Abstract
Background
Adenosine Deaminase Activity (ADA) is a commonly used marker for the diagnosis of tuberculous pleural effusion. There has been concern about its usefulness in immunocompromised patients, especially HIV positive patients with very low CD4 counts. The objective of this study was to evaluate the sensitivity of ADA in pleural fluid in patients with low CD4 counts.
Materials and Methods
This was a retrospective case control study. Medical files of patients with tuberculous pleuritis and non-tuberculous pleuritis were reviewed. Clinical characteristics, CD4 cell counts in blood and biochemical markers in pleural fluid, including ADA were recorded.
Results
One ninety seven tuberculous pleuritis and 40 non- tuberculous pleuritis patients were evaluated. Using the cut-off value of 30 U/L, the overall sensitivity, specificity, positive likelihood ratio, and negative likelihood ratio of ADA was 94%, 95%, 19, and 0.06 respectively. The mean CD4 cell counts among TB pleuritis patients was 29 and 153 cells/microL in patients with CD4 <50 cells/microL and >50 cells/microL, (p<0.05) respectively. The corresponding mean ADA values for these patients were 76 U/L and 72 U/L respectively (p>0.5). There was no correlation between ADA values and CD4 cell counts (r = −0.120, p = 0.369).
Conclusion
ADA analysis is a sensitive marker of tuberculous pleuritis even in HIV patients with very low CD4 counts in a high TB endemic region. The ADA assay is inexpensive, rapid, and simple to perform and is of great value for the immediate diagnosis of tuberculous pleuritis while waiting for culture result and this has a positive impact on patient outcome.
Introduction
Tuberculous pleuritis occurs in about 25% of total tuberculosis (TB) cases in South Africa [1], [2]. Rapid diagnosis and prompt treatment is needed to reverse the morbidity and mortality due to TB. The diagnosis of tuberculous pleuritis remains a challenge because of the small numbers of bacilli in the pleural fluid [3], [4], [5]. Pleural fluid staining for acid fast bacilli (AFB) and culture has low sensitivity [4] and culture takes more than a week even when using automated systems. Pleural biopsy culture and typical histology of necrotizing granuloma is more sensitive than pleural fluid culture [4]. However, the invasiveness of the procedure, inability to get representative pleural tissue and the risk of complications are limitations in obtaining pleural biopsies. We have recently demonstrated that immunohistochemistry with the anti- MPT64 antibody as diagnostic tool offers a reasonable high sensitivity for the diagnosis of TB pleuritis [6].
A well established biological marker for diagnosis of tuberculous pleuritis is Adenosine Deaminase Activity (ADA) [4], [7], [8], [9], [10]. The ADA assay principle is based on the detection of either hydrogen peroxide or ammonia after enzymatic deamination of adenosine to inosine by ADA. The unit cost of different commercial assays in South Africa is about South African rands nine (equivalent to U.S dollars 1.2). Production of the enzyme Adenosine Deaminase in pleural fluid reflects the presence of activated T lymphocytes and monocytes [11]. Therefore, it is expected that ADA will be lower in HIV co-infected patients and/or other immunocompromised patients with low blood CD4 counts. It has been shown that ADA values correlate with CD4 counts in the pleural fluid of TB patients [12]. Hsu et al [13] has shown lower sensitivity of ADA for the diagnosis of TB pleural effusion in 10 immunocompromised patients with chronic illness such as diabetes, cirrhosis, renal failure and leukaemia. In contrast, Riantawan et al [14] did not find any difference in the performance of ADA in 37 HIV positive patients with mean CD4 counts above 100 cells/microL. A recent study by Reuter et al [15] reported low sensitivity of ADA in tuberculous pericarditis patients with advanced HIV disease and median CD4 count of 59 (6–115) cells/microL. CD4 counts below 50 are regarded as very low and are an indication for disease progression [16]. However, it is not known how the ADA will perform for the diagnosis of tuberculous pleuritis, a common extra-pulmonary location in severely immunocompromised HIV patients. The aim of this study was to evaluate the sensitivity of ADA in tuberculous pleuritis patients with low CD4 counts and to determine whether there was any correlation between CD4 cell counts and ADA values.
Materials and Methods
Patients were identified through tracing of pleural fluid specimens sent for culture to the microbiology laboratory at the Dr George Mukhari (DGM) Hospital Ga-Rankuwa, Pretoria, South Africa. All patients presenting with pleural effusion had pleural tap and subsequently pleural fluid examination. Patients were put on TB treatment after pleural tap if there was strong suspicion of TB based on clinical features such as, history of contact with TB patients, TB history, chronic cough, night sweats, fever and loss of weight.
The diagnosis of TB pleuritis was confirmed if culture of Mycobacterium tuberculosis in pleural fluid or tissue was positive or there was caseous granuloma in the pleural biopsy and was probable if; (1) there were clinical features such as history of TB contact, past history of TB, chronic cough, night sweats, fever and loss of weight; and (2) had clinical diagnosis of TB and started on TB treatment in the absence of any other cause of effusion. The control non- TB pleuritis group include; (1) malignant pleural effusion confirmed by cytological examination of pleural fluid/histological examination of pleural biopsy; (2) Pleural effusion of other known etiology such as congestive cardiac failure or para-pneumonic effusion. Cases without ADA results were excluded from the analysis.
The clinical records of patients as described above for the period January 2004 to January 2007 were reviewed. Data regarding clinical presentation (cough, fever, chest pain, night sweats, loss of weight, diarrhoea, oral thrush), acid fast staining (AFB) and culture results of pleural fluid, HIV status, blood CD4 cell counts, and biochemical parameters in pleural fluid (ADA, lactate dehydrogenase , total protein) were collected. The ADA was analysed using a commercial colorimetric assay kit (Diazyme General Atomics, CA). The cut-off value for positive ADA result was 30 U/L according to clinical practice at this hospital [17]. The patients were stratified according to the CD4 count as follows, <50 and >50, cells/microL as referred above [16].
The study was evaluated and approved by the Research, Ethics and Publications Committee at the DGM Hospital, University of Limpopo, South Africa and the Regional Committee for Ethics in Medical Research in Bergen, Norway.
Statistical analysis
Data analysis was made by SPSS (SigmaStat; SPSS Inc; Chicago, IL). Mean, correlation coefficient, and group comparison was made with non parametric Mann-Whitney U test.
Results
A total of 300 pleural fluid samples were submitted to the laboratory for the diagnosis of TB over the 3 year study period of which 237 with available ADA results were included in the study. There were 197 TB pleuritis patients: mean age 35 years with 107 females and 40 non-TB pleuritis patients: mean age 50 years with 19 females. Among the TB pleuritis patients, 72 were confirmed TB pleuritis while the non-TB pleuritis included 26 malignancies (adenocarcinoma, mesothelioma, squamous cell carcinoma, metastatic liver carcinoma, bronchial carcinoma and Kaposi sarcoma), 10 congestive cardiac failures, 2 hydrothorax, 1 Streptococcus pneumoniae effusion and 1 Klebsiella pneumoniae empyema. The mean ADA for each category is shown in Table 1. The ADA value was positive, above the cut-off of 30 U/L in 186 of the 197 TB patients giving a sensitivity of 94% (Table 2). The mean ADA value was 71.2U/L whilst the mean total protein value was 51 g/dl.
Table 1. The mean ADA values in the different diagnostic category.
| Cases | Mean ADA (U/L) ±SD |
| Malignancy* (N = 26) | 12.9±13.1 |
| Congestive heart failure (N = 12) | 7.16±6.2 |
| Streptococcus pneumoniae effusion (N = 1) | 11 |
| Klebsiella pneumoniae empyema (N = 1) | 200 |
| Total TB pleuritis (N = 197) | 71.2±39.6 |
SD = standard deviation;
Kaposi sarcoma is the only malignancy with ADA of 73 above the cut-off.
Table 2. Validity of ADA as a diagnostic test.
| Cases | Sensitivity % (CI) | Specificity % (CI) | PPV % (CI) | NPV (%) (CI) | LR+ (CI) | LR− (CI) |
| Confirmed TB pleuritis (n = 72) | 94 (86–98) | 95 (82–99) | 97 (89–99) | 90 (76–97) | 18.9 (4.9–73 | 0.06 (0.02–0.15) |
| Clinical TB (n = 125) | 94 (88–97) | 95 (82–99) | 98 (93–100) | 84 (70–93) | 18.9 (4.9–73.0) | 0.06 (0.03–0.12) |
| Total TB Pleuritis (n = 197) | 94 (90–97) | 95 (82–99) | 99 (96–100 | 77.5 (63–88) | 18.9 (4.9–72.9) | 0.06 (0.03–0.10) |
PPV = positive predictive value; NPV = negative predictive value; CI = 95% confidence interval; LR+ = positive likelihood ratio; LR− = negative likelihood ratio.
Among the 125 probable TB pleuritis patients; 89 were HIV positive, 18 were HIV negative and HIV was not available for 18 patients. The median ADA values were 64 U/L and 58 U/L for HIV positive and HIV negative respectively.
CD4 cell counts were available for 58 of the 72 patients among the confirmed TB pleuritis patients. Fifty-one (88%) of the patients with known CD4 cell counts were confirmed HIV positive, whereas two were HIV negative with CD4 cell counts of 482 and 512 cells/microL. For five of the TB pleuritis patients the HIV was not documented, but as the patients had CD4 cell counts in the range of 19–208 cells/microL they were most likely HIV positive.
The ADA values with corresponding CD4 cell counts <50 and >50 cells/microL among confirmed TB pleuritis patients are presented in table 3. The mean CD4 cell counts in the confirmed TB pleuritis cases was 26 cells/microL in patients with CD4<50 cells/microL and 158 cells/microl in patients with CD4 count >50 cells/microL, (p<0.05) and the corresponding values among total TB cases was 29 and153 (p<0.05) respectively.
Table 3. HIV status, CD4 counts and ADA values for the 2 groups with <50 (N = 25) and >50 (N = 33) CD4 counts among confirmed TB pleuritis patients.
| CD4<50 cells/microL | CD4 >50 cells/microL | ||||||
| Case | HIV | CD4 | ADA | HIV | CD4 | ADA | |
| 1 | pos | 1 | 46 | 1 | pos | 55 | 38 |
| 2 | pos | 2 | 55 | 2 | pos | 55 | 60 |
| 3 | pos | 4 | 41 | 3 | pos | 57 | 60 |
| 4 | pos | 7 | 86 | 4 | pos | 60 | 112 |
| 5 | pos | 13 | 4 | 5 | pos | 64 | 112 |
| 6 | pos | 12 | 98 | 6 | NA | 65 | 61 |
| 7 | pos | 15 | 200 | 7 | pos | 66 | 133 |
| 8 | pos | 18 | 21 | 8 | pos | 66 | 51 |
| 9 | pos | 19 | 42 | 9 | pos | 70 | 36 |
| 10 | pos | 19 | 210 | 10 | pos | 74 | 112 |
| 11 | NA | 19 | 55 | 11 | pos | 75 | 73 |
| 12 | pos | 20 | 71 | 12 | pos | 77 | 43 |
| 13 | pos | 30 | 52 | 13 | pos | 79 | 53 |
| 14 | pos | 32 | 56 | 14 | pos | 80 | 186 |
| 15 | pos | 33 | 51 | 15 | pos | 80 | 38 |
| 16 | NA | 33 | 55 | 16 | pos | 105 | 1 |
| 17 | pos | 35 | 132 | 17 | pos | 115 | 88 |
| 18 | pos | 38 | 124 | 18 | pos | 121 | 44 |
| 19 | pos | 40 | 48 | 19 | pos | 132 | 53 |
| 20 | pos | 40 | 112 | 20 | pos | 163 | 64 |
| 21 | pos | 40 | 93 | 21 | pos | 168 | 35 |
| 22 | pos | 41 | 70 | 22 | pos | 179 | 44 |
| 23 | pos | 43 | 200 | 23 | NA | 180 | 38 |
| 24 | pos | 45 | 71 | 24 | pos | 200 | 107 |
| 25 | pos | 46 | 32 | 25 | pos | 200 | 49 |
| 26 | NA | 208 | 67 | ||||
| 27 | pos | 229 | 96 | ||||
| 28 | pos | 241 | 113 | ||||
| 29 | pos | 251 | 102 | ||||
| 30 | pos | 302 | 100 | ||||
| 31 | pos | 401 | 35 | ||||
| 32 | neg | 482 | 70 | ||||
| 33 | neg | 512 | 28 | ||||
pos = positive, neg = negative, NA = not available, ADA = adenosine deaminase.
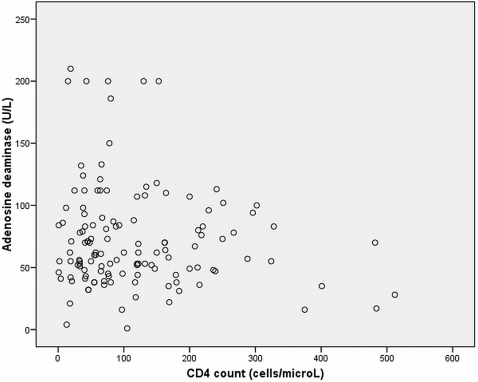
The sensitivity and specificity of ADA was 94% and 95% respectively as shown in table 2. The positive and negative likelihood ratio was 19 and 0.06 respectively. The mean ADA values for the different categories is shown in table 3. The mean ADA value was highest in tuberculous pleuritis except for Kaposi sarcoma, and empyema in which the ADA gave false positive result. The sensitivity of ADA was not affected by low CD4 cell count. In addition, there was no significant difference in the mean ADA value in patients with lower CD4 counts (81 U/L±55.1); (76 U/L±45.3) compared to those with higher CD4 cell counts (70 U/L±37.8); (72 U/L±40.6), (p>0.5) among confirmed and total TB pleuritis respectively. The four patients with CD4 cell counts of less than 10 cells/microL (1, 2, 4, and 7 cells/microL) among confirmed TB pleuritis patients had ADA values above the cut-off with values of 46, 55, 41, and 86 U/L, respectively (Table 3). There was no correlation between ADA values and blood CD4 cell counts (Figure 1).
Figure 1. Correlation plot of ADA in pleural fluid and blood CD4 counts in TB pleuritis patients (N = 129).
There was no statistical difference in the absolute blood lymphocyte count; pleural fluid lymphocytes number or percentage, lactate dehydrogenase and total protein between patients with CD4 cell counts <50 and >50 cells/microL when group comparison was made with non parametric Mann-Whitney U test (Table 4).
Table 4. Group comparison of laboratory markers among total TB pleuritis patients with <50 (N = 41) and >50 (N = 88) CD4 counts.
| Laboratory test (N) | Mann-Whitney U values | p- values |
| In pleural fluid | ||
| Adenosine deaminase U/L (129) | 1725.5 | 0.691 |
| Lymphocyte cells/microL (86) | 586.0 | 0.060 |
| Lymphocyte % (68) | 401.5 | 0.216 |
| Lactate dehydro-genase U/L (115) | 1315.0 | 0.325 |
| Total protein g/L (105) | 1211 | 0.925 |
| In blood | ||
| CD4 count cells/microL (129) | <0.05* | <0.05* |
| Absolute Lymphocyte count X109/L (75) | 552.0 | 0.181 |
| Red blood cells X 1012/L (111) | 1044.5 | 0.054 |
= significant difference between the 2 groups.
Furthermore there was no correlation between ADA values and blood CD4 counts, absolute lymphocyte count and red cell counts in blood or lymphocyte counts and percentage, lactate dehydrogenase and total protein in pleural fluid (Table 5).
Table 5. Correlation (r) values for ADA and other blood and pleural fluid markers in tuberculous pleuritis patients (N = 197).
| Test | r | p-values |
| In blood | ||
| CD4 counts cells/microL | −0.14 | 0.114 |
| Absolute blood lymphocytes x 10 9/L | −0.049 | 0.667 |
| Red blood cells x 1012/L | −0.044 | 0.644 |
| In pleural fluid | ||
| Leucocytes | 0.028 | 0.798 |
| LYM% | 0.110 | 0.373 |
| Lactate dehydro-genase U/L | 0.152 | 0.104 |
| Total protein g/L | 0.045 | 0.649 |
LYM% = pleural fluid lymphocyte percentage of total leucocytes.
Discussion
We have evaluated the usefulness of the ADA assay in predominantly HIV positive patients with very low CD4 counts. The overall sensitivity of the ADA assay in diagnosing tuberculous pleuritis was 94%. This finding is similar to that reported in a number of studies [12], [14], [18], [19], [20]. However this is the first study demonstrating high sensitivity of the ADA assay in HIV patients with very low CD4 cell counts. As shown in our study there was no significant difference in sensitivity when comparing cases with CD4 cell counts of less than 50 cells/microL to those with CD4 counts greater than 50 cells/microL. In fact, the mean ADA value was higher in the group with the lower CD4 cell count.
The ADA is useful for the diagnosis of TB pleuritis since the result is available on the same day compared to culture which takes about 2 weeks. This allows for rapid institution of TB treatment leading to improvement in patient outcome[21]. Furthermore the positive likelihood ratio (LR) was >10 and the negative likelihood ratio was <0.1, this implies that the ADA is useful in clinical practice to rule in and rule out TB pleuritis. The advantage of LR is that it is not affected by prevalence of disease as opposed to positive predictive value.
Previous studies evaluating the usefulness of ADA for the diagnosis of tuberculous pleuritis have either a very small number of HIV positive cases [22] or patients with higher mean CD4 counts [14]. Hsu et al [13] have shown a low sensitivity for ADA in tuberculous pleural effusion in immunocompromised chronic diseases patients, however only 10 patients were evaluated and the HIV status was not determined. Corral et al [23] reported a similar low sensitivity in HIV positive tuberculous meningitis cases whilst Reuter et al [15] reported low sensitivity of ADA in tuberculous pericarditis patients with advanced HIV disease compared to cases with higher CD4 cell count. The difference in the outcome of our retrospective study compared with that of Reuter et al may be due to the fact that majority of the patients in this study had lower CD4 cell counts.
Different cut-off values ranging from10 to 71 U/L have been used for positive ADA test, but the type of ADA assays has not shown any variations in sensitivity [24]. The cut-off value of 30 U/L for tuberculous pleuritis as used in this study is expected to offer a specificity of 98% [17] and is used in the clinical setting at our institution and by most laboratories in South Africa.
We did not find any correlation between the ADA values and CD4 cell counts. Even at CD4 counts of less than 10cells/microL, the ADA values were still above the cut-off. We also did not find any correlation between ADA values and CD4 cell counts in another cohort of South African HIV infected TB pleuritis patients [25]. This may be due to higher immune activation in HIV positive patients with very low CD4 counts [26]. Furthermore, we did not find any correlation between ADA values and pleural fluid lymphocyte numbers or percentage in agreement with the study by Lee et al [27] , but in contrast to other studies [12], [28]. One explanation could be that the isoenzyme ADA-2 which contributes significantly to total ADA in diagnosing TPE is found mainly in the monocytes [29] which are not significantly affected in HIV patients compared to CD4 T-lymphocytes.
Interferon gamma is considered to be a better marker than ADA for the diagnosis of tuberculous pleuritis, since its estimation is not affected by immununosuppression [30], [31]. However, in our study the ADA assay performed well even at very low CD4 count below 10 cells/microL and the cost of estimating ADA is 20 times less than that of interferon gamma estimation. Therefore ADA analysis is more cost effective than the estimation of interferon gamma for the diagnosis of tuberculous pleuritis in developing countries [18].
We have shown that ADA offers a high sensitivity and is rapid for the diagnosis of tuberculous pleuritis in HIV patients with low CD4 counts in a high TB endemic region. This is an important finding for countries with high burden of TB and HIV co-infection. Furthermore ADA is inexpensive, rapid, and simple to perform and is of great value in the diagnosis of tuberculous pleuritis for early institution of TB treatment.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was funded by QUOTA fellowship programme at the University of Bergen, Norway and thanks to the Regional Health Authorities of Western Norway who funded the running costs. The funders did not play any role in the study design, data analysis and preparation of manuscript.
References
- 1.WHO. Global tuberculosis Control: surveillance, planning, financing. WHO Report 2005. 2005. Geneva: World Health Organization WHO/HTM/TB/2005.349 WHO/HTM/TB/2005.349.
- 2.Alvarez GG, Thembela BL, Muller FJ, Clinch J, Singhal N, et al. Tuberculosis at Edendale Hospital in Pietermaritzburg, Kwazulu Natal, South Africa. Int J Tuberc Lung Dis. 2004;8:1472–1478. [PubMed] [Google Scholar]
- 3.Valdes L, Pose A, San Jose E, Marti, nez Vazquez JM. Tuberculous pleural effusions. Eur J Intern Med. 2003;14:77–88. doi: 10.1016/S0953-6205(03)00018-9. [DOI] [PubMed] [Google Scholar]
- 4.Valdes L, Alvarez D, San Jose E, Penela P, Valle JM, et al. Tuberculous pleurisy: a study of 254 patients. Arch Intern Med. 1998;158:2017–2021. doi: 10.1001/archinte.158.18.2017. [DOI] [PubMed] [Google Scholar]
- 5.Ibrahim WH, Ghadban W, Khinji A, Yasin R, Soub H, et al. Does pleural tuberculosis disease pattern differ among developed and developing countries. Respir Med. 2005;99:1038–1045. doi: 10.1016/j.rmed.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Baba K, Dyrhol-Riise AM, Sviland L, Langeland N, Hoosen AA, et al. Rapid and specific diagnosis of tuberculous pleuritis with immunohistochemistry by detecting Mycobacterium tuberculosis complex specific antigen MPT64 in patients from a HIV endemic area. AIMM In press. 2008 doi: 10.1097/PAI.0b013e31816c3f79. [DOI] [PubMed] [Google Scholar]
- 7.Burgess LJ, Maritz FJ, Le Roux I, Taljaard JJ. Combined use of pleural adenosine deaminase with lymphocyte/neutrophil ratio. Increased specificity for the diagnosis of tuberculous pleuritis. Chest. 1996;109:414–419. doi: 10.1378/chest.109.2.414. [DOI] [PubMed] [Google Scholar]
- 8.Piras MA, Gakis C, Budroni M, Andreoni G. Adenosine deaminase activity in pleural effusions: an aid to differential diagnosis. Br Med J. 1978;2:1751–1752. doi: 10.1136/bmj.2.6154.1751-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perez-Rodriguez E, Perez Walton IJ, Sanchez Hernandez JJ, Pallares E, Rubi J, et al. ADA1/ADAp ratio in pleural tuberculosis: an excellent diagnostic parameter in pleural fluid. Respir Med. 1999;93:816–821. doi: 10.1016/s0954-6111(99)90267-6. [DOI] [PubMed] [Google Scholar]
- 10.Valdes L, San Jose E, Alvarez D, Sarandeses A, Pose A, et al. Diagnosis of tuberculous pleurisy using the biologic parameters adenosine deaminase, lysozyme, and interferon gamma. Chest. 1993;103:458–465. doi: 10.1378/chest.103.2.458. [DOI] [PubMed] [Google Scholar]
- 11.Ungerer JP, Oosthuizen HM, Retief JH, Bissbort SH. Significance of adenosine deaminase activity and its isoenzymes in tuberculous effusions. Chest. 1994;106:33–37. doi: 10.1378/chest.106.1.33. [DOI] [PubMed] [Google Scholar]
- 12.Gaga M, Papamichalis G, Bakakos P, Latsi P, Samara I, et al. Tuberculous effusion: ADA activity correlates with CD4+ cell numbers in the fluid and the pleura. Respiration. 2005;72:160–165. doi: 10.1159/000084047. [DOI] [PubMed] [Google Scholar]
- 13.Hsu WH, Chiang CD, Huang PL. Diagnostic value of pleural adenosine deaminase in tuberculous effusions of immunocompromised hosts. J Formos Med Assoc. 1993;92:668–670. [PubMed] [Google Scholar]
- 14.Riantawan P, Chaowalit P, Wongsangiem M, Rojanaraweewong P. Diagnostic value of pleural fluid adenosine deaminase in tuberculous pleuritis with reference to HIV coinfection and a Bayesian analysis. Chest. 1999;116:97–103. doi: 10.1378/chest.116.1.97. [DOI] [PubMed] [Google Scholar]
- 15.Reuter H, Burgess LJ, Carstens ME, Doubell AF. Adenosine deaminase activity–more than a diagnostic tool in tuberculous pericarditis. Cardiovasc J S Afr. 2005;16:143–147. [PubMed] [Google Scholar]
- 16.Miller V, Mocroft A, Reiss P, Katlama C, Papadopoulos AI, et al. Relations among CD4 lymphocyte count nadir, antiretroviral therapy, and HIV-1 disease progression: results from the EuroSIDA study. Ann Intern Med. 1999;130:570–577. doi: 10.7326/0003-4819-130-7-199904060-00005. [DOI] [PubMed] [Google Scholar]
- 17.Blake J, Berman P. The use of adenosine deaminase assays in the diagnosis of tuberculosis. S Afr Med J. 1982;62:19–21. [PubMed] [Google Scholar]
- 18.Sharma SK, Suresh V, Mohan A, Kaur P, Saha P, et al. A prospective study of sensitivity and specificity of adenosine deaminase estimation in the diagnosis of tuberculosis pleural effusion. Indian J Chest Dis Allied Sci. 2001;43:149–155. [PubMed] [Google Scholar]
- 19.Burgess LJ, Maritz FJ, Le Roux I, Taljaard JJ. Use of adenosine deaminase as a diagnostic tool for tuberculous pleurisy. Thorax. 1995;50:672–674. doi: 10.1136/thx.50.6.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diacon AH, Van de Wal BW, Wyser C, Smedema JP, Bezuidenhout J, et al. Diagnostic tools in tuberculous pleurisy: a direct comparative study. Eur Respir J. 2003;22:589–591. doi: 10.1183/09031936.03.00017103a. [DOI] [PubMed] [Google Scholar]
- 21.Pfaller MA. Application of new technology to the detection, identification, and antimicrobial susceptibility testing of mycobacteria. Am J Clin Pathol. 1994;101:329–337. doi: 10.1093/ajcp/101.3.329. [DOI] [PubMed] [Google Scholar]
- 22.Villena V, Navarro-Gonzalvez JA, Garcia-Benayas C, Manzanos JA, Echave J, et al. Rapid automated determination of adenosine deaminase and lysozyme for differentiating tuberculous and nontuberculous pleural effusions. Clin Chem. 1996;42:218–221. [PubMed] [Google Scholar]
- 23.Corral I, Quereda C, Navas E, Martin-Davila P, Perez-Elias MJ, et al. Adenosine deaminase activity in cerebrospinal fluid of HIV-infected patients: limited value for diagnosis of tuberculous meningitis. Eur J Clin Microbiol Infect Dis. 2004;23:471–476. doi: 10.1007/s10096-004-1110-z. [DOI] [PubMed] [Google Scholar]
- 24.Goto M, Noguchi Y, Koyama H, Hira K, Shimbo T, et al. Diagnostic value of adenosine deaminase in tuberculous pleural effusion: a meta-analysis. Ann Clin Biochem. 2003;40:374–381. doi: 10.1258/000456303766477011. [DOI] [PubMed] [Google Scholar]
- 25.Baba K, Sørnes S, Hoosen AA, Lekabe JM, Mpe MJ, et al. Evaluation of Immune Responses in HIV-infected Patients with Pleural Tuberculosis by the QuantiFERON TB-Gold Interferon-gamma assay. BMC Infect Dis In press. 1998 doi: 10.1186/1471-2334-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tarhan G, Gumuslu F, Yilmaz N, Saka D, Ceyhan I, et al. Serum adenosine deaminase enzyme and plasma platelet factor 4 activities in active pulmonary tuberculosis, HIV-seropositive subjects and cancer patients. J Infect. 2006;52:264–268. doi: 10.1016/j.jinf.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 27.Lee YC, Rogers JT, Rodriguez RM, Miller KD, Light RW. Adenosine deaminase levels in nontuberculous lymphocytic pleural effusions. Chest. 2001;120:356–361. doi: 10.1378/chest.120.2.356. [DOI] [PubMed] [Google Scholar]
- 28.Baganha MF, Pego A, Lima MA, Gaspar EV, Cordeiro AR. Serum and pleural adenosine deaminase. Correlation with lymphocytic populations. Chest. 1990;97:605–610. doi: 10.1378/chest.97.3.605. [DOI] [PubMed] [Google Scholar]
- 29.Gakis C, Calia GM, Naitana AG, Ortu AR, Contu A. Serum and pleural adenosine deaminase activity. Correct interpretation of the findings. Chest. 1991;99:1555–1556. doi: 10.1378/chest.99.6.1555. [DOI] [PubMed] [Google Scholar]
- 30.Villena V, Lopez-Encuentra A, Pozo F, Echave-Sustaeta J, Ortuno-de-Solo B, et al. Interferon gamma levels in pleural fluid for the diagnosis of tuberculosis. Am J Med. 2003;115:365–370. doi: 10.1016/s0002-9343(03)00423-6. [DOI] [PubMed] [Google Scholar]
- 31.Villena V, Lopez-Encuentra A, Echave-Sustaeta J, Martin-Escribano P, Ortuno-de-Solo B, et al. Interferon-gamma in 388 immunocompromised and immunocompetent patients for diagnosing pleural tuberculosis. Eur Respir J. 1996;9:2635–2639. doi: 10.1183/09031936.96.09122635. [DOI] [PubMed] [Google Scholar]