Abstract
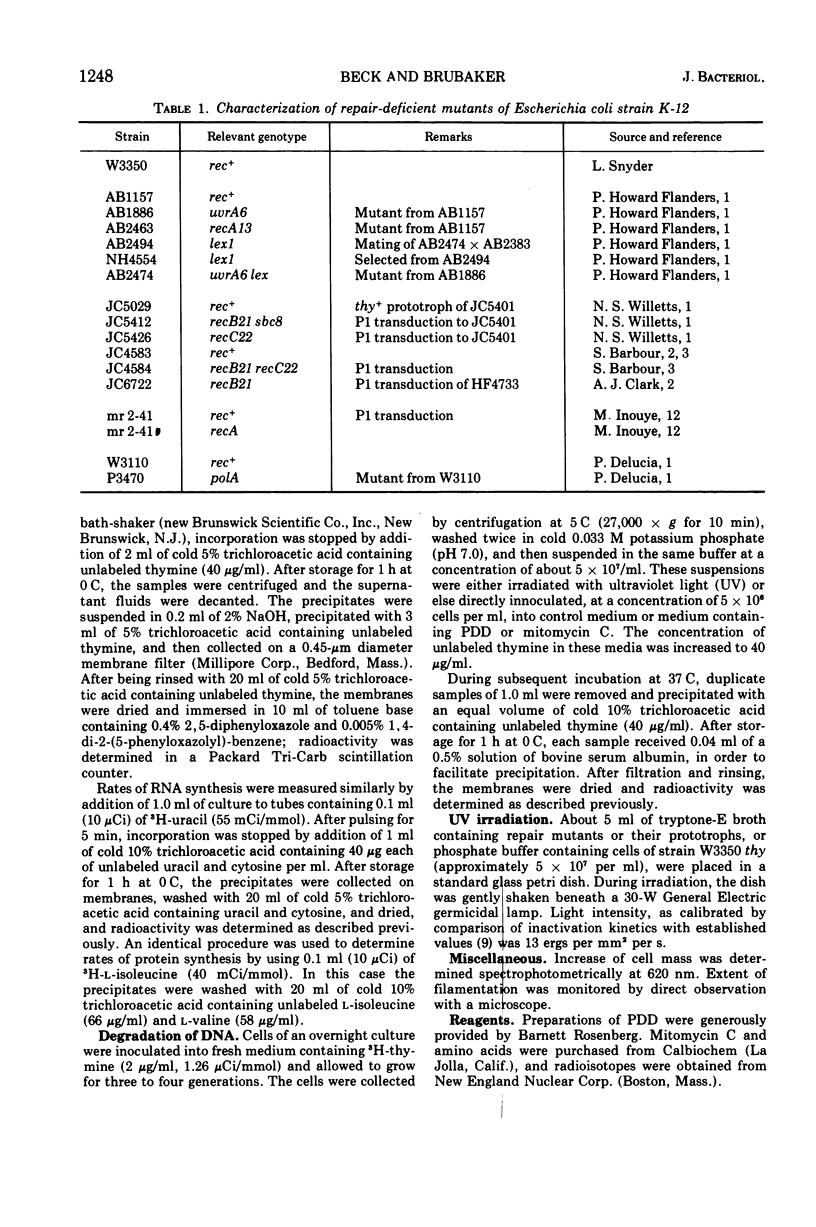
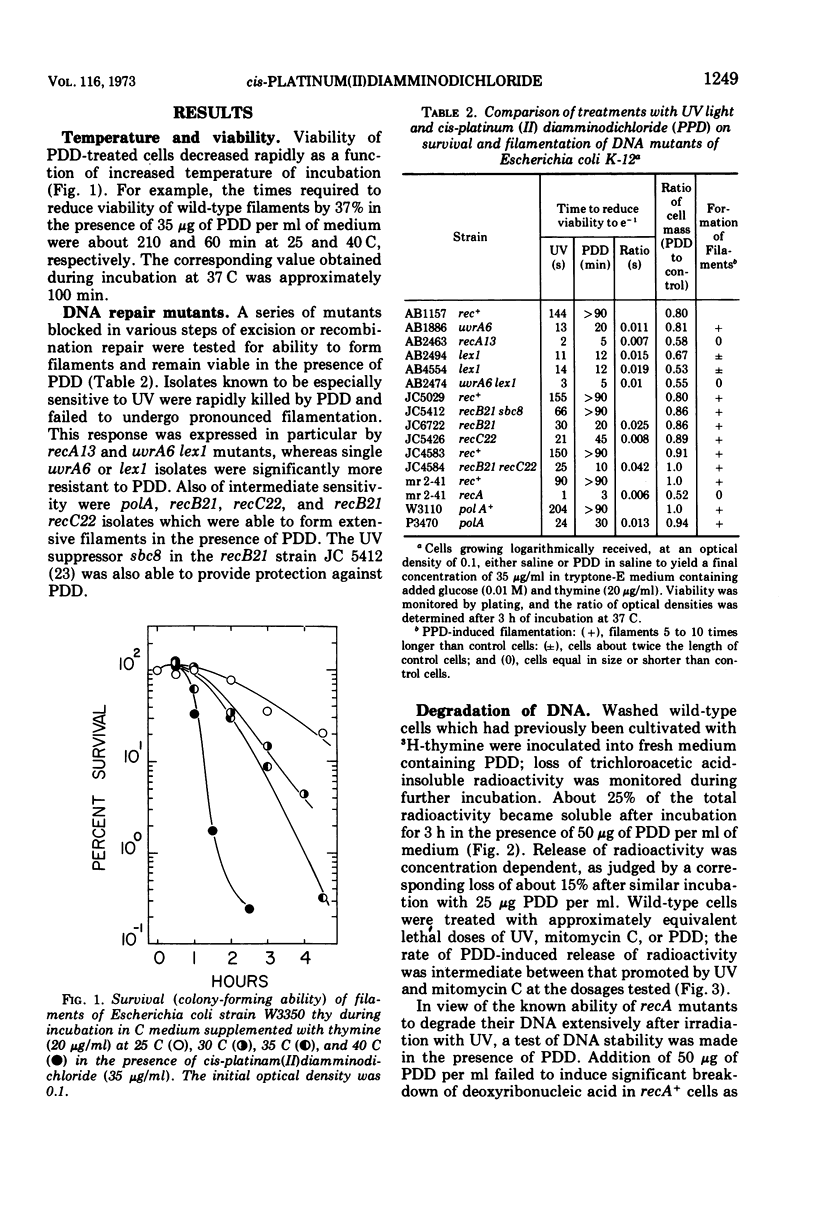
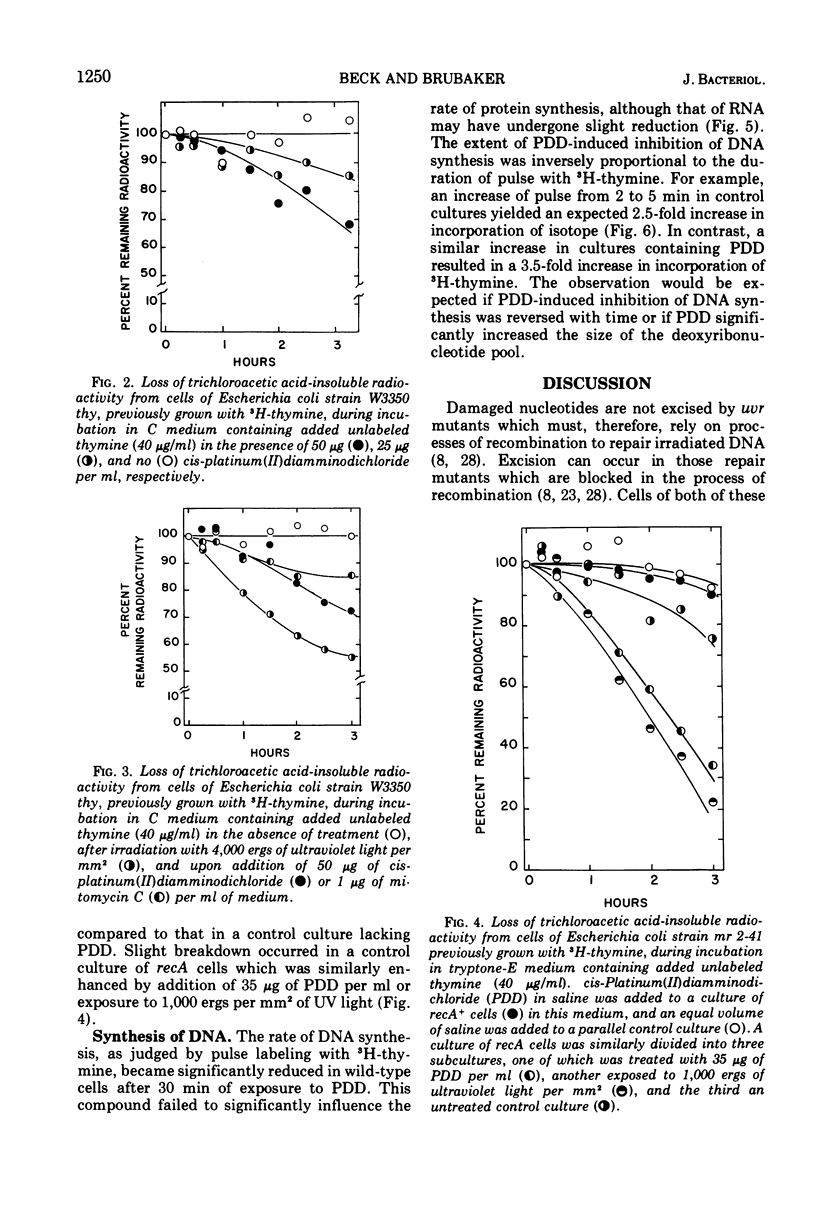
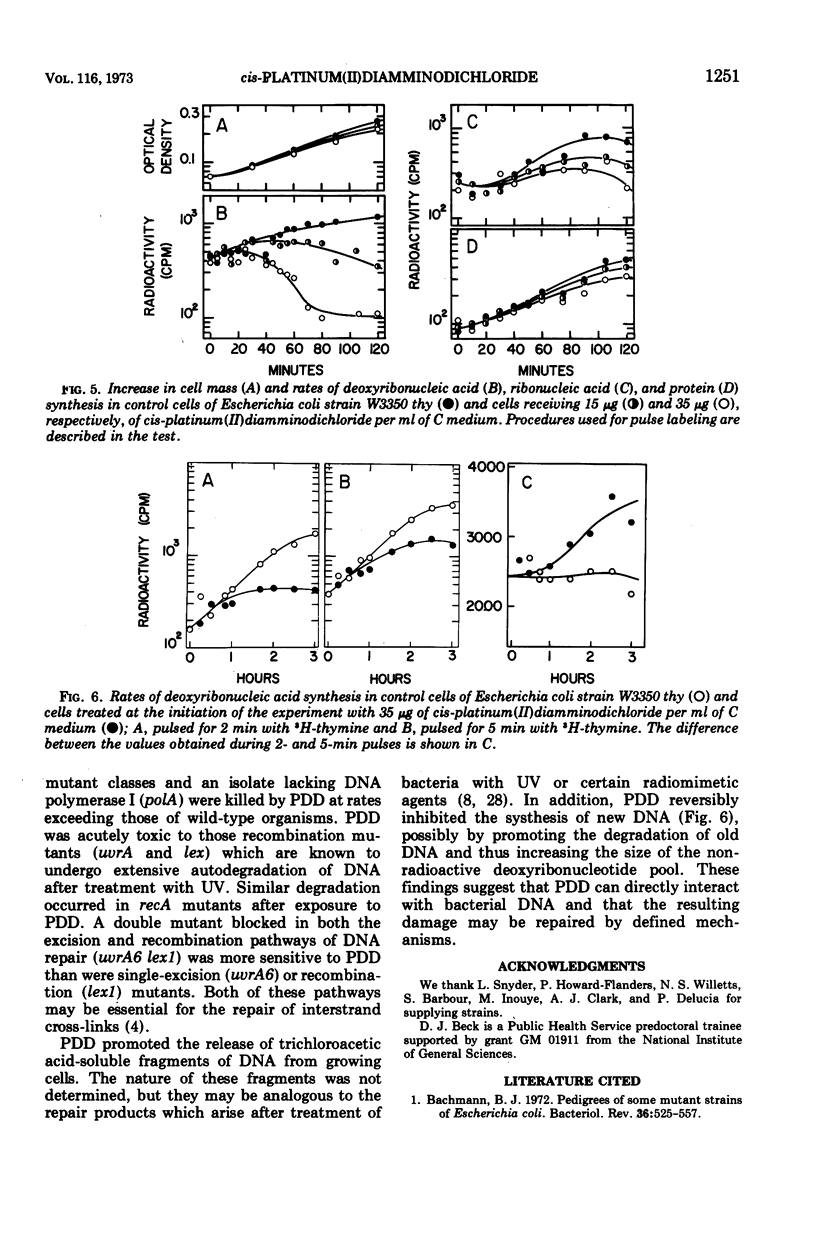
The anti-tumor drug cis-platinum(II)diamminodichloride (PDD) induced extensive filamentation in wild-type Escherichia coli and in mutants lacking certain deoxyribonucleic acid (DNA) repair functions (uvrA, recB, recC, and polA); viability of repair-deficient mutants treated with PDD was significantly less than that of wild-type cells. PDD was highly toxic to lex1, lex1 uvrA6 (where its effect was cummulative), and recA13 mutants, all of which were killed without formation of filaments. 3H-thymine incorporated into DNA of cells subsequently treated with PDD became trichloroacetic acid-soluble at rates similar to those observed after exposure to comparable doses of ultraviolet light (UV) or mitomycin C. PDD, like UV, induced extensive degradation of DNA in recA organisms. After a 30-min lag, PDD inhibited significantly the synthesis of DNA but not of ribonucleic acid or protein in E. coli. However, the relative differences between rates of DNA synthesis observed in PDD-treated and control cells decreased substantially when the duration of pulses (3H-thymine) was prolonged from 2 to 5 min. These observations suggest that PDD-induced damage to DNA is reversible, possibly by defined mechanisms of excision and recombination repair.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour S. D., Clark A. J. Biochemical and genetic studies of recombination proficiency in Escherichia coli. I. Enzymatic activity associated with recB+ and recC+ genes. Proc Natl Acad Sci U S A. 1970 Apr;65(4):955–961. doi: 10.1073/pnas.65.4.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldo-Kimball F., Barbour S. D. Involvement of recombination genes in growth and viability of Escherichia coli K-12. J Bacteriol. 1971 Apr;106(1):204–212. doi: 10.1128/jb.106.1.204-212.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R. S. Repair of DNA containing interstrand crosslinks in Escherichia coli: sequential excision and recombination. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1064–1068. doi: 10.1073/pnas.70.4.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobník J., Urbánková M., Krekulová A. The effect of cis-dichlorodiammineplatinum(II) on Escherichia coli B. The role of fil, exr and hcr markers. Mutat Res. 1973 Jan;17(1):13–20. doi: 10.1016/0027-5107(73)90248-0. [DOI] [PubMed] [Google Scholar]
- HOWARD-FLANDERS P., SIMSON E., THERIOT L. A LOCUS THAT CONTROLS FILAMENT FORMATION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI K-12. Genetics. 1964 Feb;49:237–246. doi: 10.1093/genetics/49.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder H. C., Rosenberg B. Inhibitory effects of anti-tumor platinum compounds on DNA, RNA and protein syntheses in mammalian cells in virtro. Int J Cancer. 1970 Sep 15;6(2):207–216. doi: 10.1002/ijc.2910060207. [DOI] [PubMed] [Google Scholar]
- Horácek P., Drobník J. Interaction of cis-dichlorodiammineplatinum (II) with DNA. Biochim Biophys Acta. 1971 Dec 16;254(2):341–347. doi: 10.1016/0005-2787(71)90842-2. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P. DNA repair. Annu Rev Biochem. 1968;37:175–200. doi: 10.1146/annurev.bi.37.070168.001135. [DOI] [PubMed] [Google Scholar]
- Howle J. A., Gale G. R. Cis-dichlorodiammineplatinum (II). Persistent and selective inhibition of deoxyribonucleic acid synthesis in vivo. Biochem Pharmacol. 1970 Oct;19(10):2757–2762. doi: 10.1016/0006-2952(70)90102-4. [DOI] [PubMed] [Google Scholar]
- Howle J. A., Thompson H. S., Stone A. E., Gale G. R. Cis-dichlorodiammineplatinum [II]: inhibition of nucleic acid synthesis in lymphocytes stimulated with phytohemagglutinin. Proc Soc Exp Biol Med. 1971 Jul;137(3):820–825. doi: 10.3181/00379727-137-35675. [DOI] [PubMed] [Google Scholar]
- Inouye M. Pleiotropic effect of the rec A gene of Escherichia coli: uncoupling of cell division from deoxyribonucleic acid replication. J Bacteriol. 1971 May;106(2):539–542. doi: 10.1128/jb.106.2.539-542.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kociba R. J., Sleight S. D., Rosenberg B. Inhibition of Dunning asc itic leukemia and Walker 256 carcinosarcoma with cis-diamminedichloroplatinum (NSC-119875). Cancer Chemother Rep. 1970 Oct;54(5):325–328. [PubMed] [Google Scholar]
- Kohiyama M. DNA synthesis in temperature sensitive mutants of Escherichia coli. Cold Spring Harb Symp Quant Biol. 1968;33:317–324. doi: 10.1101/sqb.1968.033.01.036. [DOI] [PubMed] [Google Scholar]
- ROSENBERG B., VANCAMP L., KRIGAS T. INHIBITION OF CELL DIVISION IN ESCHERICHIA COLI BY ELECTROLYSIS PRODUCTS FROM A PLATINUM ELECTRODE. Nature. 1965 Feb 13;205:698–699. doi: 10.1038/205698a0. [DOI] [PubMed] [Google Scholar]
- Reslová S. The induction of lysogenic strains of Escherichia coli by cis-dichloro-diammineplatinum (II). Chem Biol Interact. 1971 Dec;4(1):66–70. doi: 10.1016/0009-2797(71)90034-2. [DOI] [PubMed] [Google Scholar]
- Roberts J. J., Pascoe J. M. Cross-linking of complementary strands of DNA in mammalian cells by antitumour platinum compounds. Nature. 1972 Feb 4;235(5336):282–284. doi: 10.1038/235282a0. [DOI] [PubMed] [Google Scholar]
- Rosenberg B., Renshaw E., Vancamp L., Hartwick J., Drobnik J. Platinum-induced filamentous growth in Escherichia coli. J Bacteriol. 1967 Feb;93(2):716–721. doi: 10.1128/jb.93.2.716-721.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg B., Van Camp L., Grimley E. B., Thomson A. J. The inhibition of growth or cell division in Escherichia coli by different ionic species of platinum(IV) complexes. J Biol Chem. 1967 Mar 25;242(6):1347–1352. [PubMed] [Google Scholar]
- Rosenberg B., VanCamp L. The successful regression of large solid sarcoma 180 tumors by platinum compounds. Cancer Res. 1970 Jun;30(6):1799–1802. [PubMed] [Google Scholar]
- Rosenberg B., VanCamp L., Trosko J. E., Mansour V. H. Platinum compounds: a new class of potent antitumour agents. Nature. 1969 Apr 26;222(5191):385–386. doi: 10.1038/222385a0. [DOI] [PubMed] [Google Scholar]
- Shlaes D. M., Anderson J. A., Barbour S. D. Excision repair properties of isogenic rec mutants of Escherichia coli K-12. J Bacteriol. 1972 Sep;111(3):723–730. doi: 10.1128/jb.111.3.723-730.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shooter K. V., Howse R., Merrifield R. K., Robins A. B. The interaction of plantinum II compounds with bacteriophages T7 and R17. Chem Biol Interact. 1972 Oct;5(5):289–307. doi: 10.1016/0009-2797(72)90069-5. [DOI] [PubMed] [Google Scholar]
- Welsch C. W. Growth inhibition of rat mammary carcinoma induced by cis-platinum diamminodichloride-II. J Natl Cancer Inst. 1971 Nov;47(5):1071–1078. [PubMed] [Google Scholar]
- Willetts N. S., Mount D. W. Genetic analysis of recombination-deficient mutants of Escherichia coli K-12 carrying rec mutations cotransducible with thyA. J Bacteriol. 1969 Nov;100(2):923–934. doi: 10.1128/jb.100.2.923-934.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]



