Abstract
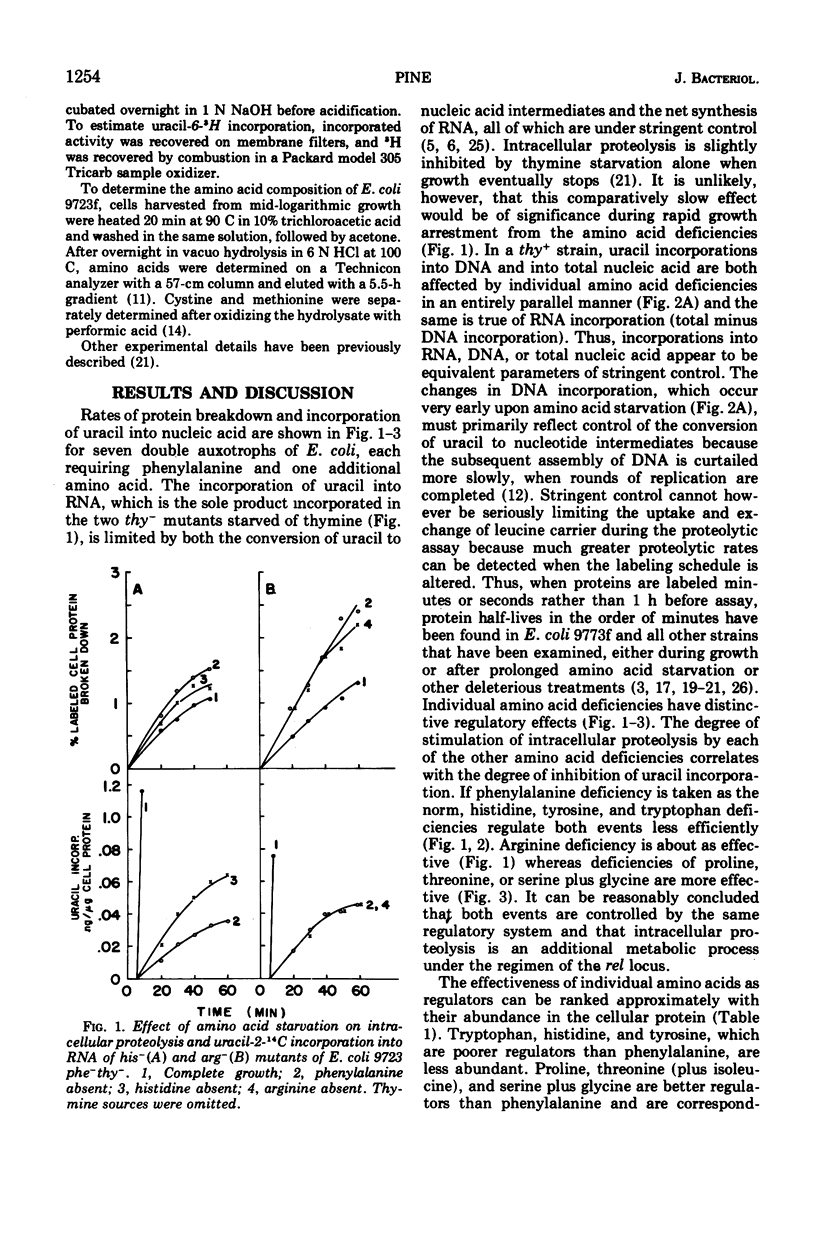
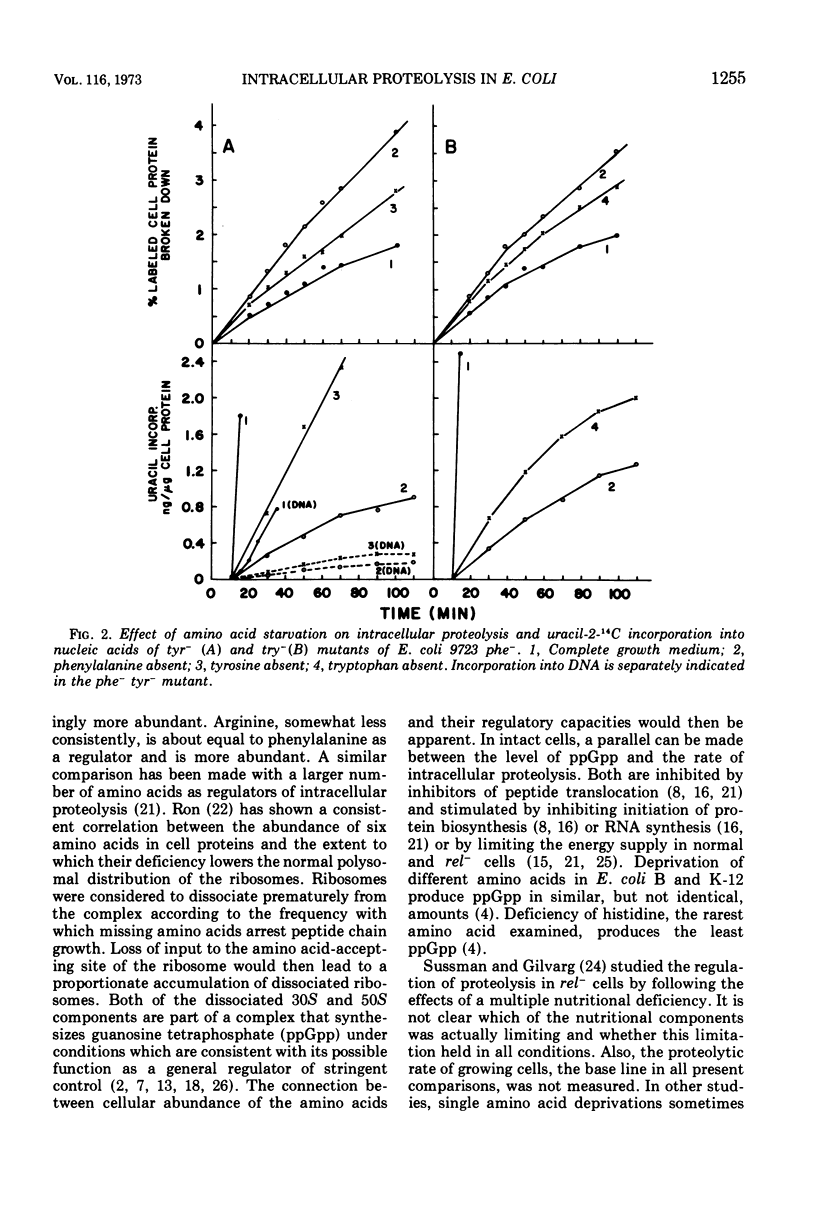
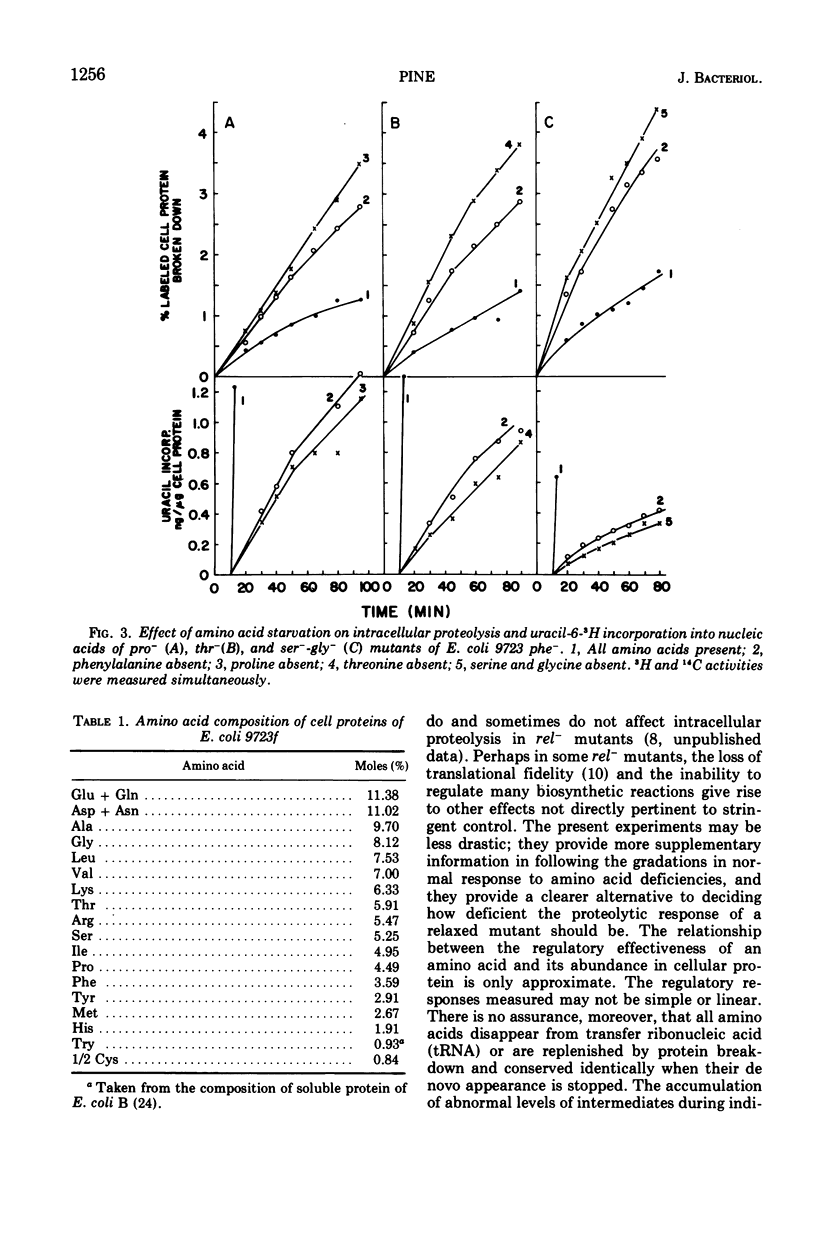
Regulation of intracellular proteolysis has been compared during amino acid deficiencies in seven double auxotrophs of Escherichia coli 9723f with a common phenylalanine requirement. Individual deficiencies were either more effective than, less effective, or equal to phenylalanine deficiency in stimulating intracellular proteolysis. For each amino acid, the same relationship prevailed in inhibiting uracil incorporation into nucleic acids, a reaction series regulated by the rel gene for stringent control. The three amino acids least abundant in the cellular protein were the least effective regulators. These findings are interpreted as supportive evidence for stringent control of intracellular proteolysis by the rel gene.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumenthal T., Landers T. A., Weber K. Bacteriophage Q replicase contains the protein biosynthesis elongation factors EF Tu and EF Ts. Proc Natl Acad Sci U S A. 1972 May;69(5):1313–1317. doi: 10.1073/pnas.69.5.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunschede H., Bremer H. Synthesis and breakdown of proteins in Escherichia coli during amino-acid starvation. J Mol Biol. 1971 Apr 14;57(1):35–57. doi: 10.1016/0022-2836(71)90118-5. [DOI] [PubMed] [Google Scholar]
- Cashel M. The control of ribonucleic acid synthesis in Escherichia coli. IV. Relevance of unusual phosphorylated compounds from amino acid-starved stringent strains. J Biol Chem. 1969 Jun 25;244(12):3133–3141. [PubMed] [Google Scholar]
- Edlin G., Broda P. Physiology and genetics of the "ribonucleic acid control" locus in escherichia coli. Bacteriol Rev. 1968 Sep;32(3):206–226. doi: 10.1128/br.32.3.206-226.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlin G., Neuhard J. Regulation of nucleoside triphosphate pools in Escherichia coli. J Mol Biol. 1967 Mar 14;24(2):225–230. doi: 10.1016/0022-2836(67)90328-2. [DOI] [PubMed] [Google Scholar]
- GORINI L., KAUFMAN H. Selecting bacterial mutants by the penicillin method. Science. 1960 Feb 26;131(3400):604–605. doi: 10.1126/science.131.3400.604. [DOI] [PubMed] [Google Scholar]
- Gallant J., Irr J., Cashel M. The mechanism of amino acid control of guanylate and adenylate biosynthesis. J Biol Chem. 1971 Sep 25;246(18):5812–5816. [PubMed] [Google Scholar]
- Goldberg A. L. A role of aminoacyl-tRNA in the regulation of protein breakdown in Escherichia coli. Proc Natl Acad Sci U S A. 1971 Feb;68(2):362–366. doi: 10.1073/pnas.68.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANAWALT P. C., MAALOE O., CUMMINGS D. J., SCHAECHTER M. The normal DNA replication cycle. II. J Mol Biol. 1961 Apr;3:156–165. doi: 10.1016/s0022-2836(61)80042-9. [DOI] [PubMed] [Google Scholar]
- Hall B., Gallant J. Defective translation in RC - cells. Nat New Biol. 1972 May 31;237(74):131–135. doi: 10.1038/newbio237131a0. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Block R., Gilbert W., Weber K. MSI and MSII made on ribosome in idling step of protein synthesis. Nature. 1972 Aug 18;238(5364):381–384. doi: 10.1038/238381a0. [DOI] [PubMed] [Google Scholar]
- Lazzarini R. A., Cashel M., Gallant J. On the regulation of guanosine tetraphosphate levels in stringent and relaxed strains of Escherichia coli. J Biol Chem. 1971 Jul 25;246(14):4381–4385. [PubMed] [Google Scholar]
- Lund E., Kjeldgaard N. O. Metabolism of guanosine tetraphosphate in Escherichia coli. Eur J Biochem. 1972 Jul 24;28(3):316–326. doi: 10.1111/j.1432-1033.1972.tb01916.x. [DOI] [PubMed] [Google Scholar]
- Nath K., Koch A. L. Protein degradation in Escherichia coli. II. Strain differences in the degradation of protein and nucleic acid resulting from starvation. J Biol Chem. 1971 Nov 25;246(22):6956–6967. [PubMed] [Google Scholar]
- Pedersen F. S., Lund E., Kjeldgaard N. O. Codon specific, tRNA dependent in vitro synthesis of ppGpp and pppGpp. Nat New Biol. 1973 May 2;243(122):13–15. [PubMed] [Google Scholar]
- Pine M. J. Heterogeneity of protein turnover in Escherichia coli. Biochim Biophys Acta. 1965 Jul 8;104(2):439–456. doi: 10.1016/0304-4165(65)90349-1. [DOI] [PubMed] [Google Scholar]
- Pine M. J. Regulation of intracellular proteolysis in Escherichia coli. J Bacteriol. 1973 Jul;115(1):107–116. doi: 10.1128/jb.115.1.107-116.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine M. J. Steady-state measurement of the turnover of amino acid in the cellular proteins of growing Escherichia coli: existence of two kinetically distinct reactions. J Bacteriol. 1970 Jul;103(1):207–215. doi: 10.1128/jb.103.1.207-215.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron E. Z. Polysome turnover during amino acid starvation in Escherichia coli. J Bacteriol. 1971 Oct;108(1):263–268. doi: 10.1128/jb.108.1.263-268.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPAHR P. F. Amino acid composition of ribosomes from Escherichia coli. J Mol Biol. 1962 May;4:395–406. doi: 10.1016/s0022-2836(62)80020-5. [DOI] [PubMed] [Google Scholar]
- Sussman A. J., Gilvarg C. Protein turnover in amino acid-starved strains of Escherichia coli K-12 differing in their ribonucleic acid control. J Biol Chem. 1969 Nov 25;244(22):6304–6306. [PubMed] [Google Scholar]
- Willetts N. S. Intracellular protein breakdown in growing cells of Escherichia coli. Biochem J. 1967 May;103(2):462–466. doi: 10.1042/bj1030462. [DOI] [PMC free article] [PubMed] [Google Scholar]


