et al. 2004). However, many social birds and mammals give frequent ‘close’ calls while foraging (Palombit et al. 1999). Although these calls may have evolved for a different primary function, e.g. the regulation of spacing between foraging competitors (Radford 2004), they could also provide valuable information on the spatial position of other group members. The possibility that vocal cues might be used by foragers when deciding how much time to spend vigilant for predators is largely unexplored.
The pied babbler (Turdoides bicolor), a cooperatively breeding bird from southern Africa, provides an ideal opportunity to investigate whether individuals in foraging groups use close calls to assess the presence and the position of other group members and whether this information influences their vigilance behaviour. The 3–11 individuals in a pied babbler group generally forage on the ground within 20 m of one another (Radford & Ridley 2006), making it possible to monitor simultaneously the position of all group members. All foraging individuals produce frequent (4–20 min−1) close calls, termed ‘chucks’ (Radford & Ridley submitted). Groups can be habituated to the close presence of observers, facilitating clear observations and playback experiments to test directly the importance of vocal cues.
Here, we address two main questions. First, is the vigilance behaviour of foraging pied babblers influenced by their group size, their spatial position within a group and the proximity of other group members? Second, do vocal cues (specifically chuck calls) signalling the presence and position of other group members influence the vigilance behaviour of foragers?
2. Material and methods
(a) Observational data
Fieldwork was carried out in the southern Kalahari, South Africa (26°58′ S, 21°49′ E) on 12 colour-ringed habituated groups of pied babblers (mean±s.e. group size 6.3±0.8, range 2–11). We conducted 5 min focal watches on foraging individuals (mean±s.e. watches per individual 35±4, range 10–93, n=84 individuals). Before each focal watch, we recorded ‘foraging group size’, thus omitting individuals that were temporarily missing (e.g. incubating). During focal watches, we recorded the start and end of each vigilance bout (when the focal individual scanned the surrounding area with its head up rather than foraging with its head lowered). Whenever a focal individual moved to a new ‘foraging patch’ (i.e. moved more than 20 cm between foraging attempts), we estimated the distance to its nearest neighbour (0–2, 2–5, 5–10, greater than 10 m) and recorded its spatial position in the group (‘centre’ or ‘edge’).
To assess the variables influencing the proportion of time spent vigilant, we used a linear mixed model (LMM) based on 2931 focal watches from 84 individuals in 12 groups. We used as the response term the proportion of time spent vigilant during the first period of a focal watch in which the focal individual spent more than 30 s within the same nearest-neighbour distance category and spatial position. See electronic supplementary material for further details.
(b) Playback experiments
We constructed playback loops using Wavelab v. 2 (Steinberg Media Technologies, Hamburg, Germany) by editing recordings of natural calls. Playbacks were of calls from members of the focal individual's group, and there was no temporal overlap of calls from different individuals. The focal individual was required to be foraging at least 10 m away from the rest of the group before playback commenced, and in each trial, we recorded the proportion of time spent vigilant by the focal individual during the playback. All three experiments were conducted on 12 adults from different groups and the order of trial presentation within an experiment was randomized. See electronic supplementary material for further details.
(i) Experiment 1
To test whether foragers use chuck calling to assess group size and whether these vocal cues influence their vigilance behaviour, individuals were presented with three trials: 1 min of background noise; 1 min of chuck calling by one individual (rate=18 calls per min); and 1 min of chuck calling by three different individuals (each at 6 calls per min, total rate=18 calls per min). Playbacks were from a single speaker 2 m from the focal individual.
(ii) Experiment 2
To test whether individuals use chuck calling to assess their spatial position (centre versus edge) and whether these vocal cues influence their vigilance behaviour, individuals were presented with two trials. Both involved 1 min of chuck calling by two different individuals (each calling at 18 calls per min) played from two speakers 2 m from the focal individual. In one trial, the speakers were next to one another on one side of the focal individual; in the other, the speakers were on opposite sides of the focal individual.
(iii) Experiment 3
To test whether individuals use chuck calling to assess the proximity of other group members and whether these vocal cues influence their vigilance behaviour, individuals were presented with three trials. Each involved 1 min of chuck calling by one individual (rate=18 calls per min), from a speaker 1, 3 or 5 m from the focal individual.
3. Results
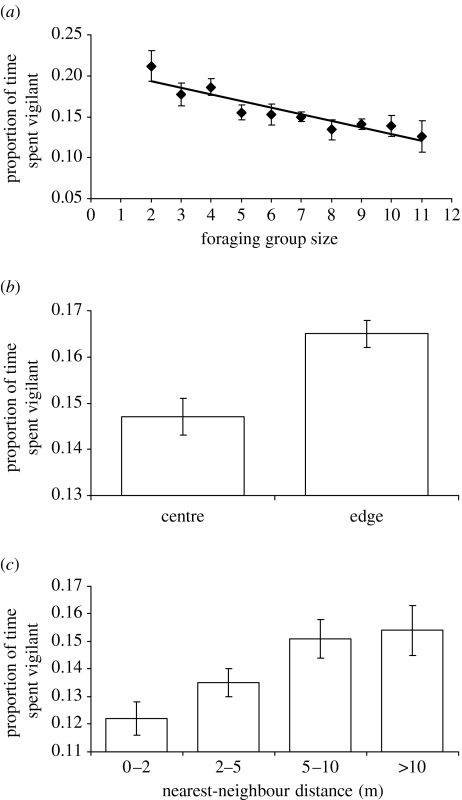
Individuals in larger groups, in the centre of a group and in closer proximity to another group member spent significantly less time vigilant than those in smaller groups, on the edge of a group and further away from other group members (table 1, figure 1). Individuals also spent significantly less time vigilant when there were no dependent fledglings in the group (dependent fledglings present 0.17±0.03; no dependent fledglings 0.13±0.02; table 1), and when the daily temperature was higher (table 1).
Table 1.
Terms affecting the proportion of time spent vigilant by foraging individuals. (Group and individual identity were included as random terms in the LMM.)
| full model | d.f. | Χ2 | p |
|---|---|---|---|
| foraging group size | 1 | 22.86 | <0.001 |
| spatial position (centre, edge) | 1 | 12.83 | <0.001 |
| presence of dependent fledglings (yes, no) | 1 | 11.91 | <0.001 |
| maximum daily temperature (°C) | 1 | 5.36 | 0.021 |
| nearest-neighbour distance (0–2, 2–5, 5–10, greater than 10 m) | 3 | 9.67 | 0.022 |
| status (dominant adult, subordinate adult, independent fledgling) | 2 | 1.46 | 0.482 |
| body weight (g) | 1 | 0.09 | 0.764 |
| sex | 1 | 0.07 | 0.792 |
| month | 5 | 2.23 | 0.816 |
| total rainfall in the preceding week (mm) | 1 | 0.01 | 0.907 |
| minimal model | effect | s.e. | |
|---|---|---|---|
| constant | 0.366 | 0.007 | |
| foraging group size | −0.008 | 0.002 | |
| spatial position | |||
| centre | 0 | 0 | |
| edge | 0.025 | 0.005 | |
| presence of dependent fledglings | |||
| no | 0 | 0 | |
| yes | 0.023 | 0.007 | |
| maximum daily temperature (°C) | −0.001 | 0.001 | |
| nearest-neighbour distance (m) | |||
| 0–2 | 0 | 0 | |
| 2–5 | 0.012 | 0.009 | |
| 5–10 | 0.031 | 0.006 | |
| >10 | 0.036 | 0.013 |
Figure 1.
The influence of (a) foraging group size, (b) spatial position and (c) nearest-neighbour distance on the mean (±s.e.) proportion of time spent vigilant by 84 foraging pied babblers.
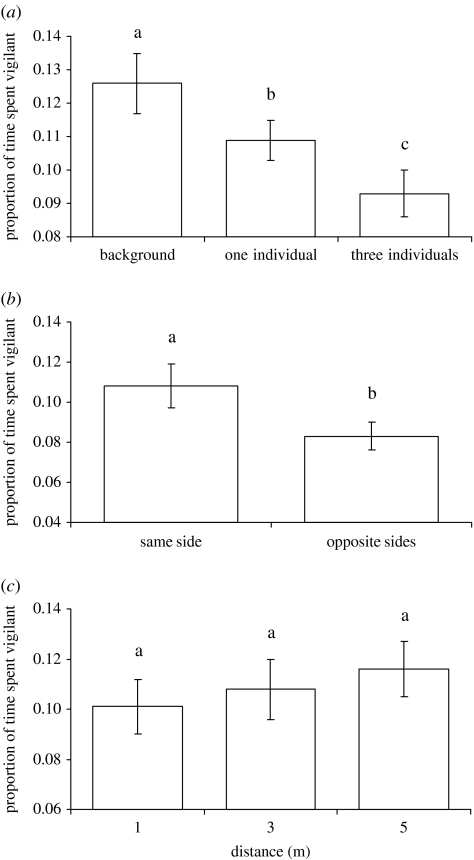
Foragers were significantly less vigilant when there were more individuals in the playback (experiment 1, Friedman test: Χ22=7.68, p=0.021; figure 2a) and when playback of two individuals occurred on opposite sides of them when compared with those on the same side (experiment 2, Wilcoxon test: W=51.0, N=12, p=0.019; figure 2b). There was no significant difference in the amount of time spent vigilant by foragers when chuck calling was played back at different distances (experiment 3, Friedman test: Χ22=1.35, p=0.510; figure 2c).
Figure 2.
Mean (±s.e.) proportion of time spent vigilant by 12 adult pied babblers during experimental playbacks of (a) background noise, one individual chuck calling and three individuals chuck calling, (b) two individuals chuck calling on the same side or opposite sides of the focal bird, and (c) chuck calling of one individual at different distances. Letters above bars indicate which trials differed significantly.
4. Discussion
As expected from theoretical models (e.g. Pulliam 1973) and previous empirical studies (Krause & Ruxton 2002; Beauchamp 2003), foraging pied babblers were less vigilant when in larger groups, in the centre of a group and in closer proximity to another group member. Our results are consistent with the idea that individuals foraging in these circumstances are less vulnerable to predation (Krause & Ruxton 2002). Theoretically, individuals in larger groups might increase their foraging time owing to increased potential competition, which might translate into a decrease in vigilance. However, group-size effects have been shown to remain even when competition for food is eliminated (Lima et al. 1999). We believe, therefore, that our results represent a change in anti-predator vigilance. Playback experiments suggested that foragers adjust their vigilance depending on cues provided by the chuck calling of others: they were less vigilant in response to the playbacks of more individuals and when they were between two speakers, although not when a speaker was closer.
It has been generally assumed that individuals in foraging groups use visual scanning to assess their need for anti-predator vigilance (Fernández-Juricic et al. 2004). Recent studies have demonstrated that some species can even use their peripheral vision for this task, preventing the need to suspend foraging while monitoring group mates (e.g. Bednekoff & Lima 2005). However, many species forage in habitats (e.g. forests) where lines of sight may be regularly obscured. Two previous studies have hinted that vocalizations might prove useful when assessing the need for anti-predator vigilance in such habitats. Downy woodpeckers (Picoides pubescens) appear to use close calls to determine the presence of other individuals when in mixed-species flocks (Sullivan 1984), while Diana monkeys (Cercopithecus diana) may use auditory cues to obtain information about the location of out-of-sight group members (Uster & Zuberbühler 2001). Although pied babblers live in a more open habitat, individuals spend much of their time foraging with their head in a hole, searching for prey beneath the sand's surface. Consequently, their peripheral vision may be greatly reduced and they would need to remove their head from the hole to monitor other group members visually. In such situations, using vocal cues to assess the presence of other group members would prevent the need to suspend foraging.
Our playback results suggest that pied babblers can assess group size and spatial position from the close calling of others. Since call rates were matched between trials, the implication is that individuals have distinct calls (Dhondt & Lambrechts 1992). Vocal cues may prove useful in some ways, therefore, but they are unlikely to provide all the information necessary for foragers when assessing the need for anti-predator vigilance. For example, experiment 3 suggested that the proximity of another individual is difficult to determine from vocal cues. Although sound attenuates with distance and this change is used as a ranging cue by several species involved in long-distance communication (Naguib & Wiley 2001), acoustic differences over short distances, such as those tested in this study, may be too subtle to be useful. Close calls are also unlikely to provide information on the behaviour (e.g. the alertness) of other individuals. In contrast to ‘sentinel’ calls, which announce the presence of an individual devoted to vigilance (Manser 1999), the chuck calling of foraging pied babblers may simply indicate the presence of others and therefore the likelihood of shared vigilance. Visual scanning may be required if information on the proximity and behaviour of others is important.
Close calls are given frequently by foraging individuals in many social species (e.g. Palombit et al. 1999; Radford 2004), but their potential usefulness when assessing how much time to devote to anti-predator vigilance has been generally overlooked. The pied babbler chuck call is likely to have evolved for another function (the regulation of spacing between foraging competitors; Radford & Ridley in review), but our study suggests that individuals may modify their vigilance behaviour depending on the information provided by close calls. Although such vocal cues are likely to be most important when visual scanning is difficult, future studies of anti-predator vigilance should consider the relative importance of both for effective social monitoring.
Acknowledgments
We thank Mr and Mrs Kotze, the Kalahari Research Trust and Tim Clutton-Brock for access to land, the Northern Cape Conservation Authority for research permission, Isa-Rita Russo for molecular sexing, Nichola Raihani for help in establishing the habituated population and Tim Fawcett, Sarah Hodge, Linda Hollén and Andy Young for their helpful comments on the manuscript. A.N.R. was funded by an ASAB grant and a JRF from Girton College, Cambridge. A.R.R. was funded by the PFIAO Centre of Excellence, University of Cape Town.
Supplementary Material
References
- Beauchamp G. Group-size effects on vigilance: a search for mechanisms. Behav. Process. 2003;63:111–121. doi: 10.1016/s0376-6357(03)00002-0. doi:10.1016/S0376-6357(03)00002-0 [DOI] [PubMed] [Google Scholar]
- Bednekoff P.A, Lima S.L. Testing for peripheral vigilance: do birds value what they see when not overtly vigilant? Anim. Behav. 2005;69:1165–1171. doi:10.1016/j.anbehav.2004.07.020 [Google Scholar]
- Dhondt A.A, Lambrechts M.M. Individual recognition in birds. Trends Ecol. Evol. 1992;7:178–179. doi: 10.1016/0169-5347(92)90068-M. doi:10.1016/0169-5347(92)90068-M [DOI] [PubMed] [Google Scholar]
- Fernández-Juricic E, Erichsen J.T, Kacelnik A. Visual perception and social foraging in birds. Trends Ecol. Evol. 2004;19:25–31. doi: 10.1016/j.tree.2003.10.003. doi:10.1016/j.tree.2003.10.003 [DOI] [PubMed] [Google Scholar]
- Krause J, Ruxton G.D. Oxford University Press; Oxford, UK: 2002. Living in groups. [Google Scholar]
- Lima S.L, Zollner P.A, Bednekoff P.A. Predation, scramble competition, and the group size effect in anti-predator vigilance. Behav. Ecol. Sociobiol. 1999;46:110–116. doi:10.1007/s002650050599 [Google Scholar]
- Manser M.B. Response of foraging group members to sentinel calls in suricates, Suricata suricatta. Proc. R. Soc. B. 1999;266:1013–1019. doi:10.1098/rspb.1999.0737 [Google Scholar]
- Naguib M, Wiley R.H. Estimating the distance to a source of sound: mechanisms and adaptations for long-range communication. Anim. Behav. 2001;62:825–837. doi:10.1006/anbe.2001.1860 [Google Scholar]
- Palombit R.A, Cheney D.L, Seyfarth R.M. Male grunts as mediators of social interaction with females in wild chacma baboons (Papio cynocephalus ursinus) Behaviour. 1999;136:221–242. doi:10.1163/156853999501298 [Google Scholar]
- Pulliam H.R. On the advantage of flocking. J. Theor. Biol. 1973;38:419–422. doi: 10.1016/0022-5193(73)90184-7. doi:10.1016/0022-5193(73)90184-7 [DOI] [PubMed] [Google Scholar]
- Radford A.N. Vocal mediation of foraging competition in the cooperatively breeding green woodhoopoe, Phoeniculus purpureus. Behav. Ecol. Sociobiol. 2004;56:279–285. doi:10.1007/s00265-004-0785-6 [Google Scholar]
- Radford A.N, Ridley A.R. Recruitment calling: a novel form of extended parental care in an altricial species. Curr. Biol. 2006;16:1700–1704. doi: 10.1016/j.cub.2006.06.053. doi:10.1016/j.cub.2006.06.053 [DOI] [PubMed] [Google Scholar]
- Radford, A. N & Ridley, A. R. Submitted. Close calling regulates spacing between foraging competitors in the group-living pied babbler.
- Sullivan K.A. Information exploitation by downy woodpeckers in mixed-species flocks. Behaviour. 1984;91:294–311. [Google Scholar]
- Treves A. Theory and method in studies of vigilance and aggregation. Anim. Behav. 2000;60:711–722. doi: 10.1006/anbe.2000.1528. doi:10.1006/anbe.2000.1528 [DOI] [PubMed] [Google Scholar]
- Uster D, Zuberbühler K. The functional significance of Diana monkey ‘clear calls’. Behaviour. 2001;138:741–756. doi:10.1163/156853901752233389 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.