Abstract
Chronic stressors such as caregiving have been associated with reduced antibody production after vaccination and elevated interleukin (IL)-6 in older adults. However, individual differences in repetitive thought, that is, frequent or prolonged thought about oneself and one’s world, can modify perception and effects of stress. For example, worry during stressful circumstances has been associated with poorer immune outcomes, whereas cognitive processing has been associated with better outcomes. The present study tested the relationship of caregiving and two types of repetitive thought, negative (e.g., worry) and neutral (e.g., reflection), to pre- and post-influenza vaccine antibody and IL-6. Dementia caregivers (n = 14) and controls (n = 30) were interviewed and had blood drawn pre- and post-vaccine in a multi-wave study. Multi-level models found that caregivers had higher IL-6 than controls after vaccination (t(23) = 2.36, p < .05). There were several interactions between caregiver status and repetitive thought in predicting both depression and immune responses to vaccination. Among caregivers, negative repetitive thought predicted more depression and lower antibody titers, whereas neutral repetitive thought predicted less depression and higher antibody titers, but also higher post-vaccination IL-6. Among controls, negative repetitive thought predicted more depression but higher antibody titers, whereas neutral repetitive thought predicted less depression and lower post-vaccination IL-6. In mediational tests, depression did not account for the effects of repetitive thought. Results generally support beneficial effects of neutral repetitive thought and detrimental effects of negative repetitive thought, but those effects may be reduced or even reversed depending on life circumstances.
Keywords: caregiving, depression, antibody, interleukin-6, worry, rumination, reflection, processing, stress, aging, vaccination
Stressful life events are associated with changes in the human immune system such as lower proliferative, cytotoxic, and antibody responses by lymphocytes, and age may increase vulnerability to stressor-related immune change (Segerstrom and Miller, 2004). Caregiving is perhaps the most frequently investigated stressor in older adults with regard to health and immune outcomes. Caregiving for dementia patients increases the risk for early mortality (Schulz and Beach, 1999), and caregiving is associated with suppression of potentially beneficial immune functions, including lymphocyte proliferation, interleukin (IL)-2 production, natural killer cell cytotoxicity, and response to vaccines (Segerstrom and Miller, 2004; Vitaliano et al., 2003).
Caregivers also have elevated inflammatory cytokines, particularly IL-6. Caregiving stress has associated with higher basal IL-6 and a larger increase in basal IL-6 over time (Kiecolt-Glaser et al., 2003; Lutgendorf et al., 1999). IL-6 has been implicated as an important component of stress-related physiological dysregulation (Mastorakos and Ilias, 2006). Higher IL-6 associated with caregiving is particularly relevant for older adults’ health, as elevated serum IL-6 increases morbidity for a number of diseases associated with aging, including cardiovascular disease, myeloma, osteoporosis, and Alzheimer’s disease. It also contributes to aspects of frailty in old age, including decreased lean body mass and anemia (see Ershler and Keller, 2000; Robles et al., 2005; Papanicolaou et al., 1998, for reviews).
However, the health consequences of caregiving are only loosely related to qualities of the caregiving experience itself. For example, duration and degree of caregiving (e.g., whether the patient is living at home) and patient impairment are typically not related to caregivers’ psychological adjustment (Schulz et al., 1995). Patient institutionalization did not improve caregiver health and adjustment in a 2-year longitudinal study, and spouses of institutionalized patients were characterized as more “at risk” immunologically than spouses caring for patients at home (Kiecolt-Glaser et al., 1991; Lieberman and Fisher, 2001). These studies indicate that although caregiving is itself stressful, there are individual differences among caregivers that may be important in determining their psychological and physiological reactions to caregiving.
The present study examines the relationship of repetitive thought to antibody and serum IL-6 responses to influenza vaccination among dementia caregivers and non-caregivers. Repetitive thought is defined as frequent, prolonged thoughts about oneself and one’s environment (Segerstrom et al., 2003). Many types of repetitive thought, such as worry and rumination, are negative insofar as they seem to compromise psychological adjustment and have adverse effects on immunity. For example, among individuals who had experienced the 1994 Northridge earthquake, higher levels of trait worry were associated with lower numbers of NK cells up to 5 months following the earthquake (Segerstrom et al., 1998). Worry has also been associated with lower helper T cell counts in men infected with HIV (Segerstrom and Kemeny, 2006). One study of older adults reported that intrusive, negative thoughts were inversely related to cytokine-stimulated natural killer cell activity in bereaved caregivers (Esterling et al., 1994).
Not all repetitive thought is detrimental, however. Adaptive forms of repetitive thought, such as cognitive and emotional processing, are characterized by controllable process and less negative content and are associated with improvements in psychological adjustment and self-reported health (Segerstrom et al., 2003; Stanton et al., 2000). Bower and colleagues (1998) found that HIV seropositive gay men who engaged in cognitive processing after bereavement were more likely to find positive meaning in the death. Finding meaning, in turn, was associated with maintenance of CD4+ T cell counts and decreased mortality risk at follow-up.
Both the amount and kind of repetitive thought in which people engage may largely result from individual differences in stable repetitive thought styles. Measures of how people typically think, such as the Penn State Worry Questionnaire, report high test-retest stability (Molina and Borkovec, 1994), and measures of “trait” repetitive thought correlate with personality traits such as neuroticism and openness to experience (Segerstrom et al., 2003). However, although there appear to be stable repetitive thought styles, the effects of these styles may be context-dependent. For example, HIV seropositive men who had negative repetitive thoughts about their disease had the fastest disease progression if they also experienced bereavement (Reed et al., 1999). Therefore, the psychosocial context, such as bereavement or caregiving, may moderate the effects of repetitive thought on immunity.
We predicted that, consistent with previous research, caregiving would be associated with lower antibody response to vaccination and to higher serum IL-6 both pre- and post-influenza vaccination. Mean increases in antibody responses are expected after vaccination, but mean increases in serum IL-6 are not expected, and IL-6 may even decrease after vaccination (Krakauer and Russo, 2001). However, variability in this response means that some people may have lower IL-6 after vaccination, whereas others may have higher IL-6, and these differences may be related to psychosocial factors (e.g., Glaser et al., 2003). Furthermore, increases in post-vaccination IL-6 may reflect a propensity toward a proinflammatory cytokine profile that could promote the health problems summarized above.
We also hypothesized that repetitive thought would predict antibody response and IL-6 along with caregiving status. For example, people who engage in chronic, negative repetitive thought may amplify stress or even generate stress de novo (Brosschot et al., 2005; Reed et al., 1999), resulting in lower antibody response and higher IL-6 response. Conversely, more neutral repetitive thought may facilitate adjustment, self-knowledge, and positive meaning, resulting in higher antibody response and lower IL-6 response. Finally, the effects of repetitive thought may be more pronounced in the context of a stressful situation such as caregiving, in which negative repetitive thought can amplify, and neutral repetitive thought neutralize, the effects of the stressor.
Method
Participants
The sample (n = 44) included 14 dementia caregivers and 30 controls. There were slightly more females (57%) than males (43%) and all participants were Caucasian (100%). Mean age of the sample was 74.52 years (SD = 7.11). The sample was generally middle class and well educated, with mean income of $56,069 (SD = $34,849) and 15.97 (SD = 3.08) years of education. The mean time since diagnosis for caregivers’ spouses at the first visit was 6.57 years (SD = 2.68).
There were no significant demographic differences between the groups (see Table 1), although controls tended to be slightly more affluent than caregivers. Table 1 also shows Problem Frequency and Caregiver Reaction scores for the three subscales of the Revised Memory and Behavior Problems Checklist at baseline: memory, depression, and disruptive behavior. Scores are similar to norms for caregivers of patients who are at most mildly reliant on a caregiver (Johnson et al., 2001). Therefore, participants on average entered the study at an early stage of caregiving.
Table 1.
Demographic comparisons of caregiver and control groups and description of patient status at baseline.
| Group
| ||||
|---|---|---|---|---|
| Caregiver (n = 14) | Control (n =30) | t/χ2 | p | |
| M (SD) | M (SD) | |||
| Age | 73.43 (7.35) | 75.03 (7.07) | .69 | .49 |
| Sex (% Male) | 50% | 40% | .39 | .53 |
| Income | $43,642 ($31,994) | $62,282 ($35,088) | 1.67 | .10 |
| Education | 15.38 (3.73) | 16.25 (2.76) | .89 | .38 |
| Years Since | 6.57 (2.68) | |||
| Diagnosis | ||||
| R-MBPC | ||||
| PF-Memory | 2.98 (1.04) | |||
| PF-Distress | 0.71 (0.67) | |||
| PF-Disruption | 0.46 (0.43) | |||
| CR-Memory | 1.28 (0.69) | |||
| CR-Distress | 0.66 (0.79) | |||
| CR-Disruption | 0.58 (0.57) | |||
Note. R-MBPC = Revised Memory and Behavior Problems Checklist; PF = Problem Frequency; CR = Caregiver Reaction.
Method
Participants were accrued and followed over a period of 5 years. Candidate dementia caregivers were identified by the Alzheimer’s Disease Research Center, a specialty secondary and tertiary care clinic located at the University of Kentucky that provides diagnostic and treatment assessments for referring physicians as well as ongoing care. All eligible caregivers (based on demographics) were identified at both consultation and care visits and contacted by letter regarding the study. A follow-up telephone call determined interest in participation and eligibility based on health criteria. Eligible caregivers were spouses of diagnosed patients at the center, living with their spouses, 65 years of age or older, and met health criteria including lack of immunologically mediated disease (e.g., autoimmunity), no history of chemotherapy or radiation in the prior 5 years, no history of general anesthesia in the past 6 months, willingness to get a flu shot, and no psychotropic or steroid medication. Other medications were allowed in order to meet enrollment targets and preserve generalizability to the population. Of spousal caregivers contacted by telephone, 49% were enrolled (n = 21), 28% were ineligible, and 23% declined. Based on demographic characteristics of enrolled caregivers, two controls who were identical to the target caregiver in sex and race and as close as possible in age and family income and meeting the same eligibility criteria were enrolled from a pool of research volunteers maintained by the Sanders-Brown Center on Aging at the University of Kentucky. Of the research volunteers contacted to participate, 33% were enrolled (n = 42), 13% were ineligible, and 54% declined. The present study reports on 44 spousal caregivers and controls for whom antibody and IL-6 data were available. All participants were interviewed in their homes at 6-month intervals. They were vaccinated annually against influenza, and serum was collected pre- and 4 weeks post-vaccination. Some participants (n = 19) were missing IL-6 data because they dropped out before the first vaccination or because they obtained the flu shot elsewhere before being vaccinated by the study. Vaccinations and blood draws took place in the late afternoon and early evening to control for any circadian effects.
The present results concern participants’ antibody titers and serum IL-6 pre-vaccination and at 4 weeks post-vaccination (cf., Glaser et al., 2003) over all study years for which they had data available. Included participants contributed 1 (n = 25), 2 (n = 12), 3 (n = 5), or 5 (n = 2) years of data, with proportions not varying between caregivers and controls. Responses to repetitive thought questionnaires and demographic information were collected at the first interview and current medications, health behaviors, and depression were assessed at each interview.
Data analysis
Data were analyzed using multi-level modeling (MLM) with SAS PROC MIXED (Singer, 2002), in which the relevant observations from each individual person’s data (level 1; e.g., intra-individual IL-6) become the basis of analysis at the group level (level 2; e.g., inter-individual IL-6). The model accounts for two important aspects of these data: level-1 covariates (e.g., medications) and differing numbers of level-1 observations from different individuals. MLM provides an estimate γ, which is analogous to an unstandardized beta weight. Because γ varies as a function of scaling, the effect size estimate η is also provided from the Type III sums of squares F test for that effect. η can be interpreted in the same manner as r: small effect sizes are around .10; medium effect sizes, .30; and large effect sizes, .50 (Cohen, 1987). In the reported models, repetitive thought at baseline was a person-level (i.e., level 2) predictor. As for statistical controls, age was a person-level predictor, whereas depression and medications were level-1 predictors matched to the particular assessment time point. Pre-vaccination antibody titers and IL-6 were always included as level-1 covariates in analyses of post-vaccination antibody titers and IL-6, respectively.
Covariates
Potentially important medications used by the sample included statins, non-steroidal anti-inflammatories (including aspirin), nasal steroid sprays, estrogen replacement, ACE inhibitors, antidepressants, benzodiazepines, and thyroid replacement. Note that although antidepressants and benzodiazepines were initial exclusion criteria, participants were not dropped from the study for later initiation of these medications. However, only antidepressants and estrogen replacement were used at a higher rate among caregivers (31% and 23%, respectively) than controls (8% and 2%). Of these two, only estrogen replacement was meaningfully related to IL-6. However, the effects of estrogen replacement were largely within people at level 1 (i.e., effects of taking and then not taking estrogen), whereas effects of repetitive thought were necessarily between people at level 2; therefore, these effects were non-overlapping and not discussed further.
Potentially important demographic covariates included sex, age, family income, body mass index, and smoking status. Of these, only age was meaningfully related to IL-6 (γ= .017, t (27) = 2.84, p < .05, η= .48). Therefore, pre-vaccine IL-6 analyses were repeated after controlling for age. Age was also meaningfully related to antibody titers to H1N1 and H3N2. Therefore, antibody analyses were performed controlling for age. There were two other, more idiosyncratic associations: Income was meaningfully related only to H3N2 antibody titer, and BMI was meaningfully related only to B antibody titer. However, controlling for these factors did not affect the results, and so they are not discussed further. Prior vaccination was also considered, but only one participant had not been vaccinated in the year prior to study entry and all participants were vaccinated annually during the study, and so the sample was basically homogeneous in its vaccination history.
Repetitive thought
All questionnaire measures were administered verbally during interviews at participants’ homes in order to avoid visual or literacy problems. Several measures of trait repetitive thought were administered at baseline and every 2 years thereafter. These included the Rumination-Reflection Questionnaire (Trapnell and Campbell, 1999), the planning and uncontrolled thought factors from the Rumination Scale (Martin et al., 1993; Segerstrom et al., 2003), the Penn State Worry Questionnaire (Meyer et al., 1990), Emotional Approach Coping (trait administration; Stanton et al., 2000), and the self-reproach/brooding and self-analysis/pondering subscales of the Response Styles Questionnaire (RSQ), a measure of depressive rumination (Segerstrom et al., 2003; Treynor et al., 2003). Except for planning, all scales showed adequate internal reliability (α= .64 – .94). Because planning did not show adequate reliability, it was dropped from further analysis. In a subset of 15 participants with 2-year re-test data, test-retest correlations ranged from .37 to .77, showing that the questionnaires were capturing stable individual differences in repetitive thought.
Depression
As a measure of psychological adjustment, participants completed the Geriatric Depression Scale, a measure of depression appropriate for use with older adults. In order to avoid criterion contamination with repetitive thought measures, two items, “Do you frequently worry about the future?” and “Do you worry a lot about the past?” were not included in the depression score. Good reliability and validity have been reported for this scale (Kieffer and Reese, 2002); consistent with these reports, internal reliability at the first interview was good at α = .80. The depression score at the interview most proximal to vaccination was used for analysis.
Antibody titers
Sera were frozen at −80° C and later thawed for analysis at the Center for Vaccine Development at St. Louis University. Hemagglutination inhibition tests were performed for each of 3 vaccine components: H1N1, H3N2, and B. Component types did not change across the course of the study; however, strains used to generate the H3N2 and B antigens did change, and so strain type was included in all analyses to control for differences in antigenicity. Serum antibody is stable over a period of weeks to months (Nommensen et al., 1989) and so was expected to be measured reliably from single assessments.
IL-6
Sera were frozen at −80° C and later thawed for analysis at the General Clinical Research Center at the University of Kentucky. Sera were analyzed in annual batches so that participant was not confounded with batch. High-sensitivity ELISA kits (R&D Systems, Minneapolis, MN) were used except for a few high-concentration samples that were re-run on regular sensitivity kits to obtain more accurate results. Median intraindividual CV for all assays was 2.8% (range = 0.0 – 14.1). IL-6 results were log-10 transformed to improve normality. Serum IL-6 has good generalizability over a period of several weeks in older adults (Rao et al., 1994) and so was expected to be measured from single assessments with adequate reliability.
Results
Repetitive thought in caregivers and controls
Based on previous work (Segerstrom et al., 2003), two subsets of repetitive thought were expected: neutral repetitive thought consisting of emotional approach coping, self-analysis/pondering, and reflection; and negative repetitive thought consisting of self-reproach/brooding, worry, uncontrolled thought, and rumination. The expected subsets emerged in a principal factor analysis with oblique rotation. Neutral repetitive thought (α = .73) and negative repetitive thought (α = .86) factors were only modestly correlated with each other (r = .16). The small correlation indicates that degree of neutral repetitive thought is independent of degree of negative repetitive thought: a single individual may engage in one, both, or neither. Furthermore, there were no significant differences between caregivers and controls in their levels of negative repetitive thought (t (42) = 1.24, p > .05) or neutral repetitive thought (t (42) = 0.51, p > .05). Therefore, propensity for repetitive thought was not a proxy for caregiving.
Effects of caregiving on antibody titers and interleukin-6
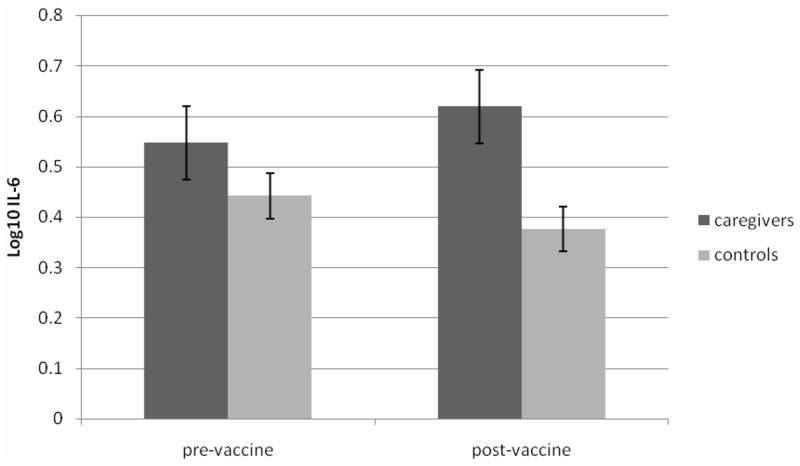
Controlling for age, caregiver status was not associated with antibody titer to any component of the influenza vaccine. Caregiving was associated with higher levels of pre-vaccination IL-6 with a medium effect size, although this relationship did not reach a conventional level of significance (γ = .13, t (27) = 1.50, p > .05, η = .28). Caregiving was a more robust predictor of increases in IL-6 at 4 weeks post-vaccination. After controlling for pre-vaccination IL-6, caregivers had significantly higher IL-6 than controls (γ = .16, t(23) = 2.36, p < .05, η = .44). Figure 1 shows mean IL-6 pre-vaccination and 4 weeks post-vaccination in controls and caregivers. Caregivers showed a prolonged IL-6 elevation after vaccination, whereas controls, if anything, had lower IL-6 by 4 weeks post-vaccination (cf., Krakauer and Russo, 2001).
Figure 1.
Effects of repetitive thought on antibody titers
There was one main effect of repetitive thought on antibody titers, in which neutral repetitive thought was significantly associated with higher H1N1 antibody titers (γ = 1.23, t(23) = 2.53, p < .05, η = .39). This main effect was, however, moderated by a more robust interaction between caregiver status and repetitive thought.
Both negative and neutral repetitive thought interacted with caregiving status to predict antibody titers. Negative repetitive thought and caregiving predicted antibody against H1N1 (γ = 1.48, t(23) = 2.31, p < .05, η = .43) and B (γ = 1.76, t(20) = 3.22, p < .05, η = .58). Neutral repetitive thought and caregiving predicted antibody against H1N1 (γ = −1.21, t(23) = −2.01, p = .05, η = .39) and H3N2 (γ = −1.43, t(20) = −2.18, p < .05, η = .44). Figure 2 shows the predicted value of H1N1 titers for caregivers and controls having high (+1 SD) or low (−1 SD) neutral repetitive thought; the patterns were very similar for the other components, so they are not shown.
Figure 2.
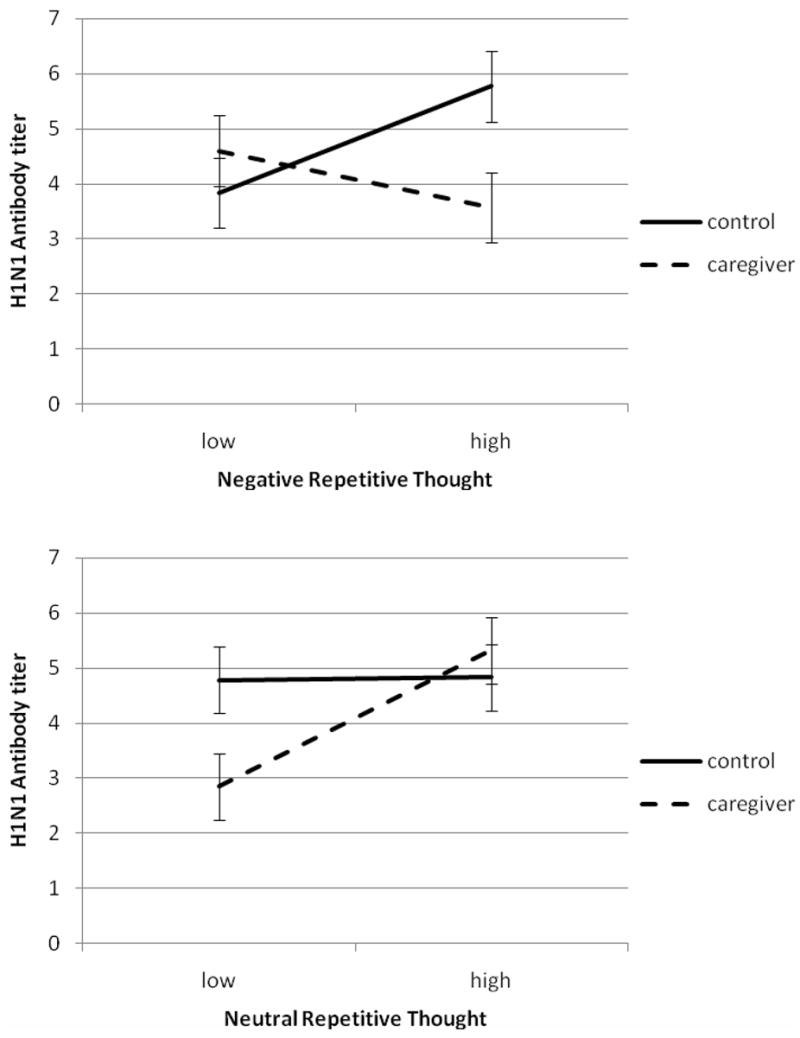
When repetitive thought included worry, rumination, and uncontrolled thought, such thoughts associated with lower antibody titers, but only among caregivers. Surprisingly, among controls, higher negative repetitive thought was associated with higher antibody titers. The effects of neutral repetitive thought, including reflection, self-analysis, and emotional approach, had different effects. Among caregivers, neutral repetitive thought increased antibody titers, but among controls, neutral repetitive thought had little effect on antibody titers. Therefore, the effects of repetitive thought on the antibody response to vaccination depended both on the type of thought and the environment of the person, that is, whether he or she was or was not engaged in caregiving for a spouse with dementia. Caregivers’ antibody titers increased with increasing neutral repetitive thought, whereas controls’ antibody titers increased with increasing negative repetitive thought.
Effects of repetitive thought on interleukin-6
There were no main effects of repetitive thought on pre- or post-vaccination IL-6. Although negative repetitive thought tended to be associated with higher IL-6 (γ = .05 – .06; η = .20 for both pre- and post-vaccination IL-6), this was not a significant effect.
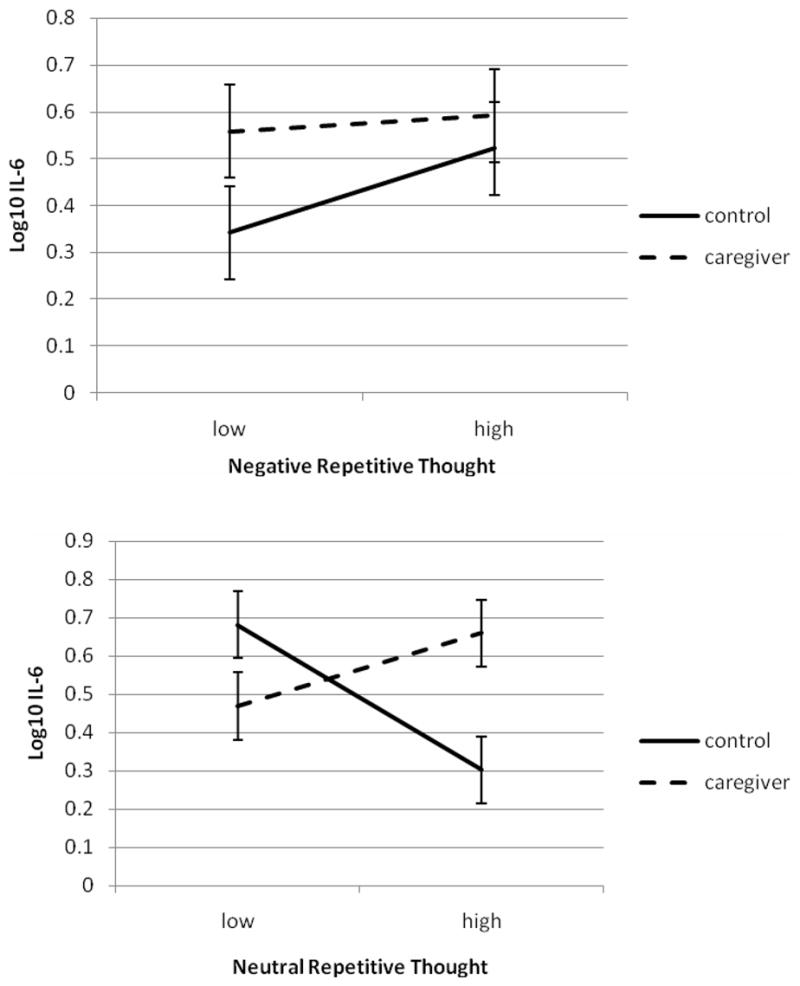
However, repetitive thought did interact with caregiver status to predict IL-6 at 4 weeks post-vaccination. Figure 3 shows the interactions between negative and neutral repetitive thought with caregiver status. There was a small and nonsignificant effect of negative repetitive thought such that controls with low levels of negative repetitive thought had the lowest levels of post-vaccine IL-6 (γ = .073, t (23) = 0.74, p > .05, η = .15). However, there was a robust effect of neutral repetitive thought in combination with caregiver status on post-vaccine IL-6 (γ = −.19, t (23) = 2.16, p < .05, η = .41). Figure 3 shows the predicted value of IL-6 for caregivers and controls having high (+1 SD) or low (−1 SD) neutral repetitive thought. For controls, increasing levels of neutral repetitive thought were associated with lower levels of IL-6. However, for caregivers, increasing levels of neutral repetitive thought were associated with increasing levels of IL-6.
Figure 3.
Depression and repetitive thought
One reasonable hypothesis is that the effects of repetitive thought depended on their effects on psychological adjustment as suggested in the Introduction: negative repetitive thought has negative implications for adjustment, but neutral repetitive thought may have more positive implications. The adverse effects of caregiving on psychological adjustment have also been described (Schulz et al., 1995). As expected, both caregiving and negative repetitive thought were associated with increased depression (caregiving: γ = 3.43, t (30) = 3.01, p < .05, η = .48; negative repetitive thought: γ = 3.06, t (29) = 4.43, p < .05, η = .55). Conversely, neutral repetitive thought was associated with decreased depression (γ = −2.97, t (30) = −2.34, p < .05, η = .34). Although these effects tended to be stronger in caregivers than in controls, there were no significant interactions between caregiving and repetitive thought in predicting depression.
Because depression had level-1 (within-person) and level-2 (between-person) variance, two depression terms were entered into the models predicting immune outcomes: a level-2 predictor that was the mean of all an individual’s GDS scores and a level-1 predictor that was the deviation at each time point from the individual’s mean score across time points. There was a significant interaction between level-2 depression and caregiving in predicting IL-6 at 4 weeks post-vaccine (γ = 0.08, t (23) = 3.82, p < .05, η = .62). This interaction took the same form as the top panel of Figure 3: higher levels of depression were associated with higher IL-6, but only among controls; caregivers had IL-6 levels equivalent to high-depression controls regardless of their depression levels. The main difference between the two interactions is that the depression effect had much smaller standard errors than the effect for negative repetitive thought and therefore was statistically significant, whereas the negative repetitive thought effect was not. Including both negative repetitive thought and level-2 depression in the model resulted in a change in the negative repetitive thought interaction term from .07 to −.04, whereas the depression interaction term remained constant. Therefore, the nonsignificant interaction effect of negative repetitive thought and caregiving on post-vaccine IL-6 appears to have occurred because of the imperfect relationship of negative repetitive thought to between-person differences in depression, which was a much more robust predictor of this parameter.
With regard to antibody responses, there was a significant main effect of level-2 depression on H1N1 titers (γ = −0.14, t (22) = 2.27, p < .05, η = .43), but neither of the others. There were no significant interactions between level-2 depression and caregiving, and so depression did not stand in for repetitive thought in terms of the latter’s effects on antibody titers.
Discussion
The present study tested the contributions of repetitive thought styles and psychosocial context – caregiving – on antibody and IL-6 responses to vaccination. Caregiving alone had limited effects on immune parameters. The pre-vaccine IL-6 difference between caregivers (log10, 0.55; raw, 3.52) and controls (log10, 0.44; raw, 2. 77) was nonsignificant and smaller than that observed by Lutgendorf et al. (1999), whose control subjects (0.39; raw, 2.45) had a similar IL-6 level to the present study, but whose caregivers (0.76; raw, 5.75) had higher IL-6 than the present sample; however, it was similar to the values predicted by Kiecolt-Glaser et al.’s (2003) model at age 75, the mean age of participants in the present study (0.36 [raw, 2.29] for controls vs. 0.51 [raw, 3.24] for caregivers). There was a significant difference between caregivers (0.62; raw, 4.16) and controls (0.38; raw, 2.38) in log10 IL-6 four weeks post-vaccination after controlling for pre-vaccination levels. Therefore, vaccination triggered the inflammatory response to a significantly greater extent in caregivers than in controls. Furthermore, this response was observed at 4 weeks post-vaccination, as opposed to the 2-week response reported elsewhere (Glaser et al., 2003), and provides evidence that immune challenges may have long-lasting consequences for individuals predisposed to proinflammatory responses. These long-lasting immunological effects may over time contribute to health problems associated with elevations in proinflammatory cytokines.
The ways in which caregivers and controls typically thought about themselves and their worlds had more extensive relationships to immune parameters, particularly in combination with caregiving. For caregivers, negative repetitive thought had uniformly negative effects, predicting more depression and lower antibody titers. The identification of repetitive thought as a risk factor among caregivers is clinically relevant. With regard to the nature of the risk, effective antibody responses to vaccination greatly reduce the risk of pneumonia, hospitalization, and death in older adults (Gross et al., 1995). With regard to interventions to reduce risk, for individuals for whom repetitive thought reaches the level of generalized anxiety disorder, FDA-approved medications such as paroxetine and escitalopram may relieve symptoms, but there is mixed evidence regarding the effects of such medications on the immune system (e.g., Kim et al., 2007; Marques-Deak et al., 2007). There are also a number of empirically validated psychological treatments for worry and rumination, including stimulus control (Borkovec et al., 1983), mindfulness (Teasdale et al., 1995), and cognitive restructuring (e.g., Ladouceur et al., 2000), some of which have been successfully applied to older adults to reduce negative repetitive thought (Stanley et al., 2003). Because exogenous sources of stress are often not modifiable (e.g., caregiving), the ability to alleviate endogenous sources (e.g., worry) is particularly important. Even where exogenous sources are modifiable, it may be important to simultaneously address endogenous sources. For example, control over repetitive thought may be important in conjunction with interventions that target relief from caregiving (e.g., respite programs), as relief periods are unlikely to be helpful if they are spent in negative repetitive thought. However, these clinical interventions to reduce repetitive thought may not be needed at all for other groups. In the present study, the effects of negative repetitive thought in controls were opposite of those for caregivers and unexpected insofar as negative repetitive thought predicted higher antibody titers.
Among caregivers, neutral repetitive thought predicted less depression and higher antibody titers, but also higher post-vaccination IL-6. The effects of neutral repetitive thought on depression and antibody responses to vaccination are consistent with previous work showing that this kind of thought has been associated with better immunity and health in the context of bereavement (Bower et al., 1998). However, neutral repetitive thought was not uniformly beneficial, as the combination of a stressful or threatening environment (caregiving) and the propensity to think about that environment (neutral repetitive thought) appears to create a physiological propensity for inflammation, and a propensity for inflammatory responses increases the risk of atherosclerosis, osteoporosis, and other diseases of aging (Ershler and Keller, 2000; Robles et al., 2005; Papanicolaou et al., 1998). This was not merely an artifact of more globally robust immune responses, as post hoc analyses indicated that IL-6 and antibody responses to vaccination were largely independent of each other (and see Krakauer and Russo, 2001, for the same finding). This independence may derive from differences in cytokine production after vaccination. Older adults are predisposed to a Th2 cytokine profile compared with younger adults, but relative preservation of Th1 cytokine production could facilitate the immune response to vaccination (Bernstein et al., 1998; Simons and Reynolds, 1990). Within the Th2 profile, more production of IL-4 by Th2 cells could result in higher antibody titers while more production of IL-10 could result in lower proinflammatory response and less IL-6.
One possibility is that directionality ran from IL-6 to repetitive thought instead of vice versa. Elevations in IL-6 are associated with cognitive dysfunction, including executive function (Marsland et al., 2006), and poor executive function is associated with increases in negative repetitive thought (Whitmer and Banich, 2007). If elevations in IL-6 compromise executive function, the result could be a relative inability to control repetitive thought. However, this possibility does not explain the pattern of results in controls. In the present study, the lowest IL-6 was among controls who engaged in neutrally valenced, frequent and prolonged thoughts about themselves and their worlds, whereas the highest IL-6 was among controls who did not engage in this kind of thought.
In general, the relationships found in this study were expected: neutral repetitive thought was beneficial for adjustment and immune parameters, whereas negative repetitive thought was detrimental for adjustment and immune parameters. For the two unexpected relationships –higher IL-6 with neutral repetitive thought in caregivers and lower antibody titer with negative repetitive thought in controls – there is a case to be made that although some people process and reflect more than others, and some people worry and ruminate more than others, the meaning and consequences of those thought styles may depend on the context. For example, “processing” or “working through” are not necessarily self-limiting and may, in the context of worsening and stressful circumstances such as caregiving, have mixed effects on psychological and physical adjustment (Brosschot et al., 2005; Wortman and Silver, 1989). On the other hand, “worrying” can contain elements of planning and coping (Davey et al., 1992) that may be more prominent than its uncontrollable, ruminative elements under less stressful circumstances. The results of this study suggest that repetitive thought measures may be capturing somewhat different and differentially adaptive constructs depending on the circumstances of the individual.
These results emphasize the main point of this special issue: biopsychosocial health must be personalized. In this study, the same individual difference that appeared to confer risk on individuals under chronic stress also appeared to be protective in individuals not experiencing chronic stress. These effects were demonstrated in a small sample of caregivers and controls and therefore require replication; the unexpected results with regard to neutral repetitive thought in caregivers and negative repetitive thought in controls particularly require further examination in larger samples. Furthermore, the size of the sample limited statistical power, but many effects were quite large and suggest the importance of both person and situation in affecting immune responses.
This study also provided two pieces of evidence that point toward future research. First, the ways that people think about themselves and their worlds have clinically relevant physiological correlates. This clinical relevance is evident, for example, in the relationship between negative repetitive thought and risk for cardiovascular disease (Kubzansky et al., 1997). The ability of physiological correlates such as IL-6 to account for clinical effects of repetitive thought will be an important piece of evidence. Second, the effects of such repetitive thought depend on context. These data demonstrate clearly that the effects of repetitive thought are dependent on both its valence and whether it is being reported by caregivers or controls. Finally, although neutral repetitive thought can have adaptive effects on adjustment and immunity (cf., Bower et al., 1998), future work on repetitive thought should include a measure of frankly positive repetitive thought, such as savoring or reminiscing (Bryant, 2003). It is possible that even individuals experiencing significant stress, such as caregivers, may benefit from thoughts whose positive valence counteract the effects of their environments.
Acknowledgments
The authors thank David Wekstein of the Sanders-Brown Center on Aging and Fran Newman of the Center for Vaccine Development, St. Louis University, for their assistance with this study and acknowledge grant support from the Dana Foundation, the National Institute on Aging (R01-AG026307, P50-AG05144), and the National Center for Research Resources (M01-RR02602).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bernstein ED, Gardner EM, Abrutyn E, Gross P, Murasko DM. Cytokine production after influenza vaccination in a healthy elderly population. Vaccine. 1998;16:1722–1731. doi: 10.1016/s0264-410x(98)00140-6. [DOI] [PubMed] [Google Scholar]
- Borkovec TD, Wilkinson L, Folensbee R, Lerman C. Stimulus control applications to the treatment of worry. Beh Res Ther. 1983;21:247–251. doi: 10.1016/0005-7967(83)90206-1. [DOI] [PubMed] [Google Scholar]
- Bower JE, Kemeny ME, Taylor SE, Fahey JL. Cognitive processing, discovery of meaning, CD4 decline, and AIDS-related mortality among bereaved HIV-seropositive men. J Consult Clin Psych. 1998;66:979–986. doi: 10.1037//0022-006x.66.6.979. [DOI] [PubMed] [Google Scholar]
- Brosschot JF, Pieper S, Thayer JF. Expanding stress theory: Prolonged activation and perseverative cognition. Psychoneuroendocrinol. 2005;30:1043–1049. doi: 10.1016/j.psyneuen.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Bryant FB. Savoring Beliefs Inventory: A scale for measuring beliefs about savouring. J Ment Health. 2003;12:175–196. [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences: Revised edition. Lawrence Erlbaum Associates; Hillsdale, NJ: 1987. [Google Scholar]
- Davey GCL, Hampton J, Farrell J, Davidson S. Some characteristics of worrying: Evidence for worrying and anxiety as separate constructs. Person Individ Diff. 1992;13:133–147. [Google Scholar]
- Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000;51:245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- Esterling BA, Kiecolt-Glaser JK, Bodnar JC, Glaser R. Chronic stress, social support, and persistent alterations in the natural killer cell response to cytokines in older adults. Health Psychol. 1994;13:291–298. doi: 10.1037//0278-6133.13.4.291. [DOI] [PubMed] [Google Scholar]
- Glaser R, Robles TF, Sheridan J, Malarkey WB, Kiecolt-Glaser JK. Mild depressive symptoms are associated with amplified and prolonged inflammatory responses after influenza virus vaccination in older adults. Arch Gen Psychiatry. 2003;60:1009–1014. doi: 10.1001/archpsyc.60.10.1009. [DOI] [PubMed] [Google Scholar]
- Gross PA, Hermogenes AQ, Sakc JS, Lau J, Levandowski RA. The efficacy of influenza vaccine in elderly persons: A meta-analysis and review of the literature. Ann Int Med. 1995;123:518–527. doi: 10.7326/0003-4819-123-7-199510010-00008. [DOI] [PubMed] [Google Scholar]
- Johnson MMS, Wackerbarth SB, Schmitt FA. The clinical usefulness, reliability, and validity of the Revised Memory and Behavior Problems Checklist. Clin Gerontol. 2001;22:87–108. [Google Scholar]
- Kiecolt-Glaser JK, Dura JR, Speicher CE, Trask OJ, Glaser R. Spousal caregivers of dementia victims: Longitudinal changes in immunity and health. Psychosom Med. 1991;53:345–362. doi: 10.1097/00006842-199107000-00001. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. P Natl Acad Sci. 2003;100:9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffer KM, Reese RJ. A reliability generalizability study of the Geriatric Depression Scale. Educ Psychol Meas. 2002;62:969–994. [Google Scholar]
- Kim YK, Na KS, Shin KH, Jung HY, Choi SH, Kim JB. Cytokine imbalance in the pathophysiology of major depressive disorder. Prog Neuro-Psychoph. 2007;31:1044–1053. doi: 10.1016/j.pnpbp.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Krakauer T, Russo C. Serum cytokine levels and antibody response to influenza vaccine in the elderly. Immunopharmacol Immunotoxicol. 2001;23:35–41. doi: 10.1081/iph-100102565. [DOI] [PubMed] [Google Scholar]
- Kubzansky LD, Kawachi I, Spiro A, Weiss ST, Vokonas PS, Sparrow D. Is worrying bad for your heart? A prospective study of worry and coronary heart disease in the Normative Aging Study. Circulation. 1997;95:818–824. doi: 10.1161/01.cir.95.4.818. [DOI] [PubMed] [Google Scholar]
- Ladouceur R, Dugas MJ, Freeston MH, Leger E, Gagnon F, Thibodeau N. Efficacy of a cognitive-behavioral treatment for generalized anxiety disorder. J Consult Clin Psych. 2000;68:957–964. [PubMed] [Google Scholar]
- Lieberman MA, Fisher L. The effects of nursing home placement on family caregivers of patients with Alzheimer’s disease. Gerontologist. 2001;41:819–826. doi: 10.1093/geront/41.6.819. [DOI] [PubMed] [Google Scholar]
- Lutgendorf SK, Garand L, Buckwalter KC, Reimer TT, Hong SY, Lubaroff DM. Life stress, mood disturbance, and elevated interleukin-6 in healthy older women. J of Gerontol. 1999;54A:M434–M439. doi: 10.1093/gerona/54.9.m434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques-Deak AH, Lotufo Neta F, Dominguez WV, Solis AC, Kurcgant D, Sato F, Ross JM, Prado EBA. Cytokine profiles in women with different subtypes of major depressive disorder. J Psychiat Res. 2007;41:152–159. doi: 10.1016/j.jpsychires.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Peterson KL, Sathanoori R, Muldoon MF, Neumann SA, et al. Interleukin-6 covaries inversely with cognitive performance among middle-aged community volunteers. Psychosom Med. 2006;68:895–903. doi: 10.1097/01.psy.0000238451.22174.92. [DOI] [PubMed] [Google Scholar]
- Martin LL, Tesser A, McIntosh D. Wanting but not having: The effects of unattained goals on thoughts and feelings. In: Wegner DM, Pennebaker JW, editors. Handbook of Mental Control. Prentice Hall; Englewood Cliffs, New Jersey: 1993. pp. 552–572. [Google Scholar]
- Mastorakos G, Ilias I. Interleukin-6: a cytokine and/or a major modulator of the response to somatic stress. Ann N Y Acad Sci. 2006;1088:373–81. doi: 10.1196/annals.1366.021. [DOI] [PubMed] [Google Scholar]
- Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn State Worry Questionnaire. Beh Res Ther. 1990;28:487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- Molina S, Borkovec TD. The Penn State Worry Questionnaire: psychometric properties and associated characteristics. In: Davey GCL, Tallis F, editors. Worrying: Perspectives on Theory, Assessment, and Treatment. Wiley; New York: pp. 265–283. [Google Scholar]
- Nommensen FE, Go ST, MacLaren DM. Half-life of HBs antibody after hepatitis B vaccination: An aid to timing of booster vaccination. Lancet. 1989;2:847–849. doi: 10.1016/s0140-6736(89)93009-2. [DOI] [PubMed] [Google Scholar]
- Reed GM, Kemeny ME, Taylor SE, Visscher BR. Negative HIV-specific expectancies and AIDS-related bereavement as predictors of symptom onset in asymptomatic HIV-positive gay men. Health Psychol. 1999;18:354–363. doi: 10.1037//0278-6133.18.4.354. [DOI] [PubMed] [Google Scholar]
- Papanicolaou DA, Wilder RL, Manolagas SC, Chrousos GP. The pathophysiologic roles of interleukin-6 in human disease. Ann Intern Med. 1998;128:127–137. doi: 10.7326/0003-4819-128-2-199801150-00009. [DOI] [PubMed] [Google Scholar]
- Rao KMK, Pieper CS, Currie MS, Cohen HJ. Variability of plasma IL-6 and crosslinked fibrin dimmers over time in community dwelling elderly subjects. Am J Clin Pathol. 1994;102:802–805. doi: 10.1093/ajcp/102.6.802. [DOI] [PubMed] [Google Scholar]
- Robles TF, Glaser R, Kiecolt-Glaser JK. Out of balance: A new look at chronic stress, depression, and immunity. Curr Dir Psychol Sci. 2005;14:111–115. [Google Scholar]
- Schulz R, O’Brien AT, Bookwala J, Fleissner K. Psychiatric and physical morbidity effects of dementia caregiving: Prevalence, correlates, and causes. Gerontologist. 1995;35:771–791. doi: 10.1093/geront/35.6.771. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, Kemeny ME. Worried to death? Stress, worry, and immune dysregulation in health and HIV. In: Plotnikoff N, Faith RE, Murgo AJ, editors. Cytokines, Stress, and Immunity. 2. CRC Press; Boca Raton, Florida: 2006. pp. 17–28. [Google Scholar]
- Segerstrom SC, Miller GE. Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychol Bull. 2004;104:601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerstrom SC, Solomon GF, Kemeny ME, Fahey JL. Relationship of worry to immune sequelae of the Northridge earthquake. J Behav Med. 1998;21:433–450. doi: 10.1023/a:1018732309353. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, Stanton AL, Alden LE, Shortridge BE. A multidimensional structure for repetitive thought: What’s on your mind, and how, and how much? J Pers Soc Psychol. 2003;85:909–921. doi: 10.1037/0022-3514.85.5.909. [DOI] [PubMed] [Google Scholar]
- Simons RJ, Reynolds HY. Altered immune status in the elderly. Sem Resp Inf. 1990;5:251–259. [PubMed] [Google Scholar]
- Singer JD. Fitting individual growth models using SAS PROC MIXED. In: Moskowitz DS, Hershberger SL, editors. Modeling intraindividual variability with repeated measures data: Methods and applications. Lawrence Erlbaum Associates; Mahwah, New Jersey: 2002. pp. 135–170. [Google Scholar]
- Stanley MA, Beck JG, Novy DM, Averill PM, Swann AC, Diefenbach GJ, Hopko DR. Cognitive-behavioral treatment of late-life generalized anxiety disorder. J Consult Clin Psych. 2003;71:309–319. doi: 10.1037/0022-006x.71.2.309. [DOI] [PubMed] [Google Scholar]
- Stanton AL, Kirk SB, Cameron CL, Danoff-Burg S. Coping through emotional approach: Scale construction and validation. J Pers Soc Psychol. 2000;78:1150–1169. doi: 10.1037//0022-3514.78.6.1150. [DOI] [PubMed] [Google Scholar]
- Teasdale JD, Segal Z, Williams JMG. How does cognitive therapy prevent depressive relapse and why should attentional control (mindfulness) training help? Beh Res Ther. 1995;33:25–39. doi: 10.1016/0005-7967(94)e0011-7. [DOI] [PubMed] [Google Scholar]
- Trapnell PD, Campbell JD. Private self-consciousness and the five-factor model of personality: Distinguishing rumination from reflection. J Pers Soc Psychol. 1999;76:284–304. doi: 10.1037//0022-3514.76.2.284. [DOI] [PubMed] [Google Scholar]
- Treynor W, Gonzalez R, Nolen-Hoeksema S. Rumination reconsidered: A psychometric analysis. Cognitive Ther Res. 2003;27:247–259. [Google Scholar]
- Vitaliano PP, Zhang J, Scanlan JM. Is caregiving hazardous to one’s physical health? A meta-analysis Psychol Bull. 2003;129:946–972. doi: 10.1037/0033-2909.129.6.946. [DOI] [PubMed] [Google Scholar]
- Wortman CB, Silver RC. The myths of coping with loss. J Consult Clin Psych. 1989;57:349–357. doi: 10.1037//0022-006x.57.3.349. [DOI] [PubMed] [Google Scholar]


