Abstract
Background
Popliteal artery entrapment syndrome (PAES) is a rare but potentially limb threatening peripheral vascular disease occurring predominantly in young adults. This study is a retrospective review of 49 limbs in 38 patients with PAES treated surgically over an 8-year period.
Patients and methods
From 1995 to 2002, 38 patients with a mean age of 21 years (range, 18–29 years) underwent surgery for PAES at a single institution. The patients’ demographic data and clinical features are recorded. The preoperative diagnosis of PAES was made based on various combinations of investigations including positional stress test, duplex ultrasonography, computed tomography, computed tomographic angiography, and angiography.
Results
Nine, 33, and 7 patients had Delaney’s type I, II, and III PAES respectively. The surgical procedures consisted of simple release of the popliteal artery in 33 limbs (67.3%), autogenous saphenous vein (ASV) patch angioplasty with or without thromboendarterectomy (TEA) in 5 limbs (10.2%) and ASV graft interposition or bypass in 11 limbs (22.5%). At a median follow up of 34 months (range, 8–42 months), there were no postoperative complications and all the patients were cured of their symptoms.
Conclusions
PAES is an unusual but important cause of peripheral vascular insufficiency especially in young patients. Early diagnosis through a combined approach is necessary for exact diagnosis. Popliteal artery release alone or with vein bypass is the treatment of choice when intervention is indicated for good operative outcome and to prevent limb loss.
Keywords: popliteal artery, entrapment syndrome, diagnosis, surgery, treatment
Introduction
Popliteal artery entrapment syndrome (PAES) is an anomalous relationship between the popliteal artery and its surrounding musculotendinous structures. In 1879, TP Anderson Stuart, an Edinburgh medical student, described an anatomical variant of the popliteal artery which he had dissected from a gangrenous limb (Stuart 1879). The significance of this anomaly was not recognized until 1959 when Hamming and Vink (1965) in the Netherlands described the clinical syndrome which is associated with entrapment of the popliteal artery. In their study of 1,200 patients suffering from calf and foot claudication, 12 (1%) were less than 30 years of age and five of these had PAES.
PAES is a rare condition, and reports of this condition in the literature have been limited to a number of small series (Schurmann et al 1990; Di Marzo et al 1997; Hoelting et al 1997; Levien and Veller 1999; Ohara et al 2001). The incidence of PAES has been reported to range from 0.17% (Bouhoutsos and Daskalakis 1981) to 3.5% Gibson et al 1977) in a review of 20,000 asymptomatic Greek soldiers and a study of autopsy specimens, respectively, leading the authors to conclude that only a small proportion of cases give rise to symptoms. The concomitant entrapment of the popliteal vein with the artery has been reported in only 7.6% of cases (Persky et al 1991).
PAES is a congenital anomaly of muscle or tendon insertion in relation to the popliteal artery that causes functional occlusion of the artery. This entity results from a developmental defect in which the popliteal artery passes medial to and beneath the medial head of the gastrocnemius muscle or a slip of that muscle, with consequent compression of the artery. Rarely, an anomalous fibrous band or the popliteus muscle deep to the medial head of the gastrocnemius is the compressing structure (Haimovici et al 1972).
The management of PAES is the surgical repair of anomalous anatomical relationship between muscle and artery in the popliteal fossa that causes functional occlusion of the popliteal artery in young people with no risk factors (Fong and Downs 1989; Murray et al 1991; Di Marzo et al 1994). The goal of surgical management is to release the popliteal artery and to prevent the limb loss.
The purpose of this retrospective review is to report our experience of patients with PAES treated surgically in an 8-year period at a single institution.
Patients and methods
Over a period of 8 years, 1995–2002, 49 limbs in 38 patients were treated surgically for PAES. There were 31 males (81.6%) and 7 females (18.4%). Their ages ranged from 18 to 29 years with a median of 21 years. Bilateral PAES was observed in 11 (29%) patients. 950 patients with leg symptoms were evaluated in order to detect PAES in 38. 70% of the 950 patients had paresthesias, 22% had claudication, and 8% had rest pain.
The clinical features of these patients are listed in Table 1. Preoperative associated risk factors were examined as follows: smoking history and presence of concomitant disease; hypertension, hyperlipidemia, diabetes mellitus, chronic renal failure, ischemic heart disease. However, none of the patients had a clinical history of these diseases except for a history of smoking in 27 (71%) patients. The chief complaints were progressive intermittent claudication and foot coldness during exercise with symptom duration ranging from 8 months to 2 years.
Table 1.
Clinical features of patients with popliteal artery entrapment syndrome
| Variable | No. of patients (n = 38) |
No. of limbs (n = 49) |
|---|---|---|
| Sex | ||
| Male | 31 (81.6) | 41 (83.7) |
| Female | 7 (18.4) | 8 (16.3) |
| Smoking | ||
| No | 11 (29) | 11 (22.5) |
| Yes | 27 (71) | 38 (77.5) |
| Laterality | ||
| Right | 15 (39.5) | |
| Left | 12 (31.5) | |
| Bilateral | 11 (29) | |
| Symptoms | ||
| Progressive intermittent claudication | 32 (84.2) | 43 (87.7) |
| Foot coldness | 6 (15.8) | 6 (12.3) |
Values in parentheses are percentages.
The preoperative diagnosis of PAES was made based on various combinations of investigations including positional stress test (PST), duplex ultrasonography (DU), computed tomography (CT), computed tomographic angiography (CTA; Figure 1 and Figure 2), and angiography.
Figure 1.
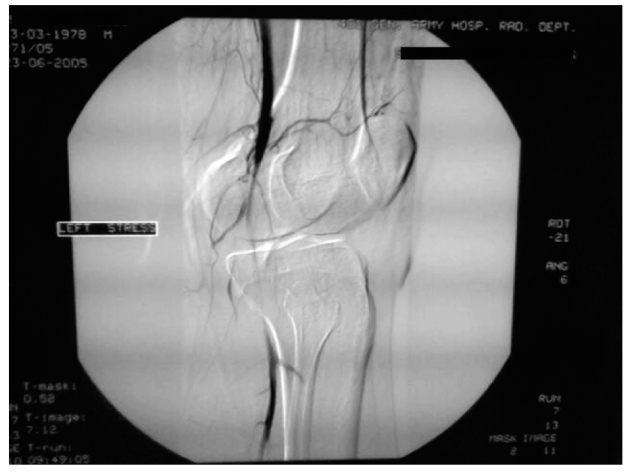
Computed tomographic angiogram findings with left popliteal artery entrapment syndrome.
Figure 2.
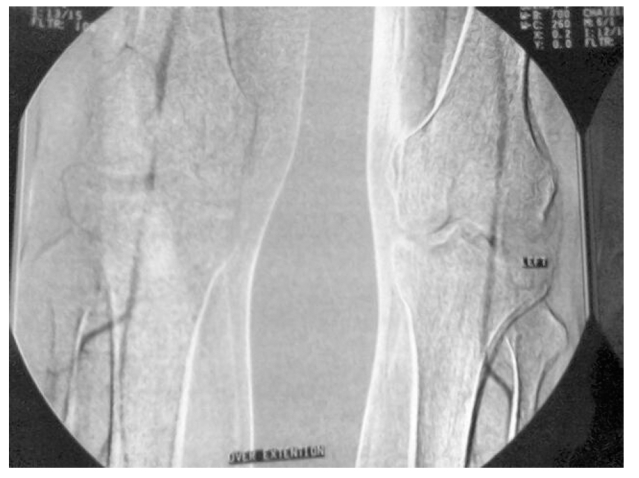
Computed tomographic angiogram findings with bilateral popliteal artery entrapment syndrome.
The PST is a clinical test that involves demonstrating the disappearance of the pedal pulses when the patient plantar flexes against resistance. All the patients had investigations performed on both limbs and 41 of 49 limbs (83.7%) showed a positive result. DU was performed in 43 limbs and it was useful in 32 (74.4%) out of them; in the rest of the patients the results were false-negative. In addition, CT was performed to confirm the diagnosis of PAES, to disclose the abnormal anatomic relationship between the popliteal artery and the medial head of the gastrocnemius muscle in all the patients. Angiographic findings in the basal position consisted of medial deviation of the proximal popliteal artery in 28 limbs (57.1%), poststenotic dilatation of the distal popliteal artery in 17 limbs (34.7%) and segmental or longitudinal occlusion of the popliteal artery in 4 limbs (8.2%).
Based on radiological and intraoperative findings, PAES was classified according to Delaney’s classification (Delaney and Gonzales 1971). PAES was divided into four variants: type I, the medial head of the gastrocnemius muscle arises normally from the upper posterior medial condyle of the femur, and the popliteal artery is displaced in an exaggerated loop passing medially around and beneath the muscle origin; type II, the medial head of the gastrocnemius muscle arises laterally, and the artery descends medially and beneath it, but its course is vertical and does not show exaggerated looping; type III, the popliteal artery is compressed by an accessory slip of muscle from the medial head of the gastrocnemius, being entrapped beneath the accessory head; and type IV, the popliteal artery is entrapped by the deeper popliteus muscle or by a fibrous band in the same location. In our study, 9 limbs of type I (18.4%), 33 limbs of type II (67.3%), 7 limbs of type III (14.3%) and none of type IV were encountered.
Musculotendonous section and popliteal artery release was performed in all the patients. In 33 limbs (67.3%), simple release of the popliteal artery by division of the medial head of gastrocnemius or other abnormal slips of muscle and tendon were performed because the vessel remained undamaged. In 5 limbs (10.2%), the popliteal artery was reconstructed by autogenous saphenous vein (ASV) patch angioplasty with or without thromboendarterectomy (TEA) because of occlusion or irreversible fibrosis of the popliteal artery. Finally, in 11 limbs (22.5%), ASV graft interposition or bypass with division of tendon in the limbs that showed irreversible fibrous thickening of the affected popliteal arterial wall was performed. The posterior approach was preferred for all the limbs, with dissection of the neurovascular bundle in the popliteal fossa, incising through any anomalous insertions or attachments involving the medial head of the gastrocnemius muscle, popliteus muscle, or other constricting bands causing compression of the popliteal vessels, unless a long vein bypass was required, whereby a medial approach was adopted. No effort was made to create any attachments of the medial head of the gastrocnemius muscle and this did not result in any disability.
None of our patients had postoperative antiplatelet therapy.
Results
The surgical outcome was good with no intraoperative or long-term postoperative complications. 8 patients had short-term postoperative complications such as wound hematoma, wound infection, and leg swelling; these patients were managed conservatively. The mean hospital stay was 9.5 days (range, 3–24 days).
The patients had a median follow-up of 34 months (range, 8–42 months). In regard to the method of follow-up within six postoperative months, all the patients underwent DU every three months as outpatients in order to determine patency of the vessels; the finding of postoperative DU was completely patent vessel in all the patients. After this postoperative period, they had been followed clinically, unless they express complains associated with popliteal vessel troubles.
All the patients were cured of their symptoms.
Discussion
The popliteal fossa is a diamond-shaped depression at the posterior of the knee that is bordered by biceps femoris tendon superolaterally, semimembranosus muscle superomedially, and medial and lateral hands of the gastrocnemius muscle inferiorly. The popliteal artery normally courses between the median and lateral heads of gastrocnemius muscle. Popliteal artery might be entrapped by neighbouring muscles and tendons due to variations that occur during embryologic development of the muscles and arteries (Elias et al 2003).
Several classifications of a number of anatomical variants of PAES have been described (Insua et al 1970; Whelan 1976; Bouhoutsos and Daskalakis 1981; Hoelting et al 1997). In the commonest of these, the popliteal artery enters the popliteal fossa by curving medial to the medial head of gastrocnemius. The artery then courses laterally, deep to the medial head, between the latter and the underlying medial condyle of the femur. In other variants, a slip of gastrocnemius, a fibrous band or the popliteus muscle may cause constriction of the artery. It is also possible for the popliteal artery to undergo compression without an embryological anomaly being present. This condition, termed physiological PAES, may be due to a hypertrophied gastrocnemius, soleus, plantaris, or semimembranosus muscle causing vascular compression and is sometimes seen in highly trained athletes (Gibson et al 1977; Erdoes et al 1994; Akkersdijk et al 1995). So PAES is most commonly found in young sportsmen or soldiers with well-developed muscles. Therefore, military surgeons have taken a special interest in this disorder, which has increased the diagnostic rate of PAES in military personnel (Love and Whelan 1965; Rich et al 1979; Rich 1982).
PAES must always be included in the list of differential diagnoses in young patients presenting with symptoms of peripheral vascular disease. This syndrome is both uncommon and difficult to diagnose. The characteristic signs and symptoms are the history of leg swelling, aching pain, pain at rest, and tiredness or cramping of the calf; but symptoms can vary and, until complications develop, physical signs are absent at rest. In the early stages, when the artery is patent except during calf-muscle contraction, symptoms in young persons are usually limited to transitory cramps or a feeling of coldness. In the later stages, when the artery is affected by stable lesions (local stenosis or occlusion, local thrombotic interruption, or poststenotic aneurysm) typical symptoms are severe acute ischemia and intermittent calf claudication, usually monolateral. Patients are usually admitted with complaint of intermittent calf claudication (walking pain). In this study, 32 patients complained for progressive intermittent claudication; while 6 patients complained for foot coldness during exercise. Intermittent calf claudication was observed in the majority of the limbs (n = 43).
Clinical examination is usually unreliable in the diagnosis of PAES. Physical examination may suggest an occluded popliteal segment with absent pulses only when the disease is advanced. The PST does not appear to be as reliable a clinical screening tool as once thought (Turnipseed 2001). However, if demonstrated, it should raise suspicion. This clinical test was performed in all of our patients and it was positive in 41 of 49 limbs (83.7%).
Angiography is the classical screening and diagnostic tool in PAES (Hoelting et al 1997). It demonstrates compression of the popliteal artery with the ankle plantar flexed, and in up to 53% of patients it may show occlusion of the popliteal artery at the time of presentation (Barabas and Macfarlane 1985). When the popliteal artery is patent, angiography may show medial displacement or compression of the artery (Persky et al 1991). Irregularity of the wall of the popliteal artery in an otherwise normal arterial tree should also raise suspicion, especially when associated with pre- or post-stenotic dilatation (Gyftokostas et al 1991). In our series, angiography was helpful in the diagnosis of PAES in all the 27 patients for whom it was performed.
CTA has been shown to be helpful in the diagnosis of PAES demonstrating the site of occlusion and anatomical abnormalities; it may also show other pathology that may mimic PAES such as adventitial cysts (Jasinski et al 1987; Beregi et al 1997). In the present study, CTA was performed in 11 patients and was successful. Angiographic findings (performing angiogram or CTA) were as follows: medial deviation of the proximal popliteal artery was demonstrated in 28 limbs, poststenotic dilatation of the distal popliteal artery in 17 limbs and segmental or longitudinal occlusion of the popliteal artery in 4 limbs.
Noninvasive imaging techniques such as DU, CT, CTA, magnetic resonance imaging (MRI) and magnetic resonance angiogram (MRA) may be used for diagnosis. Di Marzo and colleagues (1991) described the use of DU in screening in 1991. Unfortunately, there is a high rate of false-positive results especially in athletes (Akkersdijk et al 1995; Turnipseed 2001). DU proved to be useful in the present series, demonstrating popliteal artery occlusion in 32 limbs out of 43 limbs it was performed on. Finally, CT was performed to disclose the abnormal anatomic relationship between the popliteal artery and the medial head of the gastrocnemius muscle in all the patients. MRI and MRA were not performed in this study.
The management of a patient with PAES depends on the clinical picture. In symptomatic cases of PAES, surgery is definitely indicated in order to establish normal anatomy within the popliteal space and restore normal arterial flow to the extremity. The best surgical approach is a posterior S-shaped incision in the popliteal fossa, which enables complete exposure of the popliteal artery and its surrounding structures. Ideally, if the condition is recognized early and the vessel remains undamaged, simple release of the popliteal artery by division of the medial head of gastrocnemius or other abnormal slips of muscle and tendon may be all that is required (Lambert and Wilkins 1998). Reconstruction of the divided muscles does not appear to be necessary. Balloon angioplasty following thrombolysis has been reported but the rationale is unclear and the results unpredictable (Steurer et al 1995; Zund and Brunner 1995). When the artery is damaged, thromboendarterectomy and vein patching may seem attractive but this appears to give inferior results compared with interposition grafting using vein (Fowl et al 1995). When the artery is occluded, bypass is the treatment of choice because it has been shown to be superior to local therapy. If a short popliteal occlusion is present, revascularization may be carried out using the posterior approach with the patient in the prone position (Hoelting et al 1997; Lambert and Wilkins 1998). Good access is afforded to the artery and other structures of the popliteal fossa, facilitating both confirmation of the diagnosis and popliteal artery release. Furthermore, using this approach a suitable short saphenous vein may be used as an interposition graft. The medial approach to the popliteal artery is better for longer occlusions that need femoropopliteal bypass. The technical disadvantage of this approach, however, is that complete exposure of the popliteal artery and its relationship to the surrounding muscle is difficult; this can lead to the underlying entrapment being missed.
If detected and treated early, the outcome of PAES after surgery is generally good (Di Marzo et al 1997). However, if this condition is treated late when there is extensive arterial damage, then permanent claudication or even limb loss may be inevitable. Nonetheless, it is important to note that limb loss is a rare event because the arterial occlusion in PAES is usually a slow and chronic process allowing collateral formation.
Conclusions
PAES is an unusual but important cause of peripheral vascular insufficiency. It should be included in the differential diagnosis of acute popliteal artery occlusion, claudication or bizarre leg pains in young patients, especially men. Early diagnosis and surgical intervention is important for good operative outcome. It is usually diagnosed by radiological methods; angiography and CTA appears to be the most useful single investigation in the diagnosis of PAES but each imaging modality has its place and it may be appropriate to use several in combination to make a firm diagnosis. Popliteal artery release alone or with vein bypass is the treatment of choice when intervention is indicated.
References
- Akkersdijk WL, de Ruyter JW, Lapham R, et al. Colour duplex ultrasonographic imaging and provocation of popliteal artery compression. Eur J Vasc Endovasc Surg. 1995;10:342–5. doi: 10.1016/s1078-5884(05)80054-1. [DOI] [PubMed] [Google Scholar]
- Barabas AP, Macfarlane R. Popliteal artery entrapment syndrome. Br J Hosp Med. 1985;34:304–6. [PubMed] [Google Scholar]
- Beregi JP, Djabbari M, Desmoucelle F, et al. Popliteal vascular disease: evaluation with spiral CT angiography. Radiology. 1997;203:477–83. doi: 10.1148/radiology.203.2.9114108. [DOI] [PubMed] [Google Scholar]
- Bouhoutsos J, Daskalakis E. Muscular abnormalities affecting the popliteal vessels. Br J Surg. 1981;68:501–6. doi: 10.1002/bjs.1800680720. [DOI] [PubMed] [Google Scholar]
- Delaney TA, Gonzalez LL. Occlusion of popliteal artery due to muscle entrapment. Surgery. 1971;69:97–101. [PubMed] [Google Scholar]
- Di Marzo L, Cavallaro A, Sciacca V, et al. Diagnosis of popliteal artery entrapment syndrome: the role of duplex scanning. J Vasc Surg. 1991;13:434–8. doi: 10.1067/mva.1991.26375. [DOI] [PubMed] [Google Scholar]
- Di Marzo L, Cavallaro A, Sciacca V, et al. Natural history of entrapment of the popliteal artery. J Am Coll Surg. 1994;178:553–6. [PubMed] [Google Scholar]
- Di Marzo L, Cavallaro A, Mingoli A, et al. Popliteal artery entrapment syndrome: the role of early diagnosis and treatment. Surgery. 1997;122:26–31. doi: 10.1016/s0039-6060(97)90260-9. [DOI] [PubMed] [Google Scholar]
- Elias DA, White LM, Rubenstein JD, et al. Clinical evaluation and MRI imaging features of popliteal artery entrapment and cystic adventitial disease. AJR Am J Roentgenol. 2003;180:627–32. doi: 10.2214/ajr.180.3.1800627. [DOI] [PubMed] [Google Scholar]
- Erdoes LS, Devine JJ, Bernhard, et al. Popliteal vascular compression in a normal population. J Vasc Surg. 1994;20:978–86. doi: 10.1016/0741-5214(94)90236-4. [DOI] [PubMed] [Google Scholar]
- Fong H, Downs AR. Popliteal artery entrapment syndrome with distal embolization: a report of two cases. J Cardiovasc Surg. 1989;30:85–8. [PubMed] [Google Scholar]
- Fowl RJ, Kempczinski RF, Whelan TJ. Popliteal artery entrapment. In: Rutherford RB, editor. Vascular Surgery. 4. Philadelphia, Pennsylvania: Saunders WB; 1995. pp. 889–94. [Google Scholar]
- Gibson MHL, Mills JG, Johnson GE, et al. Popliteal entrapment syndrome. Ann Surg. 1977;185:341–8. doi: 10.1097/00000658-197703000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyftokostas D, Koutsoumbelis C, Mattheou T, et al. Post stenotic aneurysm in popliteal artery entrapment syndrome. J Cardiovasc Surg. 1991;32:350–2. [PubMed] [Google Scholar]
- Haimovici H, Sprayregen S, Johnson F. Popliteal artery entrapment by fibrous band. Surgery. 1972;72:789–92. [PubMed] [Google Scholar]
- Hamming JJ, Vink M. Obstruction of the popliteal artery at an early age. J Cardiovasc Surg. 1965;6:516–24. [PubMed] [Google Scholar]
- Hoelting T, Schuermann G, Allenberg JR. Entrapment of the popliteal artery and its surgical management in a 20-year period. Br J Surg. 1997;84:338–41. [PubMed] [Google Scholar]
- Insua JA, Young JR, Humphries AW. Popliteal artery entrapment syndrome. Arch Surg. 1970;101:771–5. doi: 10.1001/archsurg.1970.01340300127021. [DOI] [PubMed] [Google Scholar]
- Jasinski Masselink BA, Partridge RW, Decking BG, et al. Adventitial cystic disease of the popliteal artery. Radiology. 1987;163:153–5. doi: 10.1148/radiology.163.1.3823430. [DOI] [PubMed] [Google Scholar]
- Lambert AW, Wilkins DC. Popliteal artery entrapment syndrome: collaborative experience of the Joint Vascular Research Group. Br J Surg. 1998;85:1367–8. doi: 10.1046/j.1365-2168.1998.00944.x. [DOI] [PubMed] [Google Scholar]
- Levien LJ, Veller MG. Popliteal artery entrapment syndrome: more common than previously recognized. J Vasc Surg. 1999;30:587–98. doi: 10.1016/s0741-5214(99)70098-4. [DOI] [PubMed] [Google Scholar]
- Love JW, Whelan TJ. Popliteal artery entrapment syndrome. Am J Surg. 1965;109:620–4. doi: 10.1016/s0002-9610(65)80016-2. [DOI] [PubMed] [Google Scholar]
- Murray A, Halliday M, Croft RJ. Popliteal artery entrapment syndrome. Br J Surg. 1991;78:1414–9. doi: 10.1002/bjs.1800781204. [DOI] [PubMed] [Google Scholar]
- Ohara N, Miyata T, Oshiro H, et al. Surgical treatment for popliteal artery entrapment syndrome. Cardiovasc Surg. 2001;9:141–4. doi: 10.1016/s0967-2109(00)00110-1. [DOI] [PubMed] [Google Scholar]
- Persky JM, Kempezinski RF, Fowl RJ. Entrapment of the popliteal artery. Surg Gynecol Obstet. 1991;173:84–90. [PubMed] [Google Scholar]
- Rich NM, Collins GJ, Jr, McDonald PT, et al. Popliteal vascular entrapment: it’s increasing interest. Arch Surg. 1979;114:1377–84. doi: 10.1001/archsurg.1979.01370360031004. [DOI] [PubMed] [Google Scholar]
- Rich NM. Popliteal entrapment and adventitial cystic disease. Surg Clin North Am. 1982;62:449–65. doi: 10.1016/s0039-6109(16)42737-4. [DOI] [PubMed] [Google Scholar]
- Schurmann G, Mattfeldt T, Hofmann W, et al. The popliteal artery entrapment syndrome: presentation, morphology and surgical treatment of 13 cases. Eur J Vasc Surg. 1990;4:223–31. doi: 10.1016/s0950-821x(05)80199-9. [DOI] [PubMed] [Google Scholar]
- Steurer J, Hoffmann U, Schneider E, et al. New therapeutic approach to popliteal artery entrapment syndrome (PAES) Eur J Vasc Endovasc Surg. 1995;10:243–7. doi: 10.1016/s1078-5884(05)80120-0. [DOI] [PubMed] [Google Scholar]
- Stuart TPA. Note on a variation in the course of the popliteal artery. J Anat Physiol. 1879;13:162–5. [PMC free article] [PubMed] [Google Scholar]
- Turnipseed WD. Popliteal entrapment syndrome. J Vasc Surg. 2001;35:910–5. doi: 10.1067/mva.2002.123752. [DOI] [PubMed] [Google Scholar]
- Whelan TJ. Popliteal artery entrapment syndrome. In: Haimovici H, editor. Vascular Surgery: Principles and Techniques. New York: McGraw-Hill; 1976. pp. 493–504. [Google Scholar]
- Zund G, Brunner U. Surgical aspects of popliteal artery entrapment syndrome: 26 years of experience with 26 legs. Vasa. 1995;24:29–33. [PubMed] [Google Scholar]


