Abstract
Fracture-healing is regulated in part by mechanical factors. Study of the processes by which the mechanical environment of a fracture modulates healing can yield new strategies for the treatment of bone injuries. This article focuses on several key unanswered questions in the study of mechanotransduction and fracture repair. These questions concern identifying the mechanical stimuli that promote bone-healing, defining the mechanisms that are involved in this process, and examining the potential for cross-talk between investigations of mechanotransduction in bone-healing and in healing of other mesenchymally derived tissues. Several approaches to obtain accurate estimates of the mechanical stimuli present within a fracture callus are proposed, and our current understanding of the mechanotransduction processes involved in bone-healing is reviewed. Further study of mechanotransduction mechanisms is needed in order to identify those that are most critical and active during the various phases of fracture repair. A better understanding of the effect of mechanical factors on bone-healing will also benefit the study of healing, regeneration, and engineering of other skeletal tissues.
The Mechanical Environment of a Healing Fracture
Fracture-healing is governed by genetic as well as epigenetic factors. The mechanical environment of a healing fracture is one such epigenetic factor that is known to have a profound influence on the rate and success of the repair process. Understanding the effect of the mechanical environment, and in particular the mechanisms by which mechanical cues modulate bone-healing, has applications ranging from clinical management of fractures to bone-tissue engineering and basic science investigations of cell fate.
Multiple parameters contribute to the mechanical environment of a fracture callus. These include the stability of fixation, the geometry or type of fracture, and the type of loading. For example, highly stable fixation, such as that provided by a rigidly applied internal fixation plate and by an interfragmentary screw, results in primary cortical healing without the formation of a callus. Less stable external fixation results in a cartilaginous callus, the size of which depends heavily on the stiffness of the fixator frame1-3. The geometry or type of fracture affects how the external loads are transferred to the callus tissue. A simple example is the comparison of a transverse fracture line to an oblique fracture line. Even under the same axial compressive load, the oblique fracture will result in much higher shear strains in the callus tissue adjacent to the osteotomized or fractured bone ends.
Mechanical loading of a fracture callus occurs most commonly as a consequence of weight-bearing; however, dynamization of the fracture gap has also been investigated. Results of these studies have shown that while the effects of loading depend heavily on the rate4,5, mode6-9, and magnitude10,11 of loading, as well as gap size10, application of cyclic compressive displacements can enhance healing through increased callus formation and more rapid ossification12,13. As evidenced by the success of distraction osteogenesis in both experimental and clinical settings, application of successive tensile displacements can also promote bone formation. In contrast to the effects of cyclic compressive loading, however, bone formation in distraction osteogenesis occurs primarily via intramembranous ossification. This marked difference in the effects of tensile and compressive loading on the mode of bone formation illustrates the complexity as well as the potency of using mechanical loading to augment healing.
From the above examples, it is clear that mechanical perturbations can modulate bone-healing. However, in the context of developing effective strategies to enhance bone formation and repair, it is necessary to answer several key questions. First, what specific mechanical stimuli promote bone-healing? Second, what mechanotransduction mechanisms are involved in fracture repair? Third, what can be learned from or applied to healing and regeneration of other tissues? In the following sections, we will discuss each of these questions with respect to what is currently known, suggest and present new approaches that can be used to further current understanding of these topics, and outline existing challenges in the study of mechanotransduction processes in fracture repair.
What Specific Mechanical Stimuli Promote Bone-Healing?
Through observational and, to a lesser extent, empirical study, several theories have been developed on the role of certain mechanical stimuli in governing differentiation of pluripotent mesenchymal tissue into bone, cartilage, fibrocartilage, and fibrous tissue. For example, Carter et al.14 proposed that different combinations of hydrostatic pressure and tensile strain promote formation of different skeletal tissues, and Claes and Heigele15 postulated that these two stimuli regulate intramembranous versus endochondral ossification. Prendergast et al.16,17 instead proposed that the two key mechanical stimuli that govern mesenchymal tissue differentiation are shear strain and fluid flow. Direct comparison of the predictions of these theories to histological analyses of bone-healing suggests that the most accurate predictions are those based on shear strain and fluid flow18. However, each of these theories is unable to predict certain features of the fracture-healing process16,18, indicating that further research is needed in this area.
Testing the effects of specific mechanical variables on fracture-healing requires, above all, a method for quantifying the distribution of mechanical stimuli in the fracture callus. This is not a straightforward task, because these “tissue-level” mechanical stimuli are determined not only by the axial, transverse, and bending loads applied to the bone but also by the geometry of the bone and fracture gap and the mechanical properties of the callus tissues. A natural approach to tackling this complex problem is to estimate the mechanical stimuli via finite element analysis. In this computational approach, the investigator must supply the applied loads, the callus and bone geometry, and the tissue mechanical properties as inputs. The accuracy of these inputs must be carefully considered because errors in these inputs can substantially affect the quality of the analysis output.
Although many finite element analyses of local mechanical stimuli in bone-healing simply estimate or idealize the geometry, tissue mechanical properties, and applied loads, several techniques have been developed to measure or to obtain some of these quantities directly. Image data, such as those obtained via computed tomography, can be used to create a finite element model that captures the true geometry of the bone and callus (Figs. 1, A and 1, B). The disadvantages of this “specimen-specific” modeling approach are the increased time required to generate the model and a potentially limited extent to which the results can be applied to other specimens. However, differences in distributions of stresses, strains, and fluid flow between models with idealized geometry and those with more realistic geometry can be substantial (Figs. 1, C and 1, D), suggesting that the latter models may be necessary to rigorously test hypotheses on the role of certain mechanical stimuli in influencing bone repair and regeneration.
Fig. 1.
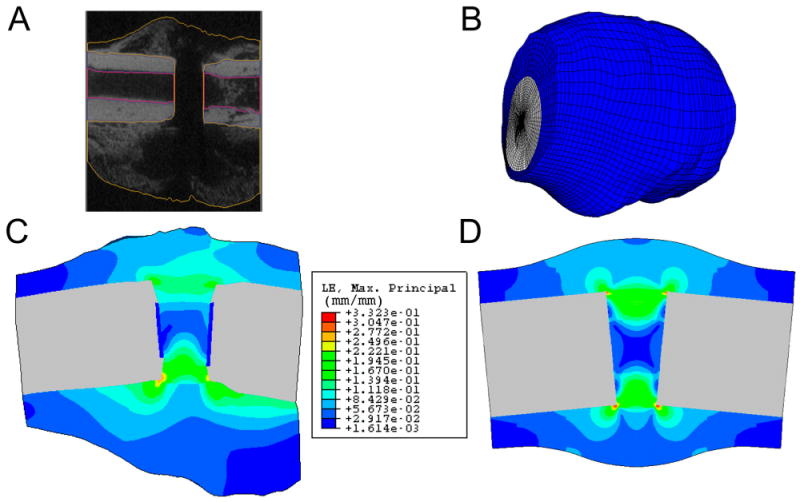
Creation of a finite element model of a rat fracture callus from micro-computed tomography (μCT) image data. A: Semiautomated image segmentation is performed to define the boundaries of the cortex and callus in each image. B: The resulting finite element mesh. C: An estimate of the distribution of maximum principal strain on a longitudinal section of the callus using the finite element mesh shown in Fig. 1, B. Displacements were applied to the left end of the cortical bone to create an 11° bending angle. D: An estimate of the distribution of maximum principal strain on a longitudinal section of a callus of idealized geometry. Displacements were applied in the same manner as in Fig. 1, C. In addition, the μCT-derived and idealized geometry meshes have identical gap size, cortical thickness, and medullary canal diameter, as well as comparable maximum callus diameters. The same tissue material properties and comparable element sizes were used in both analyses. Results are only shown in the callus tissues and not in the cortex or medullary canal.
Methods for measuring the mechanical properties of callus tissues must be able to account for the heterogeneous distribution of tissues in the callus. Particularly for fracture-healing studies that make use of small animal models, nanoindentation and microindentation are viable techniques to quantify the mechanical properties of tissue at many locations throughout the callus. For example, nanoindentation can provide highly repeatable measurements of tissue stiffness for granulation tissue as well as partially mineralized woven bone and fully mature cortical bone (Fig. 2). While stiffness is only one mechanical property, previous studies have demonstrated that indentation techniques can be used to quantify poroelastic and poroviscoelastic properties as well19,20. It is important to note that, due to the invasive specimen preparation steps involved in indentation of callus tissues, this approach is limited to ex vivo mechanical characterization. In vivo estimates of callus tissue properties have been made based on x-ray attenuation values obtained from digitized radiographs21; however, to our knowledge, no direct and noninvasive measurements of the mechanical properties of callus tissue have been reported as of the time of this writing.
Fig. 2.
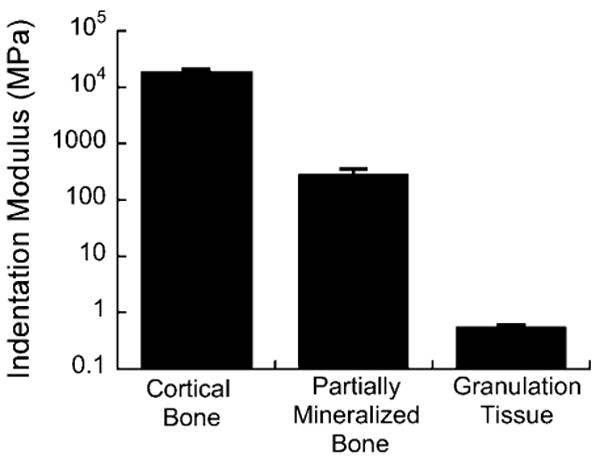
Elastic modulus of callus tissues obtained via nanoindentation. Indentations were performed on 200-μm-thick, longitudinal sections of a rat callus using a 50-μm conospherical tip. Sections were made with a sliding microtome, and no embedding, dehydration, or polishing was performed. Four indents were performed in each of two areas, one consisting of partially mineralized bone and one consisting of granulation tissue. For mature cortical bone, four indents were performed on a transverse cross section of a rat femoral diaphysis. The indentation protocol used a trapezoidal load function consisting of a 2-sec loading ramp to a specified peak force (9000, 300, 20 μN for cortical bone, partially mineralized bone, and granulation tissue, respectively), a hold period (15 sec, 15 sec, and 5 sec for cortical bone, partially mineralized bone, and granulation tissue, respectively), and a 2-sec unloading ramp to zero force. The indentation modulus was calculated using the method of Oliver and Pharr46. Shown in the graph is the mean indentation modulus for each tissue type; error bars indicate 1 standard deviation.
Measurement of applied loads or displacements also remains a major challenge. External fixators instrumented with strain gauges or displacement transducers can provide reasonably good estimates of displacements experienced by the fracture callus. However, because this approach does not measure movement of the callus relative to the fixator, these estimates likely underestimate the actual callus displacements to some degree. One class of techniques that holds promise for direct quantification of callus displacements is dynamic radiostereometric analysis, which has been used successfully to assess fracture stability and osseous union22,23. Based on recent reports24,25, the accuracy and precision of this technique may be sufficient for measuring callus displacements in humans and in large-animal models in all but the final stages of fracture-healing.
What Mechanotransduction Mechanisms Are Involved in Fracture Repair?
Mechanotransduction involves three main processes: mechanosensing (biochemical coupling), signal transduction, and effector-cell response. Within the realm of bone-healing and repair, the majority of studies to date have focused on the last of these three. For example, chondrocytes have been shown to upregulate type-II collagen in unstabilized fractures compared with stabilized fractures, and the opposite was shown to be the case for osteocalcin expression in osteoblasts and osteoblast-like cells26. Similarly, during the lengthening phase of distraction osteogenesis, presumptive osteoblasts within the regenerate have been shown to upregulate osteopontin and several bone morphogenetic proteins, including BMP-2, BMP-4, and BMP-727,28. Thus, changes in the mechanical environment of a healing bone affect expression of morphogens and extracellular matrix proteins in effector cells (i.e., chondrocytes and osteoblasts).
Less is known about the mechanosensing mechanisms and signal transduction pathways that are most critical and active during fracture repair. In particular, little is known about which cell types act as the mechanosensors during a given phase of the repair process, how these cells sense their mechanical environment, and how the act of sensing is ultimately transduced into effector-cell responses. Study of mechanotransduction in osteocytes, osteoblasts, and osteoblast progenitors has identified several candidate mechanosensing mechanisms29. These include mechanically gated ion channels30-32, integrins and focal adhesions33,34, G proteins35, and the linkage between the cytoskeleton and certain phospholipase C isoforms36,37. Recent studies have implicated focal adhesion kinase as a key mediator of mechanically induced bone formation in vivo38,39. With respect to signal transduction, several mechanotransduction pathways have been identified through in vitro studies and bone adaptation studies40-44. As with the study of mechanosensing mechanisms, the challenge remains to determine the role of these pathways during the various stages of fracture-healing.
What Can Be Learned from or Applied to Healing and Regeneration of Other Tissues?
Unlike almost all other tissues, bone has natural regenerative capacity and thus is both a logical and an unrepresentative subject for the study of mechanotransduction in skeletal tissues. Mechanical perturbations can dramatically alter the rate and course of bone-healing but often not the final outcome, which is typically a well-healed bone with near-original form and function. The tissue differentiation theories14,15,17 that have been developed to explain the effects of the mechanical environment on bone-healing also encompass formation of fibrous tissue, fibrocartilage, and cartilage. Study of mechanotransduction in differentiation and healing of these other tissues stands to provide new insights into principles governing the fate of mesenchymal stem cells and to define a much fuller spectrum of skeletal tissue responses to mechanical stimuli.
An example of a model that can be used for this type of investigation is a neoarthrosis model in which a cyclic bending motion is applied to a full-thickness bone defect in the rat femur45. The mechanical stimulation results in cartilage, fibrocartilage, and bone tissue in specific regions within the callus (Fig. 3). The mechanical stimulation also involves induction of wide ranges in values of local stresses, strains, and fluid velocities throughout the callus (Fig. 1). This diversity in stimuli, together with the characteristic pattern of tissues that are formed, indicates that this model provides an excellent platform for the investigation of relationships between mechanical stimuli and cell and molecular responses.
Fig. 3.
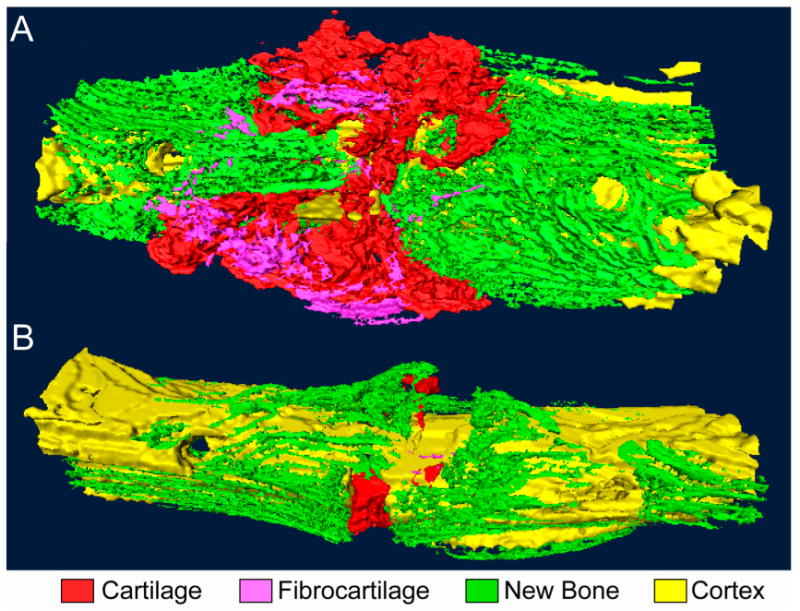
Bending stimulation of a full-thickness, transverse osteotomy gap. Three-dimensional reconstructions of serial histological sections of (A) a specimen following two weeks of bending stimulation and (B) a time-matched control that underwent continuous fixation with a four-pin external fixator. The mechanical stimulation induces formation of large amounts of cartilage within the gap and flaring outward toward the callus periphery in wedge-shaped configurations. Small amounts of fibrocartilage are found at the callus periphery, and bone formation is restricted to regions along the periosteal surface. No osseous bridging is observed. The control specimen exhibits bone formation within and surrounding the gap as well as a small amount of cartilage within the gap.
Summary
At present, the effect of mechanical factors on fracture repair has been convincingly demonstrated but the processes by which these factors influence healing have not yet been fully defined. Identities of the key mechanical stimuli still need to be proven, and the mechanotransduction mechanisms need to be elucidated. Fortunately, many tools in engineering and molecular biology are available for application to these areas of study. Much progress to date has come from a combination of in vivo and in vitro methods, and addressing the challenges that remain in understanding the role of mechanotransduction in fracture repair will continue to require a multifaceted and multidisciplinary approach. Although this research can most directly be applied to the treatment of fractures that exhibit delayed or otherwise impaired healing, a better understanding of the effect of mechanical factors on bone-healing will also undoubtedly benefit the study of healing, regeneration, and engineering of other skeletal tissues.
Footnotes
Disclosure: In support of their research for or preparation of this work, one or more of the authors received, in any one year, outside funding or grants in excess of $10,000 from the National Institutes of Health (grant #AR053353) and the Whitaker Foundation (graduate fellowship) and of less than $10,000 from Boston University (undergraduate industrial research fellowship). Neither they nor a member of their immediate families received payments or other benefits or a commitment or agreement to provide such benefits from a commercial entity. No commercial entity paid or directed, or agreed to pay or direct, any benefits to any research fund, foundation, division, center, clinical practice, or other charitable or nonprofit organization with which the authors, or a member of their immediate families, are affiliated or associated.
References
- 1.Epari DR, Schell H, Bail HJ, Duda GN. Instability prolongs the chondral phase during bone healing in sheep. Bone. 2006;38:864–70. doi: 10.1016/j.bone.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 2.Goodship AE, Watkins PE, Rigby HS, Kenwright J. The role of fixator frame stiffness in the control of fracture-healing. An experimental study. J Biomech. 1993;26:1027–35. doi: 10.1016/s0021-9290(05)80002-8. [DOI] [PubMed] [Google Scholar]
- 3.Wu JJ, Shyr HS, Chao EY, Kelly PJ. Comparison of osteotomy healing under external fixation devices with different stiffness characteristics. J Bone Joint Surg Am. 1984;66:1258–64. [PubMed] [Google Scholar]
- 4.Wolf S, Augat P, Eckert-Hübner K, Laule A, Krischak GD, Claes LE. Effects of high-frequency, low-magnitude mechanical stimulus on bone healing. Clin Orthop Relat Res. 2001;385:192–8. doi: 10.1097/00003086-200104000-00030. [DOI] [PubMed] [Google Scholar]
- 5.Goodship AE, Cunningham JL, Kenwright J. Strain rate and timing of stimulation in mechanical modulation of fracture-healing. Clin Orthop Relat Res. 1998;355(Suppl):S105–15. doi: 10.1097/00003086-199810001-00012. [DOI] [PubMed] [Google Scholar]
- 6.Augat P, Merk J, Wolf S, Claes L. Mechanical stimulation by external application of cyclic tensile strains does not effectively enhance bone healing. J Orthop Trauma. 2001;15:54–60. doi: 10.1097/00005131-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Schell H, Epari DR, Kassi JP, Bragulla H, Bail HJ, Duda GN. The course of bone healing is influenced by the initial shear fixation stability. J Orthop Res. 2005;23:1022–8. doi: 10.1016/j.orthres.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Bishop NE, van Rhijn M, Tami I, Corveleijn R, Schneider E, Ito K. Shear does not necessarily inhibit bone healing. Clin Orthop Relat Res. 2006;443:307–14. doi: 10.1097/01.blo.0000191272.34786.09. [DOI] [PubMed] [Google Scholar]
- 9.Park SH, O'Connor K, McKellop H, Sarmiento A. The influence of active shear or compressive motion on fracture-healing. J Bone Joint Surg Am. 1998;80:868–78. doi: 10.2106/00004623-199806000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Claes L, Augat P, Suger G, Wilke HJ. Influence of size and stability of the osteotomy gap on the success of fracture-healing. J Orthop Res. 1997;15:577–84. doi: 10.1002/jor.1100150414. [DOI] [PubMed] [Google Scholar]
- 11.Claes L, Eckert-Hübner K, Augat P. The effect of mechanical stability on local vascularization and tissue differentiation in callus healing. J Orthop Res. 2002;20:1099–105. doi: 10.1016/S0736-0266(02)00044-X. [DOI] [PubMed] [Google Scholar]
- 12.Goodship AE, Kenwright J. The influence of induced micromovement on the healing of experimental tibial fractures. J Bone Joint Surg Br. 1985;67:650–5. doi: 10.1302/0301-620X.67B4.4030869. [DOI] [PubMed] [Google Scholar]
- 13.Claes LE, Wilke HJ, Augat P, Rübenacker S, Margevicius KJ. Effect of dynamization on gap healing of diaphyseal fractures under external fixation. Clin Biomech (Bristol, Avon) 1995;10:227–34. doi: 10.1016/0268-0033(95)99799-8. [DOI] [PubMed] [Google Scholar]
- 14.Carter DR, Beaupré GS, Giori NJ, Helms JA. Mechanobiology of skeletal regeneration. Clin Orthop Relat Res. 1998;355(Suppl):S41–55. doi: 10.1097/00003086-199810001-00006. [DOI] [PubMed] [Google Scholar]
- 15.Claes LE, Heigele CA. Magnitudes of local stress and strain along osseous surfaces predict the course and type of fracture-healing. J Biomech. 1999;32:255–66. doi: 10.1016/s0021-9290(98)00153-5. [DOI] [PubMed] [Google Scholar]
- 16.Lacroix D, Prendergast PJ. A mechano-regulation model for tissue differentiation during fracture-healing: analysis of gap size and loading. J Biomech. 2002;35:1163–71. doi: 10.1016/s0021-9290(02)00086-6. [DOI] [PubMed] [Google Scholar]
- 17.Prendergast PJ, Huiskes R, Søballe K. Biophysical stimuli on cells during tissue differentiation at implant interfaces. J Biomech. 1997;30:539–48. doi: 10.1016/s0021-9290(96)00140-6. [DOI] [PubMed] [Google Scholar]
- 18.Isaksson H, Wilson W, van Donkelaar CC, Huiskes R, Ito K. Comparison of biophysical stimuli for mechano-regulation of tissue differentiation during fracture-healing. J Biomech. 2006;39:1507–16. doi: 10.1016/j.jbiomech.2005.01.037. [DOI] [PubMed] [Google Scholar]
- 19.Mow VC, Gibbs MC, Lai WM, Zhu WB, Athanasiou KA. Biphasic indentation of articular cartilage—II. A numerical algorithm and an experimental study. J Biomech. 1989;22:853–61. doi: 10.1016/0021-9290(89)90069-9. [DOI] [PubMed] [Google Scholar]
- 20.DiSilvestro MR, Suh JK. A cross-validation of the biphasic poroviscoelastic model of articular cartilage in unconfined compression, indentation, and confined compression. J Biomech. 2001;34:519–25. doi: 10.1016/s0021-9290(00)00224-4. [DOI] [PubMed] [Google Scholar]
- 21.Gardner TN, Stoll T, Marks L, Mishra S, Knothe Tate M. The influence of mechanical stimulus on the pattern of tissue differentiation in a long bone fracture—an FEM study. J Biomech. 2000;33:415–25. doi: 10.1016/s0021-9290(99)00189-x. [DOI] [PubMed] [Google Scholar]
- 22.Madanat R, Moritz N, Larsson S, Aro HT. RSA applications in monitoring of fracture-healing in clinical trials. Scand J Surg. 2006;95:119–27. doi: 10.1177/145749690609500207. [DOI] [PubMed] [Google Scholar]
- 23.Duffy P, Trask K, Hennigar A, Barron L, Leighton RK, Dunbar MJ. Assessment of fragment micromotion in distal femur fracture fixation with RSA. Clin Orthop Relat Res. 2006;448:105–13. doi: 10.1097/01.blo.0000224008.19798.91. [DOI] [PubMed] [Google Scholar]
- 24.Bey MJ, Zauel R, Brock SK, Tashman S. Validation of a new model-based tracking technique for measuring three-dimensional, in vivo glenohumeral joint kinematics. J Biomech Eng. 2006;128:604–9. doi: 10.1115/1.2206199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madanat R, Moritz N, Aro HT. Three-dimensional computer simulation of radiostereometric analysis (RSA) in distal radius fractures. J Biomech. 2007;40:1855–61. doi: 10.1016/j.jbiomech.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 26.Thompson Z, Miclau T, Hu D, Helms JA. A model for intramembranous ossification during fracture-healing. J Orthop Res. 2002;20:1091–8. doi: 10.1016/S0736-0266(02)00017-7. [DOI] [PubMed] [Google Scholar]
- 27.Lammens J, Liu Z, Aerssens J, Dequeker J, Fabry G. Distraction bone healing versus osteotomy healing: a comparative biochemical analysis. J Bone Miner Res. 1998;13:279–86. doi: 10.1359/jbmr.1998.13.2.279. [DOI] [PubMed] [Google Scholar]
- 28.Weiss S, Baumgart R, Jochum M, Strasburger CJ, Bidlingmaier M. Systemic regulation of distraction osteogenesis: a cascade of biochemical factors. J Bone Miner Res. 2002;17:1280–9. doi: 10.1359/jbmr.2002.17.7.1280. [DOI] [PubMed] [Google Scholar]
- 29.Duncan RL, Turner CH. Mechanotransduction and the functional response of bone to mechanical strain. Calcif Tissue Int. 1995;57:344–58. doi: 10.1007/BF00302070. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Duncan RL, Burr DB, Turner CH. L-type calcium channels mediate mechanically induced bone formation in vivo. J Bone Miner Res. 2002;17:1795–800. doi: 10.1359/jbmr.2002.17.10.1795. [DOI] [PubMed] [Google Scholar]
- 31.Rawlinson SC, Pitsillides AA, Lanyon LE. Involvement of different ion channels in osteoblasts' and osteocytes' early responses to mechanical strain. Bone. 1996;19:609–14. doi: 10.1016/s8756-3282(96)00260-8. [DOI] [PubMed] [Google Scholar]
- 32.Miyauchi A, Notoya K, Mikuni-Takagaki Y, Takagi Y, Goto M, Miki Y, Takano-Yamamoto T, Jinnai K, Takahashi K, Kumegawa M, Chihara K, Fujita T. Parathyroid hormone-activated volume-sensitive calcium influx pathways in mechanically loaded osteocytes. J Biol Chem. 2000;275:3335–42. doi: 10.1074/jbc.275.5.3335. [DOI] [PubMed] [Google Scholar]
- 33.Pavalko FM, Chen NX, Turner CH, Burr DB, Atkinson S, Hsieh YF, Qiu J, Duncan RL. Fluid shear-induced mechanical signaling in MC3T3-E1 osteoblasts requires cytoskeleton-integrin interactions. Am J Physiol. 1998;275(6 Pt 1):C1591–601. [PubMed] [Google Scholar]
- 34.Ponik SM, Pavalko FM. Formation of focal adhesions on fibronectin promotes fluid shear stress induction of COX-2 and PGE2 release in MC3T3-E1 osteoblasts. J Appl Physiol. 2004;97:135–42. doi: 10.1152/japplphysiol.01260.2003. [DOI] [PubMed] [Google Scholar]
- 35.Reich KM, McAllister TN, Gudi S, Frangos JA. Activation of G proteins mediates flow-induced prostaglandin E2 production in osteoblasts. Endocrinology. 1997;138:1014–8. doi: 10.1210/endo.138.3.4999. [DOI] [PubMed] [Google Scholar]
- 36.Jones DB, Bingmann D. How do osteoblasts respond to mechanical stimulation? Cells and Materials. 1991;1:329–40. [Google Scholar]
- 37.Hoberg M, Gratz HH, Noll M, Jones DB. Mechanosensitivity of human osteosarcoma cells and phospholipase C beta2 expression. Biochem Biophys Res Commun. 2005;333:142–9. doi: 10.1016/j.bbrc.2005.05.088. [DOI] [PubMed] [Google Scholar]
- 38.Leucht P, Kim JB, Currey JA, Brunski J, Helms JA. FAK-Mediated mechanotransduction in skeletal regeneration. PLoS ONE. 2007;2:e390. doi: 10.1371/journal.pone.0000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tong L, Buchman SR, Ignelzi MA, Jr, Rhee S, Goldstein SA. Focal adhesion kinase expression during mandibular distraction osteogenesis: evidence for mechanotransduction. Plast Reconstr Surg. 2003;111:211–24. doi: 10.1097/01.PRS.0000033180.01581.9A. [DOI] [PubMed] [Google Scholar]
- 40.Wu Q, Zhang Y, Chen Q. Indian hedgehog is an essential component of mechanotransduction complex to stimulate chondrocyte proliferation. J Biol Chem. 2001;276:35290–6. doi: 10.1074/jbc.M101055200. [DOI] [PubMed] [Google Scholar]
- 41.Robinson JA, Chatterjee-Kishore M, Yaworsky PJ, Cullen DM, Zhao W, Li C, Kharode Y, Sauter L, Babij P, Brown EL, Hill AA, Akhter MP, Johnson ML, Recker RR, Komm BS, Bex FJ. Wnt/beta-catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem. 2006;281:31720–8. doi: 10.1074/jbc.M602308200. [DOI] [PubMed] [Google Scholar]
- 42.Liedert A, Kaspar D, Blakytny R, Claes L, Ignatius A. Signal transduction pathways involved in mechanotransduction in bone cells. Biochem Biophys Res Commun. 2006;349:1–5. doi: 10.1016/j.bbrc.2006.07.214. [DOI] [PubMed] [Google Scholar]
- 43.Millward-Sadler SJ, Salter DM. Integrin-dependent signal cascades in chondrocyte mechanotransduction. Ann Biomed Eng. 2004;32:435–46. doi: 10.1023/b:abme.0000017538.72511.48. [DOI] [PubMed] [Google Scholar]
- 44.Robling AG, Castillo AB, Turner CH. Biomechanical and molecular regulation of bone remodeling. Annu Rev Biomed Eng. 2006;8:455–98. doi: 10.1146/annurev.bioeng.8.061505.095721. [DOI] [PubMed] [Google Scholar]
- 45.Cullinane DM, Fredrick A, Eisenberg SR, Pacicca D, Elman MV, Lee C, Salisbury K, Gerstenfeld LC, Einhorn TA. Induction of a neoarthrosis by precisely controlled motion in an experimental mid-femoral defect. J Orthop Res. 2002;20:579–86. doi: 10.1016/S0736-0266(01)00131-0. [DOI] [PubMed] [Google Scholar]
- 46.Oliver WC, Pharr GM. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J Mater Res. 1992;7:1564–83. [Google Scholar]


