Abstract
Chronic heart failure (HF) is a major cause of morbidity and mortality particularly in the elderly and a growing healthcare burden in Italy. The objective was to assess the cost-effectiveness of candesartan cilexetil, an angiotensin II type 1 receptor blocker (ARB) for the treatment of HF. A pre-specified economic evaluation was conducted on resource utilization (cardiovascular drug treatment, cardiovascular and non-cardiovascular hospital admission, cardiovascular procedures/operations) prospectively collected alongside the CHARM program, a series of parallel randomized clinical trials comparing candesartan with placebo (standard therapy) in patients with NYHA Class II-IV HF: CHARM-Alternative (LVEF ≤40% patients not receiving ACE inhibitors because of previous intolerance); CHARM-Added (LVEF ≤40% patients currently receiving ACE inhibitors); or CHARM-Preserved (LVEF ≥40% patients). The primary outcome for the component trials was the composite of cardiovascular death or worsening hospital admission for HF and of the overall program all-cause mortality. Adjunctive treatment with candesartan in CHARM-Alternative and CHARM-Added led to clinical benefits and to either cost-savings or a small additional cost, depending on the trial. The less certain clinical benefit in CHARM-Preserved was obtained at modest extra cost. The incremental cost-effectiveness ratios (ICERs) were estimated to range from €713 per life year gained for CHARM-Alternative to dominant for CHARM-Added and the pooled reduced LVEF trials.
Keywords: candesartan, heart failure, cost-effectiveness analysis, cost-consequence analysis, CHARM, Italy
Background
Chronic heart failure (HF) is a major cause of morbidity and mortality particularly in the elderly and a growing problem in most affluent countries given the expansion of aging populations (McMurray et al 1998; Mazza et al 2005). In Italy, one of the countries in the world with the highest proportion of persons above 65 years of age (United Nations Statistical Office 1991), HF creates a significant burden on healthcare budgets (SEOSI Investigators 1997). While advances in the management of HF in the past several decades have significantly decreased the mortality and morbidity associated with this condition, hospitalization rates due to HF have remained on an upward trend (Koelling et al 2004; Jimenez-Navarro et al 2006). This may be due to a rise in both chronic HF incidence and survival.
Cost-effective HF disease management and prevention are programs of equal importance in the drive to successfully combat the burden of the widespread disease of HF in Italy. The aim must begin with the reduction of HF hospitalization and it can therefore be expected that re-hospitalization rates are factors that will be increasingly scrutinized in the selection of HF treatments by healthcare providers in justifying the cost of treatment. The current paper describes the cost-effectiveness of candesartan cilexetil for the treatment of HF in Italy.
Results of the CHARM (Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity) program (Swedberg et al 1999; Granger et al 2003; McMurray et al 2003a, b; Pfeffer et al 2003; Yusuf et al 2003; Young et al 2004) suggest that candesartan reduces morbidity and mortality in patientswith HF and LV ejection fraction (LVEF) ≤40%, as well as those with LVEF >40%. Thus, when administered as an alternative to an angiotensin-converting enzyme (ACE) inhibitor, or as an add-on to standard therapy including an ACE inhibitor and/or beta blockers, candesartan has been shown to provide cardiovascular benefits in symptomatic HF including the decrease in the risk of hospital admission for worsening HF and deaths due to cardiovascular causes in HF. These outcomes have been further translated into economic benefits based on the analysis of cost-consequence and cost-effectiveness of the resource use data collected prospectively alongside the CHARM study (Reed et al 2005). The focus of the current paper is on the cost effectiveness of candesartan in HF from the perspective of the National Health Service (SSN Servizio Sanitario Nazionale) in Italy.
Methods
Study design
A pre-specified economic evaluation was conducted on resource utilization collected alongside the CHARM program. Methods employed in the current research complied with the published guidelines for the conduct of economic evaluations in Italy (Capri et al 2001). In addition, given the multinational scope of economic evaluations (including those previously published (McMurray et al 2006) conducted alongside the CHARM program, and the inherent methodological challenges that exist for meeting the important objectives of generalizability, transparency, and statistical power, the research methods employed were also developed in respect to the consensus frameworks that are currently being developed in the literature to address these difficulties (Reed et al 2005).
Within the CHARM program itself, patients with NYHA Class II-IV HF recruited from 26 countries were enrolled into one of three trials: CHARM-Alternative (patients with LVEF 40% or less who were not receiving ACE inhibitors because of previous intolerance) (Granger et al 2003); CHARM-Added (patients with LVEF 40% or less or who were currently receiving ACE inhibitors) (McMurray et al 2003b); or CHARM-Preserved (patients with LVEF higher than 40%) (Yusuf et al 2003). Overall, 7601 patients (7599 with data) were randomly assigned candesartan (n = 3803, titrated as tolerated to 32 mg once daily) or matching placebo (n = 3796), and followed up for at least 2 years. The primary outcome of the overall program was all-cause mortality and for the two reduced LVEF trials combined (CHARM Alternative and Added), and for each of the component trials was the composite of cardiovascular death or worsening hospital admission for HF.
Resource utilization data collection
Data on resource utilization were collected prospectively and only comprised components of direct costs including, cardiovascular drug treatment (eg, digitalis glycosides, diuretics, beta-blockers, calcium channel blockers, other vasodilators, anti-arrhythmic drugs, ACE inhibitors, angiotensin II type 1 receptor blockers [ARBs], or other cardiovascular drugs such as lipid-lowering agents and anticoagulants), cardiovascular and non-cardiovascular hospital admission (eg, proportion of patients admitted, number of admissions per patient, number of hospital days per patient) and ward type for cardiovascular admission (eg, intensive/coronary care unit, cardiology, general internal medicine), admissions for cardiovascular reasons (eg, number, duration, ward type), and cardiovascular procedures/operations. For costing non-cardiovascular hospital admissions, it was assumed that 10% of time was spent in intensive care and 90% on the general ward. Indirect costs (work productivity losses) were not considered. The time horizon over which the cost-consequence and incremental cost-effectiveness of candesartan were compared to placebo was equivalent to that observed during the period of the program (ie, no future projections were made).
Estimation of costs
Hospital admissions
For the estimation of costs, two approaches were employed (CIBIS-II Investigators and Health Economics Group 2001); these included the diagnosis-related group (DRG) costing (with data obtained from Health Ministry, Tariffa Unica Nazionale TUC, 2006) and per diem (hospital bed-day) costing (with data obtained from (Azienda Ospedaliera di Busto Arsizio, Varese – Administration Dept in a group of Hospitals in Lombardy Region). In this analysis actual recorded days in hospital were multiplied by the daily unit costs of hospital care.
Drug treatment
The source for drugs costs was Gazzetta Ufficiale n.227, 29.09.2006; these were the standard tariffs for the financial year 2006 and the costs of generic drugs were used where available. Candesartan treatment costs also accounted for the initiation and up-titration of candesartan, and included four extra GP visits and four checks of blood biochemistry (CIBIS-II Investigators and Health Economics Group).
Cardiovascular procedures
The DRG costs of cardiovascular procedures and operations were obtained from local government sources (Health Ministry, Tariffa Unica Nazionale TUC, 2006). Where there was a lapse in data given little reliable and comparable public information is available for the per diem costs of cardiovascular procedures, DRG costs were used as proxies in the per diem analysis. All costs were converted to 2006 values using the local price index with the exception of drug prices which are for 2006. Costs are presented in the local currency (Euro) and were discounted at 3% for costs and outcomes (ISTAT 2007; Capri et al 2001). The costs used in this analysis are summarized in Table 1.
Table 1.
Unit costsa used in the economic analysis of CHARM
| Event | DRG cost in Italy |
|---|---|
| Hospitalizations | |
| Worsening HF | 2669.82 |
| Myocardial infarction | 3356.12 |
| Unstable angina | 1882.16 |
| Stroke | |
| Hemorrhagic | 3391.02 |
| Ischemic/unknown/other | 3391.02 |
| Transient ischemic attack | 2124.34 |
| Cardiogenic shock | 2669.82 |
| Atrial tachyarrhythmia | 2213.55 |
| Ventricular arrhythmia | 5455.44 |
| Pulmonary embolism | 3710.82 |
| Other cardiovascular event | 1765.16 |
| Cancer (neoplasm) | 2182.41 |
| Other non-cardiovascular event | 939.69 |
| Cardiovascular procedures | |
| Cardiac catheterizations including angiography | 2722.25 |
| CABG | 17898.67 |
| PTCA with stent | 7878.69 |
| PTCA without stent | 5455.44 |
| Implantation of cardioverter defibrillator | 23876.00 |
| Implantation of pacemaker | 9438.59 |
| Heart transplantation | 61066.70 |
| Ventricular assist device | 7052.77 |
| Other cardiac surgery for HF | 19419.16 |
| Other cardiovascular procedure/operation | 9187.76 |
| Per diem costs | |
| Intensive/coronary care unit | 1860.65 |
| Cardiology ward | 600 |
| General medical ward | 200 |
| Non-cardiovascular admission | 366.07 |
| Visit general practitioner | 20.66 |
| Laboratory test – blood biochemistry | 23.81 |
| Candesartan 4 mg | 0.83 |
| Candesartan 8 mg | 0.69 |
| Candesartan 16 mg | 0.89 |
| Candesartan 32 mg | 1.13 |
All costs shown in Euro (1€ = US$1.20 and £0.67).
Abbreviations: CABG, coronary artery bypass grafting; DRG, diagnosis related group; HF, heart failure; PTCA, percutaneous transluminal coronary intervention.
Economic analysis
Effectiveness parameter employed
The effectiveness parameters employed in the analyses were as follows. For the component trials of the CHARM program the composite of cardiovascular death or hospital admission for worsening HF was employed. The candesartan to placebo hazard ratios for this outcome were as follows: CHARM-Alternative: 0.77 (95% CI 0.67–0.89, p = 0.0004) (Granger et al 2003); CHARM-Added: 0.85 (0.75–0.96, p = 0.011) (McMurray et al 2003b); CHARM-Preserved: 0.89 (0.77–1.03, p = 0.118) (Yusuf et al 2003); the overall CHARM program: 0.84 (0.77–0.91, p < 0.0001) (Pfeffer et al 2003). For the overall CHARM program and for the two reduced LVEF trials combined (CHARM-Alternative and Added) all-cause mortality was the pre-specified primary endpoint (Swedberg et al 1999). The candesartan to placebo hazard ratio for this outcome in the overall CHARM program was 0.91 (0.83–1.00, p = 0.055) (Pfeffer et al 2003) and in the reduced LVEF trials 0.88 (0.79–0.98, p = 0.018) (Young et al 2004).
Economic analyses conducted
The economic analyses were based on the comparison of placebo, ie, standard therapy for HF to candesartan added to standard therapy. Two types of economic analyses were performed: these include a cost-consequence analysis (CCA) for a disaggregated examination of resource costs and health outcomes associated with the alternative interventions; and cost-effectiveness analysis (CEA) in which the alternative interventions are examined in light of total cost per unit of health outcome. Thus CCA was performed for each component trial and for the overall CHARM program using the primary outcome of the component trials as the measure of effectiveness. For this, the annual cost per patient treated to postpone or prevent one patient experiencing a cardiovascular death or hospital admission for worsening HF within the trial was calculated (Mauskopf et al 1998). For CEA, the incremental cost-effectiveness ratios (ICERs), in terms of cost per life year gained (LYG), were estimated for the reduced LVEF trials given that there was a significant increase in survival with candesartan in these two trials combined. CEA was not performed for CHARM-Preserved given that no reduction was observed in that trial for candesartan in cardiovascular or all cause mortality.
Sensitivity analyses
Univariate sensitivity analyses were conducted by increasing the length of stay for non-cardiovascular admissions by 30% in the candesartan group to model the potential additional cost of possible adverse effects related to candesartan (White 2003); adding an additional GP visit to account for a possible adverse event or laboratory abnormality; and testing the impact of alternative discount rates for costs and benefits in the range 0% and 8% (Capri et al 2001).
Statistical methods
Statistical analyses were performed using SAS software (version 8; SAS Institute Inc., Cary, NC, USA). The group mean approach was employed to account for censored data (eg, early dropouts and missing values) (Cook et al 2004) and bootstrapping, a technique which involves re-sampling was implemented as the test for significance.
Results
Clinical outcomes
All-cause hospital admissions
The rates and number of hospital admissions in the overall CHARM program and each component trial (Table 2 and Figures 1 and 2) show that noteworthy differences between the placebo and treatment groups were obtained for the number of patients hospitalized in CHARM-Overall and CHARM-Alternative. In the CHARM-Overall trial 63.8% of patients in the placebo group were admitted to hospital at least once for any reason, compared with 62.4% in the candesartan group [odds ratio (OR) 0.94, 95% CI 0.86–1.03, p = 0.20]. In the CHARM-Alternative trial 63.3% of patients in the placebo group were admitted to hospital at least once for any reason, compared with 60.2% in the candesartan group (odds ratio [OR] 0.88, 95% CI 0.71–1.05, p = 0.15).
Table 2.
Hospital admissions and patients hospitalized in CHARM
| CHARM-Alternative | |||||
|---|---|---|---|---|---|
| Placebo (n = 1015) | Candesartan (n = 1013) | Diff (95% CI) | p-value | ||
| All patients | |||||
| Patient-years | 2582 | 2658 | |||
| No. deaths | 296 | 265 | |||
| No. admissions | 1835 | 1719 | |||
| No. hospl days | 16,816 | 15,079 | |||
| Hosp days/adm | 9.16 | 8.77 | 0.39 (−0.60, 1.38) | 0.44 | |
| Adm/patient | 1.81 | 1.70 | 0.11 (−0.10, 0.32) | 0.30 | |
| Hosp days/patient | 16.57 | 14.89 | 1.86 (−1.11, 4.47) | 0.24 | |
| Hosp days/patient-year | 6.51 | 5.67 | 0.84 (−0.27, 1.95) | 0.13 | |
| Patients hospitalized | |||||
| No. hosp patient | 643 | 610 | 0.88 (0.73, 1.05) | 0.15 | |
| Adm/patient | 2.85 | 2.82 | 0.04 (−0.25, 0.32) | 0.80 | |
| Hosp days/patient | 26.15 | 24.72 | 1.43(−2.73, 5.60) | 0.50 | |
| CHARM-Added | |||||
| Placebo (n = 1272) | Candesartan (n = 1276) | Diff (95% CI) | p-value | ||
| All patients | |||||
| Patient-years | 3721 | 3846 | |||
| No. deaths | 412 | 377 | |||
| No. admissions | 2799 | 2462 | |||
| No. hospl days | 24,161 | 21,902 | |||
| Hosp days/adm | 8.63 | 8.90 | −0.26 (−0.95, 0.43) | 0.45 | |
| Adm/patient | 2.20 | 1.93 | 0.27 (0.07, 0.47) | 0.008 | |
| Hosp days/patient | 18.99 | 17.16 | 1.83 (−0.65, 4.31) | 0.15 | |
| Hosp days/patient-year | 6.49 | 5.7 | 0.79 (−0.06, 1.64) | 0.070 | |
| Patients hospitalized | |||||
| No. hospit patient | 858 | 852 | 0.97 (0.82, 1.14) | 0.71 | |
| Adm/patient | 3.26 | 2.89 | 0.37 (0.13, 0.62) | 0.003 | |
| Hosp days/patient | 28.16 | 25.71 | 2.45 (−0.94, 5.85) | 0.16 | |
| CHARM-Preserved | |||||
| Placebo (n = 1509) | Candesartan (n = 1514) | Diff (95% CI) | p-value | ||
| All patients | |||||
| Patient-years | 4387 | 4434 | |||
| No. deaths | 244 | 237 | |||
| No. admissions | 2548 | 2510 | |||
| No. hosp days | 22,705 | 22,942 | |||
| Hosp days/adm | 8.91 | 9.14 | −0.23 (−1.02, 0.56) | 0.57 | |
| Adm/patient | 1.69 | 1.66 | 0.03 (−0.13, 0.20) | 0.71 | |
| Hosp days/patient | 15.05 | 15.15 | −0.11 (−2.20, 2.01) | 0.92 | |
| Hosp days/patient-year | 5.18 | 5.17 | 0.01 (−0.73,0.75) | 0.98 | |
| Hospitalized patients | |||||
| No. hosp patient | 922 | 9.12 | 0.96 (0.83, 1.12) | 0.63 | |
| Adm/patient | 2.76 | 2.75 | 0.01 (−0.21, 0.23) | 0.92 | |
| Hosp days/patient | 24.63 | 25.16 | −0.53 (−3.71, 2.65) | 0.74 | |
| CHARM-Overall | |||||
| Placebo (n = 3796) | Candesartan (n = 3803) | Diff (95% CI) | p-value | ||
| All patients | |||||
| Patient-years | 10,690 | 10,938 | |||
| No. deaths | 945 | 886 | |||
| No. admissions | 7182 | 6691 | |||
| No. hosp days | 63,681 | 59,923 | |||
| Hosp days/adm | 8.87 | 8.96 | −0.09 (−0.55, 0.38) | 0.71 | |
| Adm/patient | 1.89 | 1.76 | 0.13 (0.02, 0.24) | 0.018 | |
| Hosp days/patient | 16.78 | 15.76 | 1.02 (−0.38, 2.42) | 0.15 | |
| Hosp days/patient-year | 5.96 | 5.48 | 0.48 (−0.02,0.98) | 0.056 | |
| Hospitalized patients | |||||
| No.hospitalized patient | 2423 | 2374 | 0.94 (0.86, 1.03) | 0.20 | |
| Adm/patient | 2.96 | 2.82 | 0.15 (0.003, 0.29) | 0.045 | |
| Hosp days/patient | 26.28 | 25.24 | 1.04 (−0.99, 3.07) | 0.31 | |
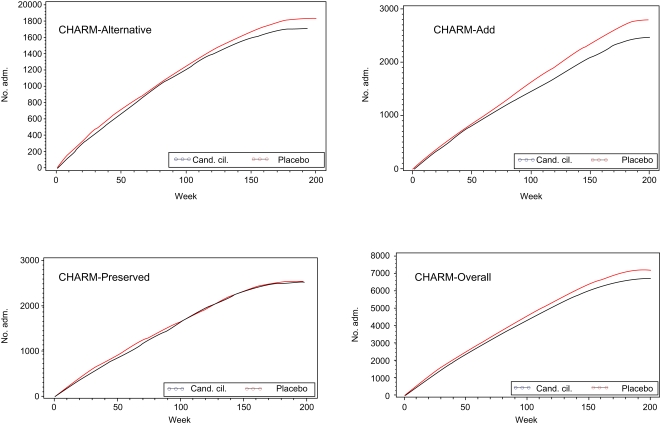
Figure 1.
Cumulative number of hospital admissions.
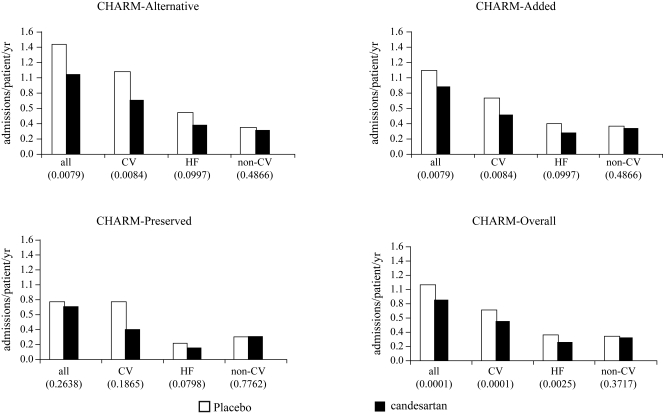
Figure 2.
Rates of hospital admission for any cause, all cardiovascular (CV) reasons, heart failure (HF) only, and non-cardiovascular reasons (p-values within brackets).
Additionally for CHARM-Overall, the number of admissions per patient hospitalized was 2.96 in the placebo group when compared with 2.82 in the candesartan group (p = 0.045). The average length of an individual admission was 8.9 days in the placebo group and 9.0 days in the candesartan group. The average number of days spent in hospital for admitted patients was 26.3 days in the placebo group and 25.2 days in the candesartan group. As a result, treatment with candesartan resulted in fewer hospital admissions (7182 for placebo vs 6691 for candesartan or 1.060 compared with 0.853 admissions per year of follow-up, p = 0.0001) and fewer days in hospital (placebo 63681, candesartan 59923; Table 2, p = ns). The number of days in hospital per patient-year of follow-up was 6.0 in the placebo group and 5.5 in the candesartan group (p = 0.056).
Cardiovascular hospital admissions
The frequency of hospital admissions for specific cardiovascular causes (Table 3 and Figure 2) shows that candesartan reduced both the proportion of patients admitted (−20%) and the number of admissions (−28%) for worsening HF. Atrial tachyarrhythmias also showed a trend toward reduction, while hospital admission for myocardial infarction showed a trend toward reduction in CHARM Added and CHARM Preserved. ‘Other’ cardiovascular admissions were fewer in the candesartan group in CHARM Alternative and CHARM-Added, and just nominally greater in the candesartan group in CHARM-Preserved. Examination of these miscellaneous admissions did not reveal an excess in any specific category of event.
Table 3.
Hospital admissions by cause – number of patients and % of patients admitted
| CHARM-Alternative | CHARM-Added | CHARM-Preserved | CHARM-Overall | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo (n = 1015) |
Candesartan (n = 1013) |
Placebo (n = 1272) |
Candesartan (n = 1514) |
Placebo (n = 1509) |
Candesartan (n = 1514) |
Placebo (n = 3796) |
Candesartan (n = 3803) |
|||||||||
| Adm | Pat (%) | Adm | Pat (%) | Adm | Pat (%) | Adm | Pat (%) | Adm | Pat (%) | Adm | Pat (%) | Adm | Pat (%) | Adm | Pat (%) | |
| Worsening CHF | 608 | 291 (28.7) | 445 | 212 (20.9)† | 836 | 382 (30.0) | 607 | 323 (25.3)** | 566 | 279 (18.5) | 402 | 230 (15.2)* | 2010 | 952 (25.1) | 1454 | 765 (20.1)† |
| Myocardial infarction | 51 | 45 (4.4) | 55 | 48 (4.7) | 64 | 50 (3.9) | 38 | 36 (2.8) | 64 | 61 (4.0) | 68 | 58 (3.8) | 179 | 156 (4.1) | 161 | 142 (3.7) |
| Unstable angina | 109 | 74 (7.3) | 137 | 94 (9.3) | 192 | 117 (9.2) | 145 | 101 (7.9) | 235 | 145 (9.6) | 244 | 154 (10.2) | 536 | 336 (8.9) | 526 | 349 (9.1) |
| Stroke | 34 | 32 (3.2) | 35 | 32 (3.2) | 38 | 34 (2.7) | 37 | 34 (2.7) | 56 | 52 (3.5) | 52 | 46 (3.0) | 128 | 118 (3.1) | 124 | 112 (3.0) |
| TIA | 16 | 15 (1.5) | 16 | 12 (1.2) | 10 | 9 (0.7) | 20 | 19 (1.5) | 25 | 21 (1.4) | 21 | 18 (1.2) | 51 | 45 (1.2) | 57 | 49 (1.3) |
| Hypotension | 14 | 14 (1.4) | 26 | 20 (2.0) | 24 | 22 (1.7) | 60 | 55 (4.3)† | 15 | 13 (0.9) | 25 | 23 (1.5) | 53 | 49 (1.3) | 111 | 98 (2.6) |
| Atrial tachyarrhythmia | 44 | 34 (3.4) | 53 | 34 (3.4) | 56 | 46 (3.6) | 61 | 49 (3.8) | 123 | 86 (5.7) | 82 | 59 (3.9)* | 223 | 166 (4.4) | 196 | 142 (3.7) |
| Ventricular arrhythmia | 55 | 39 (3.8) | 52 | 45 (4.4) | 86 | 65 (5.1) | 68 | 52 (4.1) | 16 | 14 (0.9) | 18 | 17 (1.1) | 157 | 118 (3.1) | 138 | 114 (3.0) |
| Pulmonary embolism | 9 | 8 (0.8) | 6 | 6 (0.6) | 11 | 10 (0.8) | 6 | 6 (0.5) | 9 | 9 (0.6) | 8 | 8 (0.5) | 29 | 27 (0.7) | 20 | 20 (0.53) |
| Other CV event | 244 | 181 (17.8) | 225 | 164 (16.2) | 416 | 254 (20.0) | 362 | 249 (19.5) | 368 | 247 (16.4) | 400 | 280 (18.5) | 1028 | 682 (18.0) | 987 | 693 (18.2) |
| CV unknown | 1 | 1 (0.0) | 1 | 1 (0.0) | 1 | 1 (0.0) | 1 | 1 (0.0) | ||||||||
| Cancer | 55 | 35 (3.5) | 42 | 30 (3.0) | 49 | 36 (2.8) | 83 | 52 (4.1) | 84 | 52 (3.5) | 53 | 40 (2.6) | 188 | 123 (3.2) | 178 | 122 (3.21) |
| Other non-CV event | 596 | 334 (32.9) | 627 | 350 (34.6) | 1017 | 531 (41.8) | 975 | 525 (41.1) | 986 | 546 (36.2) | 1136 | 589 (38.9) | 2599 | 1411 (37.2) | 2738 | 1464 (38.5) |
| All | 1835 | 643 (63.4) | 1719 | 610 (60.2) | 2799 | 858 (67.5) | 2462 | 852 (66.8) | 2548 | 922 (61.1) | 2510 | 912 (60.2) | 7182 | 2423 (63.8) | 6691 | 2374 (62.4) |
p < 0.05.
p < 0.01.
<0.001 (comparison of proportion of randomized patients).
Abbreviations: CV, cardiovascular; TIA, transient ischemic attack.
Procedures and operations
Other than cardiac catheterizations, the frequency of other cardiovascular procedures (Table 4), was relatively few and did not differ between treatment groups.
Table 4.
Number of cardiovascular procedures in CHARM
| Procedure | CHARM-Alternative | CHARM-Added | CHARM-Preserved | CHARM-Overall | ||||
|---|---|---|---|---|---|---|---|---|
| Placebo (n = 1015) |
Candesartan (n = 1013) |
Placebo (n = 1272) |
Candesartan (n = 1276) |
Placebo (n = 1509) |
Candesartan (n = 1514) |
Placebo (n = 3796) |
Candesartan (n = 3803) |
|
| Cardiac catheterization incl. angiography | 160 | 139 | 228 | 172 | 261 | 262 | 649 | 573 |
| CABG | 26 | 21 | 22 | 39 | 42 | 46 | 90 | 106 |
| PTCA with stent | 31 | 30 | 55 | 36 | 73 | 78 | 159 | 144 |
| PTCA without stent | 11 | 10 | 19 | 10 | 23 | 15 | 53 | 35 |
| Implantation of cardioverter defibrillator | 31 | 30 | 60 | 51 | 9 | 9 | 100 | 90 |
| Implantation of pacemaker | 50 | 49 | 75 | 80 | 54 | 67 | 179 | 196 |
| Heart transplantation | 8 | 7 | 15 | 17 | 0 | 0 | 23 | 24 |
| Ventricular assist device | 6 | 6 | 3 | 1 | 0 | 1 | 9 | 8 |
| Other cardiac surgery for heart failure | 3 | 2 | 6 | 9 | 10 | 11 | 19 | 22 |
| Other cardiovascular procedure/operation | 112 | 112 | 213 | 188 | 196 | 192 | 521 | 492 |
| Total no. CV procedures | 438 | 406 | 696 | 603 | 668 | 681 | 1802 | 1690 |
| Total no. patients with CV procedure (% of patients) | 239 (23.6) | 218 (21.5) (p = 0.27) | 349 (27.4) | 320 (25.1) (p = 0.18) | 350 (23.2) | 342 (22.6) (p = 0.69) | 938 (24.7) | 880 (23.1) (p = ??) |
Abbreviations: CABG, coronary artery bypass grafting; CV, cardiovascular; PTCA, percutaneous transluminal coronary intervention.
Economic outcomes
Costs of adjunctive candesartan treatment
The per diem cost analysis of adding candesartan to conventional treatment (Table 5) shows that in CHARM-Overall the cost of care in the candesartan group was slightly (1%) less, even taking into account the cost of candesartan. There was a net cost-saving in both CHARM-Alternative and CHARM-Added (5% reduction in cost in both trials). In CHARM-Preserved, there was a net increase in the daily cost of care (6% increase in cost). The results of the DRG analysis (Table 6) were very similar; there was a small increase observed (2%) in the net daily cost of care with candesartan.
Table 5.
Daily per patient cost (€) of treatment in CHARM – per diem analysis
| CHARM-Alternative | CHAR-Added | CHARM-Preserved | CHARM-Overall | |||||
|---|---|---|---|---|---|---|---|---|
| Placebo | Candesartan | Placebo | Candesartan | Placebo | Candesartan | Placebo | Candesartan | |
| Hospitalizations | 9.33 | 7.99 | 8.92 | 7.71 | 6.95 | 6.71 | 8.21 | 7.37 |
| Cardiovascular procedures | 4.15 | 3.77 | 4.76 | 4.43 | 2.98 | 3.04 | 3.88 | 3.71 |
| Concomitant medication | 2.03 | 1.97 | 2.62 | 2.48 | 1.99 | 1.87 | 2.22 | 2.11 |
| Study drug | 0.00 | 0.80 | 0.00 | 0.79 | 0.00 | 0.83 | 0.00 | 0.81 |
| Titration cost | 0.00 | 0.18 | 0.00 | 0.16 | 0.00 | 0.16 | 0.00 | 0.17 |
| Total | 15.51 (0.85)a | 14.71 (0.78) | 16.30 (0.66) | 15.56 (0.61) | 11.93 (0.44) | 12.61 (0.46) | 14.31 (0.36) | 14.16 (0.33) |
standard error within parentheses.
Table 6.
Daily per patient cost of treatment (€) in CHARM – DRG analysis
| CHARM-Alternative | CHARM-Added | CHARM-Preserved | CHARM-Overall | |||||
|---|---|---|---|---|---|---|---|---|
| Placebo | Candesartan | Placebo | Candesartan | Placebo | Candesartan | Placebo | Candesartan | |
| Hospitalizations | 3.85 | 3.37 | 3.88 | 3.18 | 2.80 | 2.55 | 3.43 | 2.97 |
| Cardiovascular procedures | 4.15 | 3.77 | 4.76 | 4.43 | 2.98 | 3.04 | 3.88 | 3.71 |
| Concomitant medication | 2.03 | 1.97 | 2.62 | 2.48 | 1.99 | 1.87 | 2.22 | 2.11 |
| Study drug | 0.00 | 0.80 | 0.00 | 0.79 | 0.00 | 0.83 | 0.00 | 0.81 |
| Titration cost | 0.00 | 0.18 | 0.00 | 0.16 | 0.00 | 0.16 | 0.00 | 0.17 |
| Total | 10.03 (0.50)a | 10.09 (0.44) | 11.26 (0.44) | 11.04 (0.39) | 7.77 (0.25) | 8.45 (0.25) | 9.53 (0.23) | 9.76 (0.20) |
standard error within parentheses.
CCA
The CCA of the data (Table 7) shows that adjunctive treatment with candesartan in CHARM-Alternative and CHARM-Added led to clinical benefits and to either cost-savings or a small additional annual cost, depending on the trial. The less certain clinical benefit in CHARM-Preserved was obtained at modest extra cost.
Table 7.
Cost-consequence analysis of candesartan compared with placebo in the treatment of HF – clinical benefits and annual per patient saving/cost increase (95% CI)
| Trial | Clinical benefits vs placebo | DRG costs | Per-diem costs |
|---|---|---|---|
| CHARM-Alternative | CV deaths (−15%) HF admission (−32%) | Net increase (€22 ± 245/year) | Savings (€291 ± 421/year) |
| CHARM-Added | CV deaths (−16%) HF admission (−17%) | Savings (€81 ± 214/year) | Savings (€267 ± 328/year) |
| CHARM-Preserved | CV deaths (−1%), ns HF admission (−15%) | Net increase (€249 ± 128/year) | Net increase (€249 ± 232/year) |
| CHARM-Overall | CV deaths (−12%) HF admission (−21%) | Net increase (€83 ± 111/year) | Savings (€56 ± 179/year) |
Abbreviations: CV, cardiovascular; DRG, diagnosis related group; HF, heart failure.
CEA
The CEA of the two reduced LVEF CHARM trials (Table 8) was conducted using a conservative approach of employing DRG costs only as all scenarios with per diem costs obtained results of cost-savings. Thus, following this approach in terms of the cost per LYG, the incremental cost-effectiveness ratio (ICER) was estimated to range from €713 for CHARM-Alternative to dominant for CHARM-Added and the pooled reduced LVEF data. The results for Italy are consistent with the results in France, Germany, and the UK.
Table 8.
Cost-effectiveness of candesartan in the CHARM reduced LVEF trials (based on DRG costs)
| CHARM TRIAL LYG (95% CI) | Cost per LYG (95% CI) | ||||
|---|---|---|---|---|---|
| Italy | France | Germany | UK | ||
| Alternative | 0.078 (0.003–0.15) | €713 (−7736; 431,600) | Dominant | €3881 (−17,728; 1,105,920) | €2547 (−18 171; 1 059 150) |
| Added | 0.061 (−0.002–0.12) | Dominanta | Dominant | €1427 (−14,479; −984,755) | Dominant |
| Reduced LVEF pooled | 0.068 (0.02–0.12) | Dominant | Dominant | €2997 (−19,183; 121,500) | €1348 (−16 225; 106 600) |
Dominant means a cost per LYG could not be calculated because costs were lower in the candesartan than in the placebo group.
Abbreviations: CV, cardiovascular; DRG, diagnosis related group; HF, heart failure; LVEF, left ventricular ejection fraction; LYG, life year gained.
Sensitivity analyses
The sensitivity analyses (Table 9) showed that increasing the length of stay for non-cardiovascular admissions by 30% increased the cost per day in the candesartan group by 2%. As a result, candesartan was no longer cost-saving in any comparison. Adding one GP visit for an adverse event or laboratory abnormality which led to a reduction in the dose of, or discontinuation of candesartan resulted in an increase in daily costs by €0.01. As expected the cost per day is sensitive to changes in discount rate 0% and 8%.
Table 9.
Sensitivity analysis
| Alternative | Added | Preserved | Overall | |||||
|---|---|---|---|---|---|---|---|---|
| Placebo | Candesartan | Placebo | Candesartan | Placebo | Candesartan | Placebo | Candesartan | |
| Increasing the length of stay for non-cardiovascular admissions by 30% | ||||||||
| Total | 10.03 (0.50) | 10.30 (0.45) | 11.26 (0.44) | 11.26 (0.39) | 7.77 (0.25) | 8.66 (0.25) | 9.53 (0.23) | 9.97 (0.20) |
| Adding one GP visit for an adverse event or laboratory abnormality | ||||||||
| Total | 10.03 (0.50) | 10.10 (0.44) | 11.26 (0.44) | 11.04 (0.39) | 7.77 (0.25) | 8.46 (0.25) | 9.53 (0.23) | 9.77 (0.20) |
| Sensitivity analysis 0% costs, 0% benefits | ||||||||
| Total | 10.33 (0.52) | 10.37 (0.70) | 11.65 (0.46) | 11.39 (0.40) | 7.98 (0.26) | 8.70 (0.26) | 9.82 (0.24) | 10.05 (0.21) |
| Sensitivity analysis 8% costs, 8% benefits | ||||||||
| Total | 9.56 (0.43) | 9.64 (0.48) | 10.63 (0.41) | 10.46 (0.37) | 7.42 (0.24) | 8.04 (0.24) | 9.06 (0.22) | 9.28 (0.19) |
Discussion
The CHARM program has shown that in treated patients, a substantial reduction in the proportion of patients admitted with worsening HF (and an even more marked reduction in the number of such admissions), without any increase in length of stay, contributed to a reduction in the rate of admission (and hospital bed days) for any reason, though this overall reduction was more modest. This is because the full impact of the reduction in admissions for worsening HF was attenuated by increased survival in the candesartan-treated patients (who, therefore, spent more time at risk of hospital admission for other reasons).
Via economic analysis, it has been shown that the cost-savings accruing from even this modest reduction in the rate of hospital admission for any cause largely offset the cost of candesartan in Italy. According to the method of analysis (per diem compared with DRG), candesartan was, essentially, cost-neutral in the overall-CHARM program (though clinical effectiveness was not proven in one component trial, CHARM Preserved). There was, however, heterogeneity between the component trials in the program. Although candesartan treatment was associated with either a small reduction or increase in the net overall cost of care in CHARM-Alternative and CHARM-Added, depending on the analysis, in CHARM-Preserved (in which candesartan treatment did not reduce the primary endpoint significantly) there was a consistent and modest increase in the net cost of care. There appear to be two reasons for this. Though the proportional reduction in the rate and number of admissions for worsening HF was similar in all three CHARM trials, the absolute number of admissions prevented was smaller, relative to the number of patients treated, in CHARM-Preserved (ie, the rate of admission for worsening HF was lower in CHARM-Preserved, Figure 2). Consequently, the cost-offset was less in CHARM-Preserved than in the other two trials.
Another possible explanation is the increased number of ‘other’ cardiovascular admissions in the candesartan group in CHARM-Preserved (a reduction, rather than excess, of these admissions was observed in the other CHARM trials), also observed with cardiovascular procedures in CHARM-Preserved. As no specific pattern could be observed for the excesses in these outcomes, both increases may be viewed as a chance finding. Also noteworthy is the overall net cost of treatment in CHARM-Added (candesartan added to full conventional treatment, including an ACE-inhibitor), which was comparable to the net treatment cost obtained for CHARM-Alternative; and that the essentially cost-neutral outcome of these analyses of CHARM was obtained despite adding the cost of extra clinic visits and biochemical tests to reflect the extra costs related to initiating, up-titrating the dose, and monitoring the effects of candesartan.
Our findings on the cost – consequences and cost-effectiveness of using candesartan in Italy are consistent with the results observed in the economic evaluations of using candesartan in France, Germany, and the UK (McMurray et al 2006). In CE, the ICER was second only to the result obtained for France where candesartan was dominant across both of the reduced LVEF trials as well as their pooled data. Furthermore, although broadly in keeping with economic analyses of other effective treatments for HF (Paul et al 1994; Glick et al 1995, 2002; CIBIS-II Investigators and Health Economics Group 2001), it is difficult to directly compare the current and previously published candesartan studies to those. No other placebo-controlled study included such a broad spectrum of patients, had within-trial data for such a long period of follow-up (with the exception of the ACE inhibitor enalapril in the treatment arm of the studies of LV dysfunction in the case of the latter) (Cook et al 2004), or added the drug to such extensive background treatment. Nevertheless, a consistent message from these prior economic analyses and ours is that reduction in hospital admission offsets the cost of treatment. Remarkably, the cost-offset has been sufficient with all treatments examined, to date, to be cost-saving or more or less cost-neutral. This is despite each new drug being used as an additional treatment and against a trend of falling lengths of hospital stay.
The economic results, coupled with the clinical findings of the CHARM program, have clear implications for the management of patients with HF in Italy. Not only does candesartan improve important clinical outcomes in HF but also offers these benefits at little or no additional cost to the health care system; indeed, its use in patients with HF and reduced LV systolic function may lead to an actual reduction in the direct costs of health care. This is an important finding for health-care providers and society more generally, because there is no trade-off between the interest of the individual patient and the greater population served by the health-care system.
As with any analysis of this type there were limitations. By using the full unit cost of candesartan, our analyses have reduced the cost-effectiveness of this treatment for the patient or private insurer who provides a co-payment for the cost of treatment (although this co-payment is exempt for the elderly in Italy). We did not take account of indirect costs, pension payments in those who survived were not taken account, costs related to death out of hospital. We had less detailed and complete information on non-cardiovascular procedures and drugs. However, the main driver of costs is hospital admission and we did have information on these and tried to account for lack of information on the former in our sensitivity analyses.
As with all economic analyses based on clinical trials of limited duration, there is concern that costs may only be postponed and that there may be ‘catch-up’ over the whole life-time of a patient. We believe that this is unlikely, given the relatively long-duration of follow-up of CHARM (37.7 months) compared with the average life-expectancy of patients with HF. We carried out a cost–consequence analysis of CHARM-Preserved even though the pre-specified primary outcome was not reduced significantly.
In summary, when added to currently recommended treatment, candesartan reduces hospital admissions for worsening HF and increases survival in patients with HF (Granger et al 2003; McMurray et al 2003a, b; Pfeffer et al 2003; Yusuf et al 2003), specifically in those with low LVEF (Pfeffer et al 2003; Young et al 2004) and does this at little or no extra direct cost to the Italian health-care system. Candesartan is, therefore, a clinically and economically attractive adjunctive treatment for HF in Italy, representing significant value to the individual patient as well as to health-care providers.
Acknowledgments
The CHARM Program was supported by AstraZeneca and the present analyses were supported by AstraZeneca and Takeda. The authors would like to thank Bernt Kartman (AstraZeneca), Alessandra Fionda (Takeda) and Francis Pang (Takeda) for their health economic input, Klas Svensson (freelance consultant) for statistical support and Gauri Saul (freelance writer) for editorial assistance funded by Takeda.
Disclosures
Giorgio L. Colombo, Mauro Caruggi, Chiara Ottolini, and Aldo P Maggioni have served as consultants to or received research grants and honoraria from AstraZeneca and Takeda and/or other major pharmaceutical companies.
Aldo P. Maggioni received research grants and honoraria for conferences from AstraZeneca, Novartis and Takeda.
References
- Capri S, Ceci A, Terranova L, et al. Guidelines for economic evaluations in Italy: recommendations from the Italian group of pharmacoeconomic studies. Drug Inf J. 2001;35:189–201. [Google Scholar]
- CIBIS-II Investigators and Health Economics Group*. Reduced costs with bisoprolol treatment for heart failure: an economic analysis of the second Cardiac Insufficiency Bisoprolol Study (CIBIS-II) Eur Heart J. 2001;22:1021–31. doi: 10.1053/euhj.2000.2532. [DOI] [PubMed] [Google Scholar]
- Cook J, Drummond M, Heyse JF. Economic endpoints in clinical trials. Stat Methods Med Res. 2004 Apr;13(2):157–76. doi: 10.1191/0962280204sm359ra. [DOI] [PubMed] [Google Scholar]
- Covinsky KE, Palmer RM, Fortinsky RH. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Am Geriatr Soc. 2003;51:451–8. doi: 10.1046/j.1532-5415.2003.51152.x. [DOI] [PubMed] [Google Scholar]
- Garattini L, Grilli R, Scopelliti D, et al. A proposal for Italian Guidelines in pharmacoeconomics. Pharmacoeconomics. 1995;7:1–6. doi: 10.2165/00019053-199507010-00001. [DOI] [PubMed] [Google Scholar]
- Glick H, Cook J, Kinosian B, et al. Costs and effects of enalapril therapy in patients with symptomatic heart failure: an economic analysis of the Studies of Left Ventricular Dysfunction (SOLVD) Treatment Trial. J Card Fail. 1995;1:371–80. doi: 10.1016/s1071-9164(05)80006-5. [DOI] [PubMed] [Google Scholar]
- Glick HA, Orzol SM, Tooley JF, et al. Economic evaluation of the randomized aldactone evaluation study (RALES): treatment of patients with severe heart failure. Cardiovasc Drugs Ther. 2002;16:53–9. doi: 10.1023/a:1015371616135. [DOI] [PubMed] [Google Scholar]
- Granger CB, McMurray JJ, Yusuf S, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003;362:772–6. doi: 10.1016/S0140-6736(03)14284-5. [DOI] [PubMed] [Google Scholar]
- Instituto Nazionale di Statistica. 2007 URL: http://demo.istat.it/index_e.html.
- Jimenez-Navarro MF, Munoz Garcia AJ, Garcia-Pinilla JM, et al. Trends of hospitalizations for chronic heart failure in Andalusia in the last decade. Rev Clin Esp. 2006;206:474–6. doi: 10.1157/13094894. [DOI] [PubMed] [Google Scholar]
- Koelling TM, Chen RS, Lubwama RN, et al. The expanding national burden of heart failure in the United States: the influence of heart failure in women. Am Heart J. 2004;147:74–8. doi: 10.1016/j.ahj.2003.07.021. [DOI] [PubMed] [Google Scholar]
- Lim SC, Doshi V, Castasus B, et al. Factors causing delay in discharge of elderly patients in an acute care hospital. Ann Acad Med Singapore. 2006;35:27–32. [PubMed] [Google Scholar]
- Mauskopf JA, Paul JE, Grant DM. The role of cost-consequence analysis in healthcare decision-making. Pharmacoeconomics. 1998;13:277–88. doi: 10.2165/00019053-199813030-00002. [DOI] [PubMed] [Google Scholar]
- Mazza A, Tikhonoff V, Casiglia, et al. Predictors of congestive heart failure mortality in elderly people from the general population. Int Heart J. 2005;46:419–31. doi: 10.1536/ihj.46.419. [DOI] [PubMed] [Google Scholar]
- McMurray J, Davie A. The pharmacoeconomics of ACE inhibitors in chronic heart failure. Pharmacoeconomics. 1996;9:188–97. doi: 10.2165/00019053-199609030-00002. [DOI] [PubMed] [Google Scholar]
- McMurray JJV, Petrie MC, Murdoch DR, et al. Clinical epidemiology of heart failure: public and private health burden. Eur Heart J. 1998;19:9–16. [PubMed] [Google Scholar]
- McMurray J, Ostergren J, Pfeffer M, et al. Clinical features and contemporary management of patients with low and preserved ejection fraction heart failure: baseline characteristics of patients in the Candesartan in Heart failure-Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur J Heart Fail. 2003a;5:261–70. doi: 10.1016/s1388-9842(03)00052-7. [DOI] [PubMed] [Google Scholar]
- McMurray JJ, Ostergren J, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003b;362:767–71. doi: 10.1016/S0140-6736(03)14283-3. [DOI] [PubMed] [Google Scholar]
- McMurray JJ, Andersson FL, Stewart S, et al. Resource utilization and costs in the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur Heart J. 2006;27:1447–58. doi: 10.1093/eurheartj/ehl016. [DOI] [PubMed] [Google Scholar]
- O’Meara E, Solomon S, McMurray J, et al. Effect of candesartan on New York Heart Association functional class. Results of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur Heart J. 2004;25:1920–6. doi: 10.1016/j.ehj.2004.07.025. [DOI] [PubMed] [Google Scholar]
- Paul SD, Kuntz KM, Eagle KA, et al. Costs and effectiveness of angiotensin converting enzyme inhibition in patients with congestive heart failure. Arch Intern Med. 1994;154:1143–9. [PubMed] [Google Scholar]
- Pfeffer MA, Swedberg K, Granger CB, et al. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 2003;362:759–66. doi: 10.1016/s0140-6736(03)14282-1. [DOI] [PubMed] [Google Scholar]
- Reed SD, Anstrom KJ, Bakhai A, et al. Conducting economic evaluations alongside multinational clinical trials. Toward a research consensus. Amn Heart J. 2005;149:434–43. doi: 10.1016/j.ahj.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Seguin P, Laviolle B, Chanavaz C. Factors associated with multidrug-resistant bacteria in secondary peritonitis: impact on antibiotic therapy. Clin Microbiol Infect. 2006;12:980–5. doi: 10.1111/j.1469-0691.2006.01507.x. [DOI] [PubMed] [Google Scholar]
- SEOSI Investigators. Survey on heart failure in Italian hospital cardiology units. Results of the SEOSI study. Eur Heart J. 1997;18:1457–64. doi: 10.1093/oxfordjournals.eurheartj.a015472. [DOI] [PubMed] [Google Scholar]
- Swedberg K, Pfeffer M, Granger C, et al. Candesartan in heart failure – assessment of reduction in mortality and morbidity (CHARM): rationale and design. Charm-Programme Investigators. J Card Fail. 1999;5:276–82. doi: 10.1016/s1071-9164(99)90013-1. [DOI] [PubMed] [Google Scholar]
- United Nations, Statistical Office. Demographic Yearbook. New York: United Nations; 1991. [Google Scholar]
- Vilella A, Prat A, Bare ML, Bayas JM, et al. Med Clin (Barc) Vol. 100. 1993. Risk of nosocomial infection in elderly patients admitted to a university hospital [Spanish] pp. 128–31. [PubMed] [Google Scholar]
- White HD. Candesartan and heart failure: the allure of CHARM. Lancet. 2003;362:754–5. doi: 10.1016/S0140-6736(03)14294-8. [DOI] [PubMed] [Google Scholar]
- Young JB, Dunlap ME, Pfeffer MA, et al. Mortality and morbidity reduction with Candesartan in patients with chronic heart failure and left ventricular systolic dysfunction: results of the CHARM low-left ventricular ejection fraction trials. Circulation. 2004;110:2618–26. doi: 10.1161/01.CIR.0000146819.43235.A9. Epub 2004 Oct 18. [DOI] [PubMed] [Google Scholar]
- Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777–81. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]