Abstract
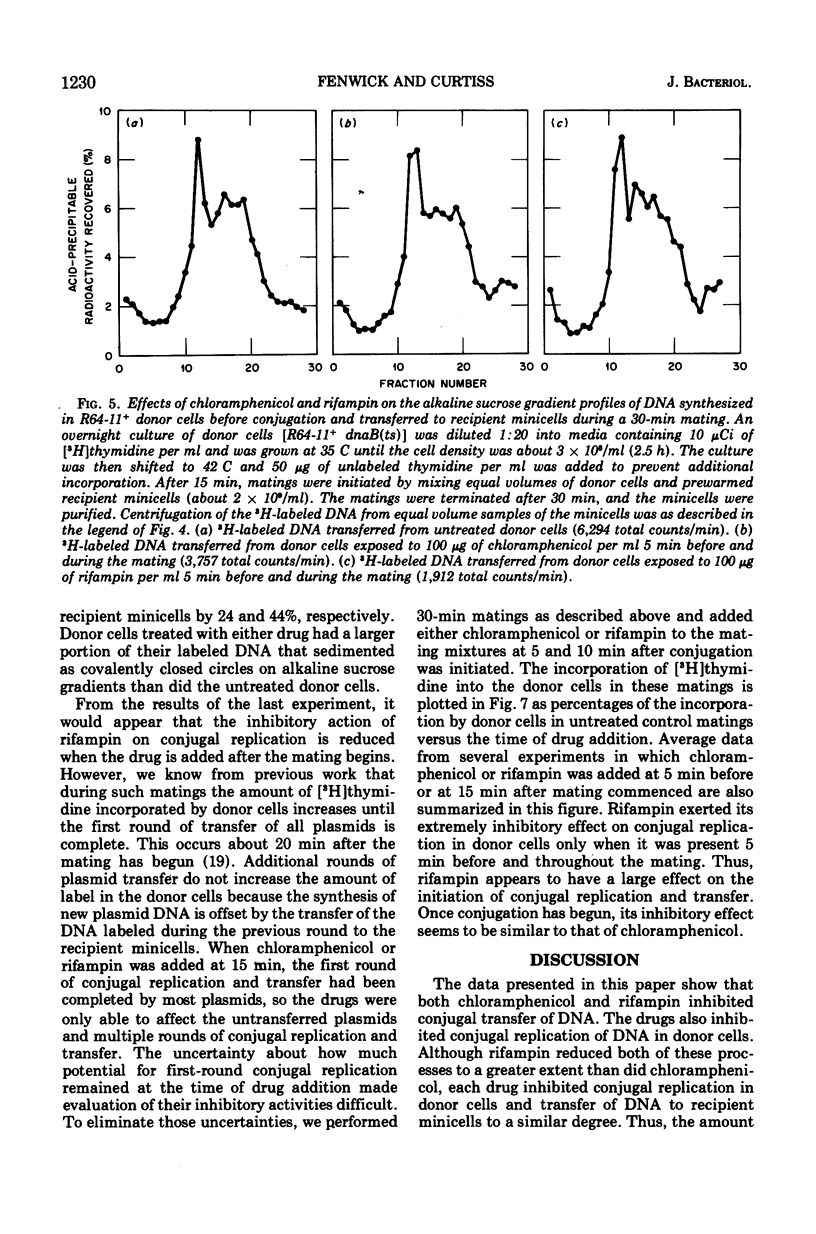
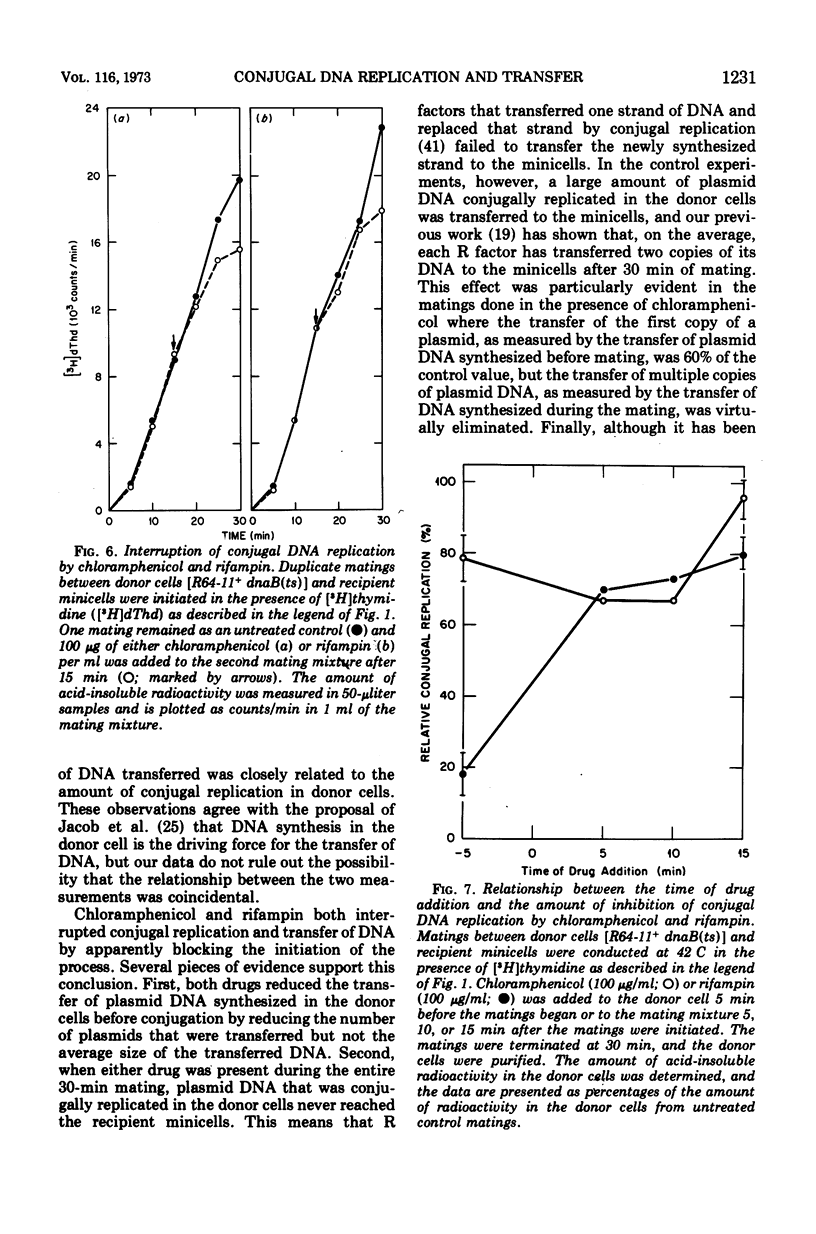
Conjugal replication of R64-11 deoxyribonucleic acid (DNA) and the concomitant transfer of R64-11 DNA to DNA-deficient minicells are dependent upon processes that are inhibited by rifampin and chloramphenicol. The rifampin-sensitive product is not present in vegetatively growing cells and is needed to initiate both conjugal DNA replication in donor cells and DNA transfer to recipient minicells. If the rifampin-sensitive product is a ribonucleic acid (RNA) molecule (rather than RNA polymerase itself), our data indicate that this RNA species required for initiation of conjugal activity does not need to be translated into a protein product. The chloramphenicol-sensitive product(s) is present in vegetatively growing cells in sufficient quantity to permit most donor cells to carry out one round of plasmid conjugal replication and transfer. The initiation of second and subsequent rounds of conjugal replication and transfer are dependent on the synthesis of both the rifampin-sensitive and chloramphenicol-sensitive products. Our results demonstrate a correspondence between the amount of conjugal DNA replication in the donor and the amount of DNA transferred to recipient minicells under all conditions, and therefore suggest but do not prove that plasmid transfer is dependent on conjugal DNA replication. The results also add additional proof that R64-11 transfer to minicells is discontinuous. All of these results are discussed in regard to further refinements of old models for the mechanism of conjugal transfer as well as a more radical departure from current dogma.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai T., Watanabe T. Effects of acriflavine on the transfer of episomes and bacterial chromosome in Escherichia coli K-12. Genet Res. 1967 Dec;10(3):241–249. doi: 10.1017/s0016672300011009. [DOI] [PubMed] [Google Scholar]
- Bazzicalupo P., Tocchini-Valentini G. P. Curing of an Escherichia coli episome by rifampicin (acridine orange-F + -F - -Hfr-lac). Proc Natl Acad Sci U S A. 1972 Feb;69(2):298–300. doi: 10.1073/pnas.69.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair D. G., Sherratt D. J., Clewell D. B., Helinski D. R. Isolation of supercoiled colicinogenic factor E 1 DNA sensitive to ribonuclease and alkali. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2518–2522. doi: 10.1073/pnas.69.9.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresler S. E., Lanzov V. A., Lukjaniec-Blinkova A. A. On the mechanism of conjugation in Escherichia coli K 12. Mol Gen Genet. 1968;102(4):269–274. doi: 10.1007/BF00433718. [DOI] [PubMed] [Google Scholar]
- Brewin N. [Catalytic role for RNA in DNA replication]. Nat New Biol. 1972 Mar 29;236(65):101–101. doi: 10.1038/newbio236101a0. [DOI] [PubMed] [Google Scholar]
- Brutlag D., Schekman R., Kornberg A. A possible role for RNA polymerase in the initiation of M13 DNA synthesis. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2826–2829. doi: 10.1073/pnas.68.11.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Evenchik B., Cranston J. W. Direct inhibition of Col E 1 plasmid DNA replication in Escherichia coli by rifampicin. Nat New Biol. 1972 May 3;237(70):29–31. doi: 10.1038/newbio237029a0. [DOI] [PubMed] [Google Scholar]
- Clowes R. C. Molecular structure of bacterial plasmids. Bacteriol Rev. 1972 Sep;36(3):361–405. doi: 10.1128/br.36.3.361-405.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A., Fisher W. D., Curtiss R., 3rd, Adler H. I. DNA isolated from Escherichia coli minicells mated with F+ cells. Proc Natl Acad Sci U S A. 1968 Sep;61(1):61–68. doi: 10.1073/pnas.61.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A., Fisher W. D., Curtiss R., 3rd, Adler H. I. The properties of DNA transferred to minicells during conjugation. Cold Spring Harb Symp Quant Biol. 1968;33:635–641. doi: 10.1101/sqb.1968.033.01.071. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Silver R. P., Sharp P. A., McCoubrey A. E. The problems of drug-resistant pathogenic bacteria. Studies on the molecular nature of R factors. Ann N Y Acad Sci. 1971 Jun 11;182:172–187. doi: 10.1111/j.1749-6632.1971.tb30655.x. [DOI] [PubMed] [Google Scholar]
- Curtiss R., 3rd Bacterial conjugation. Annu Rev Microbiol. 1969;23:69–136. doi: 10.1146/annurev.mi.23.100169.000441. [DOI] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Caro L. G., Allison D. P., Stallions D. R. Early stages of conjugation in Escherichia coli. J Bacteriol. 1969 Nov;100(2):1091–1104. doi: 10.1128/jb.100.2.1091-1104.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
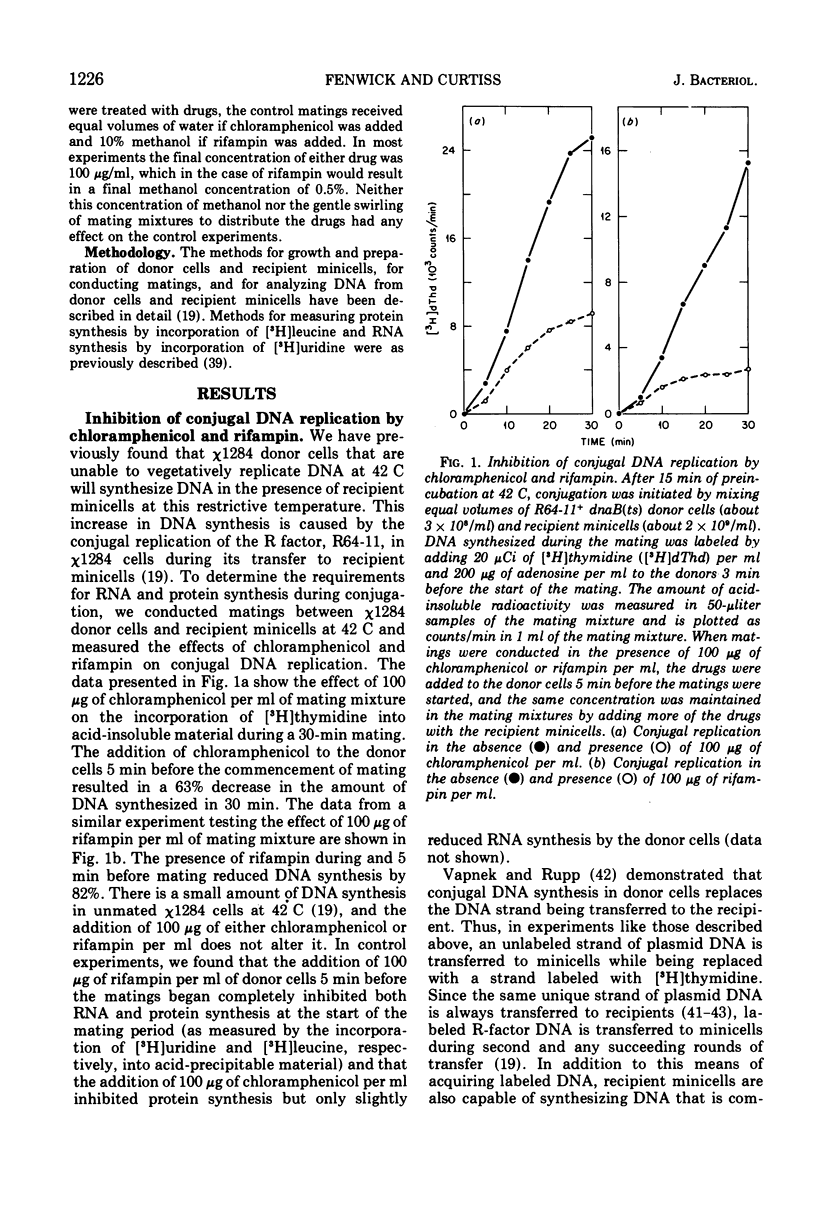
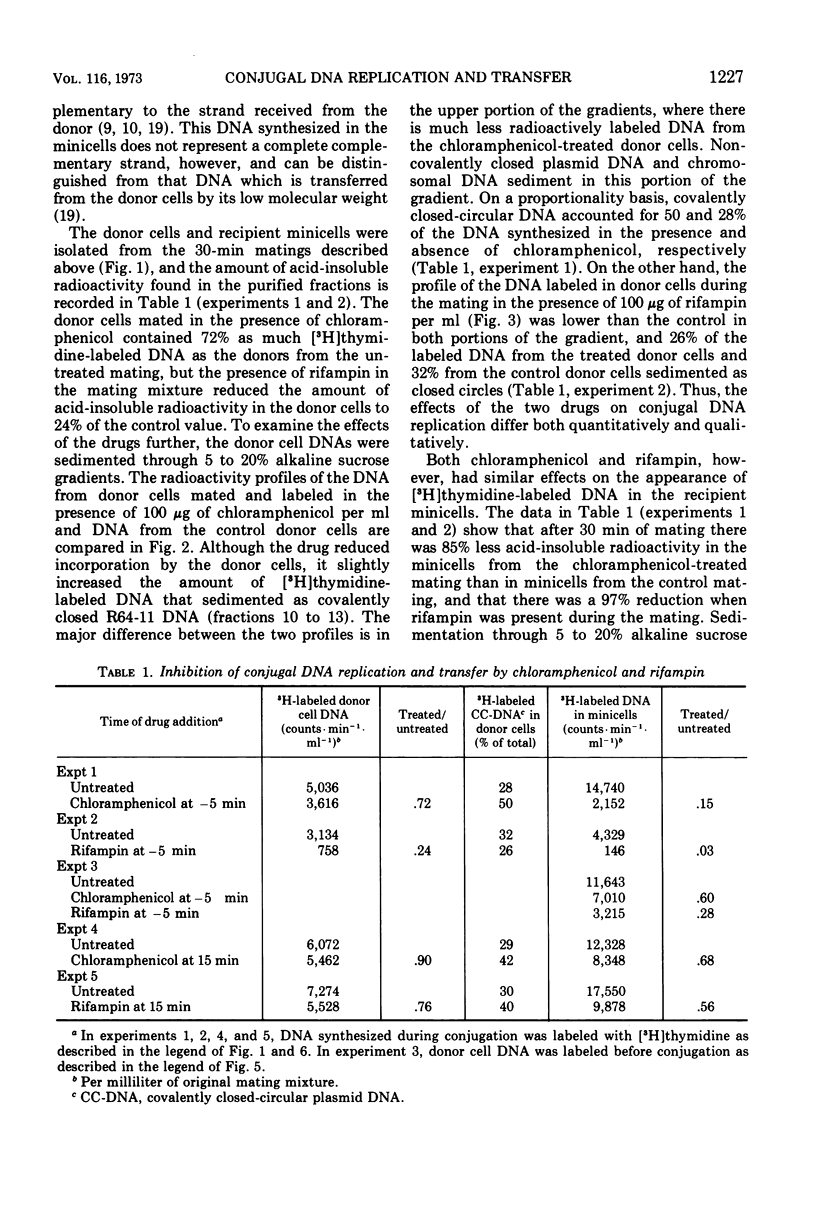
- Curtiss R., 3rd, Charamella L. J., Stallions D. R., Mays J. A. Parental functions during conjugation in Escherichia coli K-12. Bacteriol Rev. 1968 Dec;32(4 Pt 1):320–348. [PMC free article] [PubMed] [Google Scholar]
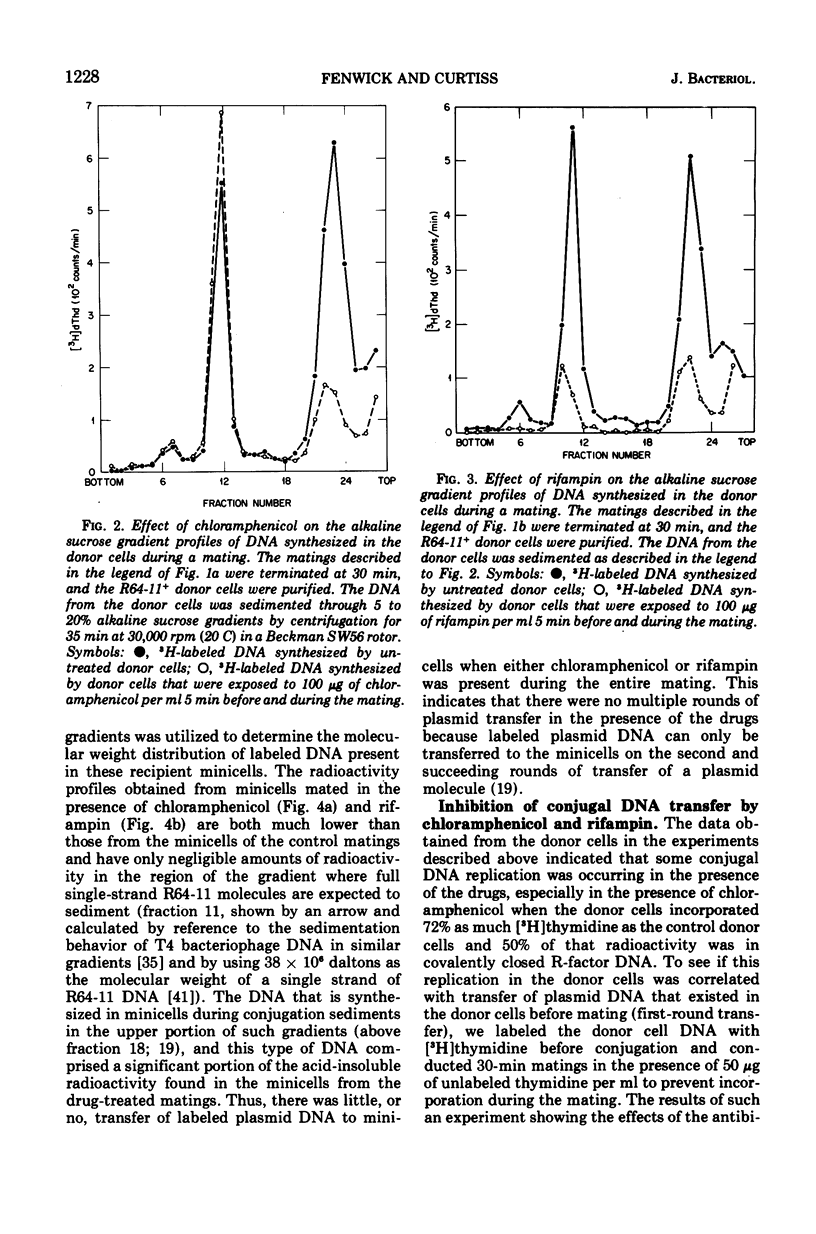
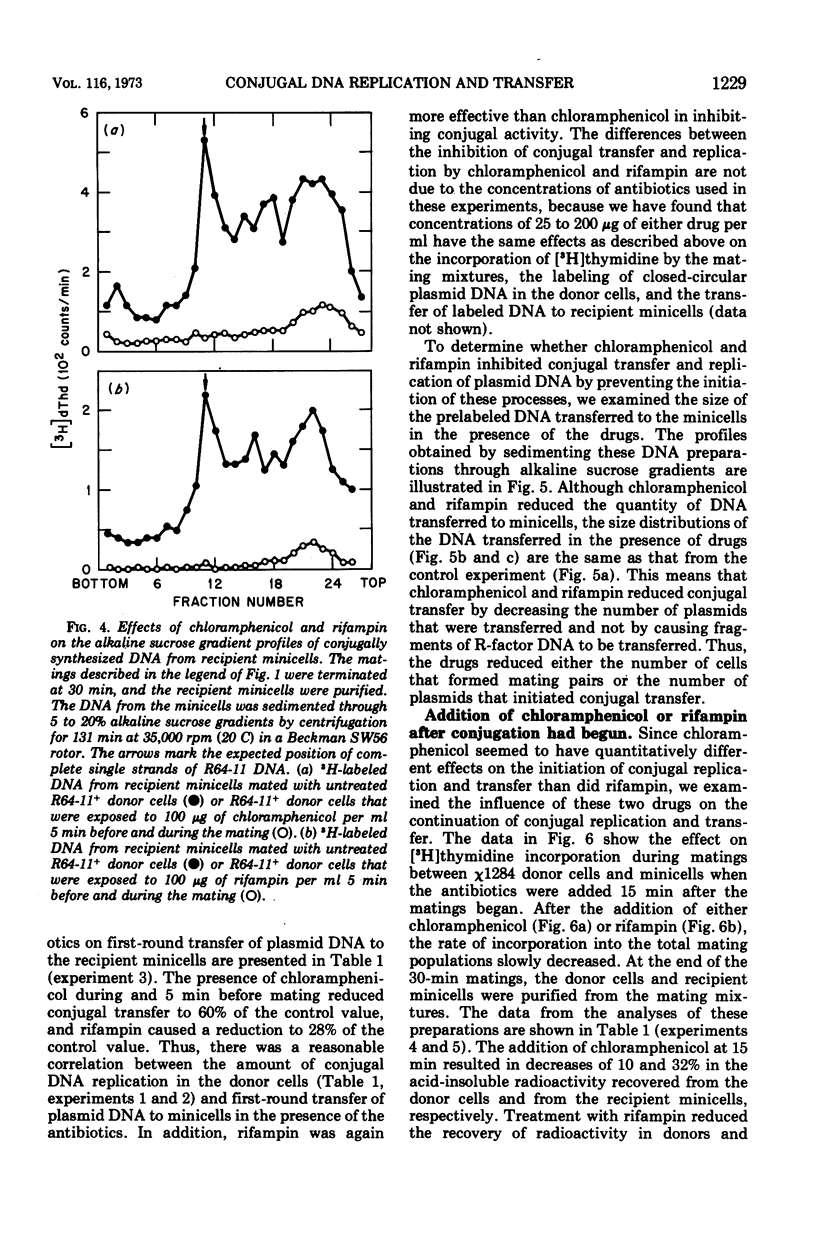
- Cuzin F., Jacob F. Inhibition par les acridines du transfert génétique par les souches donatrices d'Escherichia coli K 12. Ann Inst Pasteur (Paris) 1966 Oct;111(4):427–436. [PubMed] [Google Scholar]
- Cuzin F., Jacob F. Mutations de l'épisome F d'Escherichia coli K 12. II. Mutants à réplication thermosensible. Ann Inst Pasteur (Paris) 1967 Apr;112(4):397–418. [PubMed] [Google Scholar]
- FISHER K. W. The role of the Krebs cycle in conjugation in Escherichia coli K-12. J Gen Microbiol. 1957 Feb;16(1):120–135. doi: 10.1099/00221287-16-1-120. [DOI] [PubMed] [Google Scholar]
- Falkow S., Tompkins L. S., Silver R. P., Guerry P., Le Blanc D. J. The problems of drug-resistant pathogenic bacteria. The replication of R-factor DNA in Escherichia coli K-12 following conjugation. Ann N Y Acad Sci. 1971 Jun 11;182:153–171. doi: 10.1111/j.1749-6632.1971.tb30654.x. [DOI] [PubMed] [Google Scholar]
- Fenwick R. G., Jr, Curtiss R., 3rd Conjugal deoxyribonucleic acid replication by Escherichia coli K-12: stimulation in dnaB(ts) donors by minicells. J Bacteriol. 1973 Dec;116(3):1212–1223. doi: 10.1128/jb.116.3.1212-1223.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher K. W. Amino acid deprivation and its effect on mating ability in Escherichia coli K12. Genet Res. 1966 Aug;8(1):115–118. doi: 10.1017/s0016672300009964. [DOI] [PubMed] [Google Scholar]
- Friszke E. Mechanism of conjugation in bacteria. I. Effect of chloramphenicol on conjugation in Escherichia coli K-12. Acta Microbiol Pol A. 1970;2(3):123–131. [PubMed] [Google Scholar]
- Gilbert W., Dressler D. DNA replication: the rolling circle model. Cold Spring Harb Symp Quant Biol. 1968;33:473–484. doi: 10.1101/sqb.1968.033.01.055. [DOI] [PubMed] [Google Scholar]
- Inselburg J., Fuke M. Isolation of catenated and replicating DNA molecules of colicin factor E1 from minicells. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2839–2842. doi: 10.1073/pnas.68.11.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline B. C. Inhibition of plasmid DNA replication by rifampin in Salmonella pullorum. Biochem Biophys Res Commun. 1972 Mar 24;46(6):2019–2025. doi: 10.1016/0006-291x(72)90753-x. [DOI] [PubMed] [Google Scholar]
- Krisch R. E., Kvetkas M. J. Inhibition of bacterial mating by amino acid deprivation. Biochem Biophys Res Commun. 1966 Mar 22;22(6):707–711. doi: 10.1016/0006-291x(66)90206-3. [DOI] [PubMed] [Google Scholar]
- Lark K. G. Evidence for the direct involvement of RNA in the initiation of DNA replication in Escherichia coli 15T. J Mol Biol. 1972 Feb 28;64(1):47–60. doi: 10.1016/0022-2836(72)90320-8. [DOI] [PubMed] [Google Scholar]
- Lark K. G. Initiation and control of DNA synthesis. Annu Rev Biochem. 1969;38:569–604. doi: 10.1146/annurev.bi.38.070169.003033. [DOI] [PubMed] [Google Scholar]
- Marinus M. G., Adelberg E. A. Vegetative Replication and Transfer Replication of Deoxyribonucleic Acid in Temperature-Sensitive Mutants of Escherichia coli K-12. J Bacteriol. 1970 Dec;104(3):1266–1272. doi: 10.1128/jb.104.3.1266-1272.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y., Caro L., Berg C. M., Hirota Y. Chromosome replication in Escherichia coli. IV. Control of chromosome replication and cell division by an integrated episome. J Mol Biol. 1971 Feb 14;55(3):441–456. doi: 10.1016/0022-2836(71)90328-7. [DOI] [PubMed] [Google Scholar]
- Novotny C. P., Taylor P. F., Lavin K. Effects of growth inhibitors and ultraviolet irradiation on F pili. J Bacteriol. 1972 Dec;112(3):1083–1089. doi: 10.1128/jb.112.3.1083-1089.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohki M., Tomizawa J. Asymmetric transfer of DNA strands in bacterial conjugation. Cold Spring Harb Symp Quant Biol. 1968;33:651–658. doi: 10.1101/sqb.1968.033.01.074. [DOI] [PubMed] [Google Scholar]
- Rajchert-Trzpil M. The influence of chloramphenicol on the conjugation yield in E. coli K-12. Bull Acad Pol Sci Biol. 1965;13(4):211–213. [PubMed] [Google Scholar]
- Regan J. D., Setlow R. B., Ley R. D. Normal and defective repair of damaged DNA in human cells: a sensitive assay utilizing the photolysis of bromodeoxyuridine. Proc Natl Acad Sci U S A. 1971 Apr;68(4):708–712. doi: 10.1073/pnas.68.4.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva S., Fietta A. M., Silvestri L. G., Romero E. Effect of rifampicin on expression of some episomal genes in E. coli. Nat New Biol. 1972 Jan 19;235(55):78–80. doi: 10.1038/newbio235078a0. [DOI] [PubMed] [Google Scholar]
- Roeser J., Konetzka W. A. Chromosome transfer and the DNA replication cycle in Escherichia coli. Biochem Biophys Res Commun. 1964 Jul 1;16(4):326–331. doi: 10.1016/0006-291x(64)90034-8. [DOI] [PubMed] [Google Scholar]
- Romero E., Riva S., Fietta A. M., Silvestri L. G. Effect of R factors on rifampicin resistance in E. coli. Nat New Biol. 1971 Nov 10;234(45):56–58. doi: 10.1038/newbio234056a0. [DOI] [PubMed] [Google Scholar]
- Roozen K. J., Fenwick R. G., Jr, Curtiss R., 3rd Synthesis of ribonucleic acid and protein in plasmid-containing minicells of Escherichia coli K-12. J Bacteriol. 1971 Jul;107(1):21–33. doi: 10.1128/jb.107.1.21-33.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp W. D., Ihler G. Strand selection during bacterial mating. Cold Spring Harb Symp Quant Biol. 1968;33:647–650. doi: 10.1101/sqb.1968.033.01.073. [DOI] [PubMed] [Google Scholar]
- Vapnek D., Lipman M. B., Rupp W. D. Physical properties and mechanism of transfer of R factors in Escherichia coli. J Bacteriol. 1971 Oct;108(1):508–514. doi: 10.1128/jb.108.1.508-514.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vapnek D., Rupp W. D. Asymmetric segregation of the complementary sex-factor DNA strands during conjugation in Escherichia coli. J Mol Biol. 1970 Nov 14;53(3):287–303. doi: 10.1016/0022-2836(70)90066-5. [DOI] [PubMed] [Google Scholar]
- Vapnek D., Rupp W. D. Identification of individual sex-factor DNA strands and their replication during conjugation in thermosensitive DNA mutants of Escherichia coli. J Mol Biol. 1971 Sep 28;60(3):413–424. doi: 10.1016/0022-2836(71)90178-1. [DOI] [PubMed] [Google Scholar]
- Walmsley R. H. Physical assay of competence for specific mating-pair formation in Escherichia coli. J Bacteriol. 1973 Apr;114(1):144–151. doi: 10.1128/jb.114.1.144-151.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrli W., Staehelin M. Actions of the rifamycins. Bacteriol Rev. 1971 Sep;35(3):290–309. doi: 10.1128/br.35.3.290-309.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Mauro E., Synder L., Marino P., Lamberti A., Coppo A., Tocchini-Valentini G. P. Rifampicin sensitivity of the components of DNA-dependent RNA polymerase. Nature. 1969 May 10;222(5193):533–537. doi: 10.1038/222533a0. [DOI] [PubMed] [Google Scholar]



