Abstract
Cardiovascular malformations are one of the most common congenital birth defects observed in humans. Defects in cardiac valves disrupt normal blood flow. Zebrafish are an outstanding experimental model for studying the effects that environmental contaminants have on developmental processes. Previous research has shown that 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) causes blood regurgitation in the heart and reduces peripheral blood flow in embryonic zebrafish, suggesting some form of valve failure. To test this we used video microscopy to examine valve function and structure in developing zebrafish exposed to TCDD. TCDD exposure produced blood regurgitation at both the atrioventricular (AV) and bulboventricular (BV) junctions. In marked contrast to control embryos exposed to the vehicle dimethyl sulfoxide, embryos exposed to TCDD failed to form valve leaflets as the heart matured. In addition, whereas TCDD did not block initial formation of the bulbus arteriosus, we found that TCDD exposure prevented the normal growth and development of this portion of the outflow tract. TCDD altered the localization of endothelial cells at the AV and BV junctions and altered the localized expression of mRNAs bmp4 and notch1b normally associated with the nascent valves. Taken together, our results demonstrate that although TCDD does not prevent the initial specification of the presumptive valve locations, TCDD exposure produces severe alterations in valve development, leading to blood regurgitation and failing circulation in the developing zebrafish.
Keywords: zebrafish; 2,3,7,8-tetrachlorodibenzo-p-dioxin; heart; blood regurgitation; atrioventricular valve; bulboventricular valve
The cardiac valves play a critical role in heart function by preventing retrograde blood flow, thereby maintaining vascular hemodynamics. It is estimated that 25–30% of all cases of human cardiovascular malformation involve defects in cardiac valves (Armstrong and Bischoff, 2004). Atrioventricular (AV) canal defects are seen in approximately 5% of congenital heart disease, establishing this as the fourth most common congenital heart malformation (Emmanouilides et al., 1998). Valve defects are also observed in 16% of Down and Noonan syndrome patients as well as infants born to gestational diabetic mothers (Enciso et al., 2003; Stainier et al., 2002). The American Heart Association estimates 95,000 in patient valve procedures were performed in the United Stated alone in 2003, and about 300,000 worldwide (Thom et al., 2006; Vesely, 2005).
In humans, valve malformations include valvular stenosis where there is a narrowing of the valve opening and valvular insufficiency in which the valves do not close completely, allowing blood regurgitation (Flanagan and Pandit, 2003). Epidemiological studies suggest that people living in areas of water contaminated with halogenated hydrocarbons have an elevated risk for valvular stenosis and other cardiac malformations (Goldberg et al., 1990). Another study identified a region of Baltimore, MD, where an increased frequency of hypoplastic left heart syndrome correlated with the industrial release of solvents, dioxins and polychlorinated biphenyls (Kuehl and Loffredo, 2006). Understanding how genetic predisposition and environmental factors play a role in the development and functioning of the cardiac valves is a critical step in preventing these diseases.
Environmental contaminants such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) are bioaccumulative, lipophilic halogenated aromatic hydrocarbons that persist in the environment and cause cardiovascular malformations in mammalian, avian and aquatic species (Heid et al., 2001; Henry et al., 1997; Thackaberry et al., 2005b). Fetuses from pregnant mice exposed to TCDD exhibit a reduced heart to body weight, reduced fetal cardiocyte proliferation, and altered expression of cardiac genes involved in extracellular matrix (ECM) deposition, remodeling and cardiac homeostasis (Thackaberry et al., 2005a, b). In chickens, TCDD causes an increase in apoptosis in cells of the outflow tract and the AV canal endocardial cushions (ECs), whereas decreasing cardiomyocyte proliferation (Ivnitski et al., 2001). TCDD also reduced the growth of cells in cardiac ventricle explants isolated from embryonic chickens (Ivnitski-Steele and Walker, 2003).
Embryonic zebrafish exposed to TCDD exhibit a reduction in common cardinal vein area and reduced number of cardiomyocytes by 48 hpf (hours postfertilization). This is followed by reduced blood flow, decreased heart size, pericardial edema, impaired looping, reduced cardiac output, anemia, ventricular standstill, and ultimately death (Antkiewicz et al., 2005; Belair et al., 2001; Bello et al., 2004).
The developing zebrafish heart begins to beat at around 22 hpf and circulation is initiated shortly thereafter (Stainier et al., 1993). By 48 hpf, constrictions have clearly partitioned the heart into the sinus venosus, atrium, ventricle, and bulbous arteriosus (Hu et al., 2000). The heart consists of an outer myocardial layer that is contractile and an inner endocardial layer that is continuous with the vascular endothelium. In between these two layers lies the ECM or cardiac jelly (Yelon, 2001). The valves are formed from the endocardial cells at three sites: the sinus venosus valve at the junction between the sinus venosus and the atrium; the AV valve at the junction between the atrium and the ventricle; and the bulboventricular (BV) valve between the ventricle and the bulbus arteriosus (BA) of the outflow tract (Hu et al., 2000).
Two components of the Wnt signaling pathway, bone morphogenetic protein 4 (bmp4) and notch1b, are initially expressed throughout the heart, but become restricted to myocardial and endocardial cells marking the constrictions at the presumptive valve sites by 37 and 45 hpf, respectively (Walsh and Stainier, 2001).
There is some disagreement between reports describing the processes involved in early valve formation. For several years it has been thought that a subset of the AV endocardial cells differentiate in response to myocardial cell signals, and undergo an epithelial to mesenchymal transformation (EMT) at the site of the nascent valve. In this process, the endothelial cells delaminate and migrate into the cardiac jelly by 60–72 hpf. The delaminated cells form transient structures called ECs that help to prevent reverse blood flow (Walsh and Stainier, 2001). The ECs undergo further remodeling to form the actual valve leaflets at the AV boundary. Many aspects of this process have been observed directly and recently reported (Bartman et al., 2004; Beis et al., 2005; Hu et al., 2000; Hurlstone et al., 2003; Stainier et al., 2002; Wang et al., 2006)
However, a very recent report from Scherz et al. (2008) has called this model into question. In this paper, the authors described the use of selective plane illumination microscopy to gather evidence that valve leaflets are formed directly through an invagination process rather than through the intermediate step of forming cushions.
Blood flow and proper myocardial contractility are both believed to play a role in valve development in zebrafish. Reducing blood flow across the AV junction by obstructing the sinus venosus results in the absence of the AV valve and the BA (Hove et al., 2003). However, in other experiments, Bartman et al. (2004) found that it was possible to pharmacologically block blood flow entirely, without preventing the initial steps of valve formation.
The anecdotal report that TCDD causes blood regurgitation in the developing zebrafish heart provides a possible explanation for at least part of the circulation failure that TCDD produces in developing zebrafish (Antkiewicz et al., 2005). If TCDD altered the morphology or function of valve structures in the heart, then blood would be expected to leak backwards during systole, reducing cardiac output and blood flow. Here we report experiments designed to test this possibility. We used video microscopy to demonstrate that TCDD causes both retrograde blood flow and valve failure. We found that an initial failure of the nascent valve to form a seal was followed by a failure to form ECs or AV valve leaflets. Although markers of AV junction specification appeared normal in vehicle and TCDD-treated embryos at 48 hpf, the clusters of endothelial cells at the AV and BV junctions became progressively delocalized in the TCDD-exposed embryos at later time points. This coincided with a loss of localization and a general increase in expression of the valve specific markers bmp4 and notch1b in the region of the AV junction. These results demonstrate that TCDD exposure severely disrupts heart valve formation in the developing zebrafish, a response that would be expected to contribute to the subsequent lethality.
MATERIALS AND METHODS
Zebrafish lines.
All zebrafish embryos were kept at 28.5° in egg water (60 μg/ml Instant Ocean Salts; Aquarium Systems, Mentor, OH) with a 14-h/10-h light/dark cycle. Wild-type AB embryos were used in assessing blood regurgitation and for in situ hybridization experiments. Embryos from the vascular endothelial specific Tg(flk1:GFP) fish (kindly provided by Dr Beth Roman and Dr Jau-Nian Chen; Cross et al., 2003) were used for assessing the endothelial cell localization and for assessing AV valve leaflet development. Tg(cmlc2:gfp) embryos a kind gift from Dr C. Geoffrey Burns (Rottbauer et al., 2006) were used to assess the development of the BA.
TCDD exposure.
TCDD of > 99% purity was obtained from Chemsyn (Lenexa, KS) and dissolved in 0.1% dimethyl sulfoxide (DMSO). Embryos were placed in glass vials containing egg water and exposed to either TCDD (1 ng/ml) or 0.1% DMSO (vehicle) for 1 h on a rocker. After the 1-h exposure, the embryos were rinsed with fresh egg water and transferred to Petri dishes and raised at 28.5° in a 14-h light/10-h dark cycle. The maximum number of eggs/ml of egg water did not exceed 10 embryos. For our experiments, each group of embryos exposed in a single vial constitutes an n = 1.
Assessing blood regurgitation and endothelial cell localization.
Live embryos were mounted on a slide containing 3% methylcellulose to reduce movement and blood regurgitation across the valves was assessed by following video recordings made with a Nikon TE300 inverted microscope with a high-speed Motion Scope camera. Statistical significance of blood regurgitation incidence at the AV junction was determined via Fisher's p-test. For endothelial cell localization, Tg(flk1:GFP) embryos were dosed and collected as above but visualized using a Princeton instruments Micromax charge coupled device camera and MetaMorph imaging software (Molecular Devices, CA).
Assessing development of the BA chamber.
Tg(cmlc2::gfp) embryos were exposed to TCDD or vehicle as described above. In this strain, gfp is seen in the atrium and ventricle, but is absent in the BA. At 24 hpf embryos were transferred to fish water containing 0.003% 1-phenyl-2-thiourea (PTU) to prevent pigment formation. At 36 hpf live embryos were transferred to fish water containing 10μM diaminorhodamine-4M AM (DAR-4M AM) (Calbiochem, San Diego, CA) in fish water adjusted to pH 7.0 and kept overnight in the dark for detection of the bulbus (Grimes et al. 2006). DAR-4M AM is an in vivo cell permeable, photo-stable nitric oxide reactive dye that marks the developing BA. New fish water containing DAR-4M AM was replaced every 24 h, and the presence of the bulbus was assessed at 48, 60, 72, 76, 96, and 120 hpf using a Nikon TE300 inverted microscope connected to a Princeton instruments Micromax CCD camera.
Immunohistochemistry.
Tg(flk1:GFP) embryos were exposed to TCDD or vehicle as described above. At 24 hpf embryos were transferred to fish water containing 0.003% PTU to prevent pigment formation. At 120 hpf they were euthanized in 1.67 mg/ml Tricaine (MS 222, Sigma, St Louis, MO), rinsed in phosphate-buffered saline (PBS, Cellgro), and fixed overnight in 4% Paraformaldehyde in PBS. Whole embryos were then mounted on a glass slide and observed with a Nikon C1 laser scanning confocal microscope.
In situ hybridization.
Vehicle and TCDD-treated AB embryos were collected at 48, 60, and 72 hpf. The embryos were euthanized in 1.67 mg/ml Tricaine (MS 222, Sigma), rinsed in PBS and fixed overnight in 4% Paraformaldehyde in PBS. The next day they were washed in PBS, and bleached in a mix of 3% Hydrogen peroxide and 1% potassium hydroxide. They were then washed in PBS, and dehydrated in methanol and stored at −20°C until needed. TCDD-treated fish were tail clipped for identification after dehydration so that both treatment groups could be processed together for the in situ hybridization steps. This ensured identical staining. BMP4 and Notch1b plasmids were kindly provided by Dr Randall Peterson. Riboprobes were labeled with digoxigenin-UTP and visualized using anti-digoxigenin-AP Fab fragments (Roche Applied Science, Indianapolis, IN) with 4-nitro blue tetrazolium chloride solution and 5-bromo-4-chloro-3-indolyl-phosphate as substrates. Hybridization was carried out at 65°C. Overall for the 60 hpf samples n = 16 from two separate experiments; for the 48 and 72 hpf samples n = 26 from three separate experiments. A single representative embryo was selected for each treatment group at each time point and photographed with an Optronics MicroFire camera (Leica) mounted onto a Leica MZ16 stereomicroscope (Leica).
RESULTS
TCDD Exposure Causes Blood Regurgitation at the AV Valve
Anecdotal evidence suggested that a part of the reduction in blood flow produced by TCDD might be due to regurgitation of blood at the sites of the nascent valves in the developing heart. We used video microscopy to examine the incidence of blood regurgitation in TCDD-exposed embryos and in control embryos exposed to DMSO from 48 to 120 hpf. We found evidence that TCDD produced an overall greater incidence of blood regurgitation at the AV boundary compared with vehicle (Fig. 1).
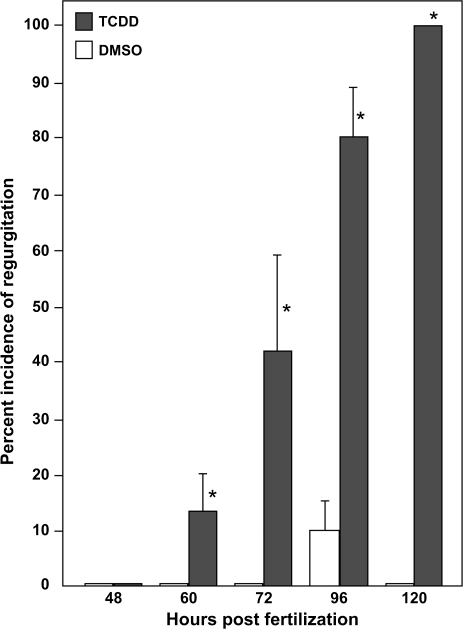
FIG. 1.
TCDD causes blood regurgitation in the developing zebrafish heart. Newly fertilized zebrafish eggs were treated with TCDD or DMSO as a vehicle control as described in the “Materials and Methods.” Videomicrographs were recorded at the indicated times during development and individuals were scored for retrograde blood flow across the AV junction. Asterisks indicate a statistical difference between TCDD and control hearts p ≤ (0.05) determined via one-tailed Fisher's p-test. Error bars indicate standard error.
We saw significant blood regurgitation at the AV boundary beginning at approximately 60 hpf in the TCDD-exposed embryos, but not in the controls. This became progressively more severe as development progressed. By 72 hpf, TCDD-exposed embryos demonstrate clear signs of valvular insufficiency. We observed that the ring of tissue at the presumptive AV valve site failed to shut completely during ventricular systole. This left a small opening that allowed blood cells to trickle back into the atrium in TCDD-exposed fish examined at 72 hpf (Fig. 2) (Supplemental Movie: 72hpfAB.mov). As the atrium contracted, blood was expelled from the atrium into the ventricle; however, as the ventricle contracted a small fraction of cells could be seen pushed in a retrograde motion from within the ventricle back into the atrium. By 96 hpf virtually all of the blood moving into the ventricle was then moved backwards into the atrium during systole, virtually abolishing blood flow (Supplementary Data, supplemental movie: 96hpfAB.mov).
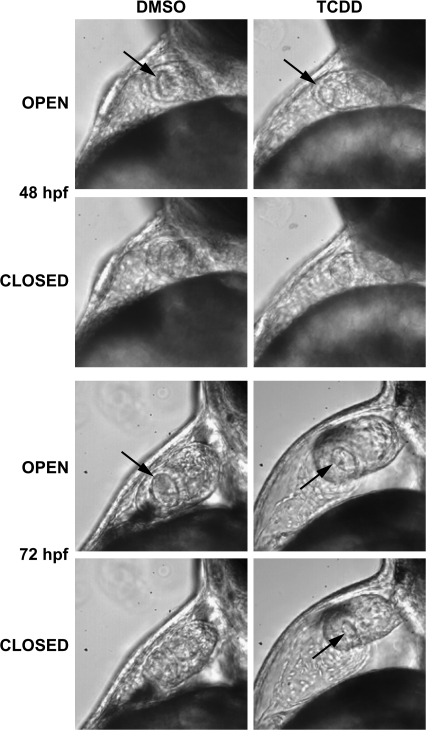
FIG. 2.
TCDD prevents closure of the nascent AV valve during systole. Newly fertilized zebrafish eggs were treated with TCDD or DMSO as a vehicle control as described in the “Materials and Methods.” Representative lateral view micrographs are shown for hearts at the indicated time in development. The arrows indicate an open passage between the atrium and ventricle. Open: the image was taken during diastole in which the AV junction is relaxed. Closed: the image was taken during systole, during which the AV junction has contracted to its fullest extent. An open passage between the ventricle and atrium was consistently observed associated with retrograde blood movement at 72 hpf (see supplemental movie: 72hpfAB.mov).
TCDD Exposure Prevents Formation of Valve Cushions and Leaflets at the AV Junction
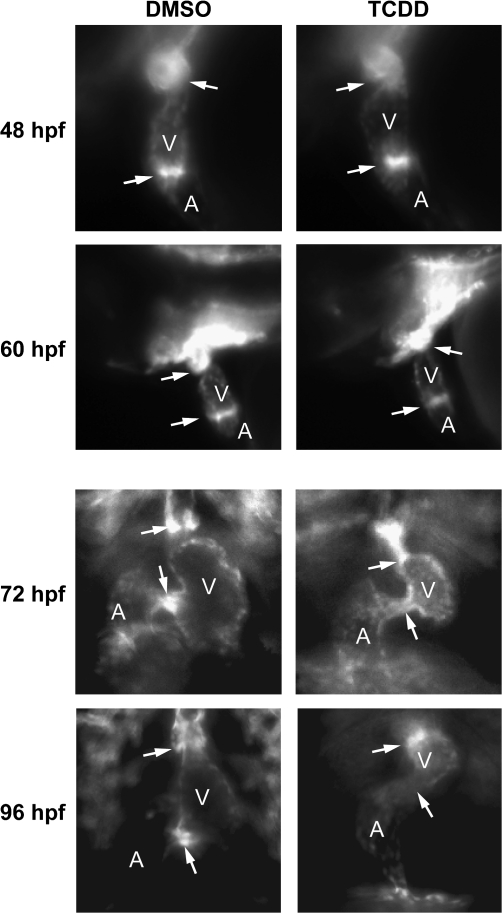
We used Tg(flk1:GFP) to determine whether TCDD prevents the distinct thickening of endothelial cells at the AV junction that has been reported to be indicative of cardiac cushion formation (Bartman et al., 2004; Stainier et al., 2002). Figure 3 shows successive images from high-speed video recordings in which the endothelial cells are marked with GFP fluorescence. In the control zebrafish, endothelial cells were observed in distinct clusters that appeared to form actual valve leaflets as early as 72 hpf, consistent with the results recently reported by Scherz et al. (2008). These leaflets were even more distinct and well developed by 96 hpf. This can be even more readily discerned in movies included as supplemental data (supplemental movies: 72hpf_flk.mov; 96hpf_flk.mov). In striking contrast, there was no evidence of either cushion or leaflet formation in the TCDD-exposed zebrafish at either 72 or 96 hpf. Furthermore, although the endothelial cells in the control hearts were clustered tightly so as to form a mass that decisively occludes the lumen during contraction, the endothelial cells in the TCDD-treated hearts were less concentrated, leaving what appears to be a small gap during contraction. By 96 hpf there was no readily discernible cluster of endothelial cells at the AV junction in the TCDD-treated hearts.
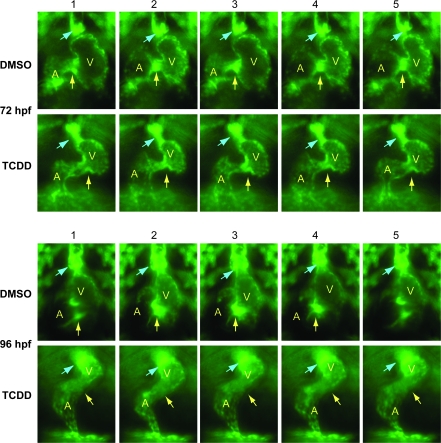
FIG. 3.
TCDD alters the arrangement of endothelial cell clusters at valve sites. Newly fertilized Tg(flk1:GFP) eggs were treated with TCDD or DMSO as a vehicle control as described in the “Materials and Methods.” The figure shows sequential frames taken at six images per second. Representative ventral view fluorescence micrographs showing endocardial cells at the AV and BV junctions are shown for 72 and 96 hpf as indicated. A indicates atrium; V indicates ventricle. Arrows show the location of the AV and BV junctions. The arrows are positioned to indicate forming valve leaflets at the junctions in the DMSO images; leaflets were not observed in the TCDD-exposed hearts.
TCDD produced similar effects at the BV junction, in which the control hearts had distinct clusters of endocardial structures forming discernible flaps by 96 hpf, whereas the TCDD-treated hearts had a more diffuse and unorganized arrangement of endothelial cells. These results are consistent with the results shown in Figures 1 and 2, demonstrating that TCDD prevents sealing at the AV junction, allowing retrograde blood flow.
We used confocal microscopy to get a better view of leaflet formation at the AV junction at 120 hpf in treated and vehicle Tg(flk1:GFP) embryos (Fig. 4). Because it was possible that TCDD had simply delayed leaflet formation, we chose a time point two days after we had first seen signs of leaflet formation in the control hearts. If TCDD were simply delaying the emergence of leaflets, one might expect to see evidence of leaflet formation in the TCDD-exposed hearts by this time point. At 120 hpf, the nascent valve leaflets were clearly visible at the AV boundary in the control hearts. We saw no signs of leaflets in the TCDD-exposed hearts; aggregation of endothelial cells at the AV boundary was greatly reduced in TCDD-treated fish.
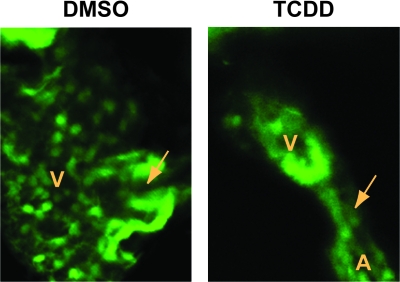
FIG. 4.
Valve leaflets fail to form in 120 hpf zebrafish exposed to TCDD. Newly fertilized Tg(flk1:GFP) eggs were treated with TCDD or DMSO as a vehicle control and ventral view confocal micrographs were recorded at 120 hpf as described in the Materials and Methods. Representative images show the AV junction, indicated by the arrow. Valve leaflets can be clearly seen flanking the AV passage in the DMSO heart. A indicates atrium; V indicates ventricle.
TCDD Exposure Inhibits BA Development
During our confocal microscopy experiments, we began to notice that TCDD produces changes in the BV valve as well as the BA chamber itself. The BA serves as a capacitor as the smooth muscle expands and relaxes (supplemental movie:dar4_120hpf.mov), protecting the gills and providing uniform blood flow (Grimes et al., 2006; Hu et al., 2000). The BA is first noticeable at the outflow side of the ventricle at around 48 hpf, when it can be observed by in vivo staining with DAR-4M AM, a membrane permeable compound that detects nitric oxide (Grimes et al., 2006).
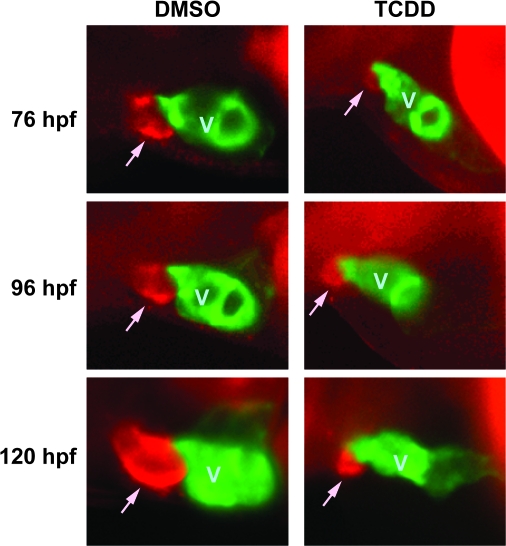
We used DAR-4M AM to determine what effects TCDD has on the formation of the BA (Fig. 5). In our hands, we could not clearly detect the BA prior to 76 hpf. By this time, TCDD-exposed embryos already exhibited a dramatically smaller BA then was observed in the vehicle exposed fish. Although the BA in vehicle treated fish steadily increased in size as seen at the 96 and 120 hpf points, the BA in the TCDD-exposed embryos exhibited little if any growth. This indicates that although TCDD did not block the initial formation of a group of DAR-4M AM staining cells at the location of the BA, it prevented any further growth of the outflow chamber.
FIG. 5.
Development of the BA is inhibited by TCDD. Tg(cmlc2::gfp) embryos were exposed to TCDD or DMSO vehicle and treated with DAR-4M AM to stain the BA as described in the Materials and Methods. Embryos were mounted in methylcellulose and visualized using epifluorescence. Lateral view images of representative vehicle and TCDD embryos are shown for 76, 96, and 120 hpf are shown. The head is toward the left of the figure, and the dorsal side is to the top. The ventricle is labeled with GFP, and indicated by a V; the DAR-4 signal identifying the BA is seen as red, and is indicated by an arrow.
TCDD Exposure Alters Endothelial Cell Localization at the AV Junction
Among the early steps of valve formation is the accumulation and clustering of endothelial cells lining the heart at the AV and BV junctions. Using Tg(flk1:GFP) we followed the migration and accumulation of endothelial cells at the AV boundary over time. We were able to clearly distinguish a cluster of endothelial cells at the AV and BV junction by 36 hpf demarcating the junction between the atrium and ventricle and between the ventricle and outflow tract (data not shown). This group of GFP-positive cells was even more distinct in lateral view images at 48 hpf, where both vehicle and TCDD-treated fish showed a distinct cluster of endothelial cells at the AV junction, and a distinct cluster of endothelial cells at the BV junction (Fig. 6). We were unable to detect any difference between the localization of endothelial cells at the AV or BV junction in control and TCDD samples at 48 hpf or before. However, after 48 hpf we observed progressive loss of localization of these GFP-positive endothelial cells in the TCDD-treated samples. This TCDD-induced delocalization of endothelial cells at the AV junction was clearly evident in the ventral view images taken at 72 hpf and 96 hpf (Figs. 3 and 6).
FIG. 6.
TCDD alters endothelial cell localization at the AV and BV junctions. Newly fertilized Tg(flk1:GFP) eggs were treated with TCDD or DMSO as a vehicle control as described in the “Materials and Methods.” Representative fluorescence micrographs showing lateral views (48 and 60 hpf) and ventral views (72 and 96 hpf) of the heart are shown. These orientations were selected to give the best view of the heart at each stage of development. A indicates atrium; V indicates ventricle. Arrows show the locations of the AV and BV junctions.
We also found that TCDD caused the GFP-positive endothelial cells at the BV junction to become progressively less restricted at the BV junction over time. At 72 hpf, vehicle exposed embryos clearly exhibited a distinct cluster of endothelial cells at the BV junction. However, in TCDD-exposed fish the endothelial cells at the BV junction were distributed across a larger region of the outflow tract compared with the vehicle control (Fig. 6) (supplemental movie: 72hpf_flk).
TCDD Alters AV Boundary Specification during the EMT Process
During early heart development genes encoding bone morphogenetic protein 4 (bmp4) and notch1b are expressed throughout the heart. However, as the heart develops, these markers become restricted to the presumptive AV valve region: bmp4 is expressed in the myocardial layer, and notch1b is found in the endocardial layer (Walsh and Stainier, 2001). We used these markers to test the hypothesis that TCDD disrupts AV boundary specification.
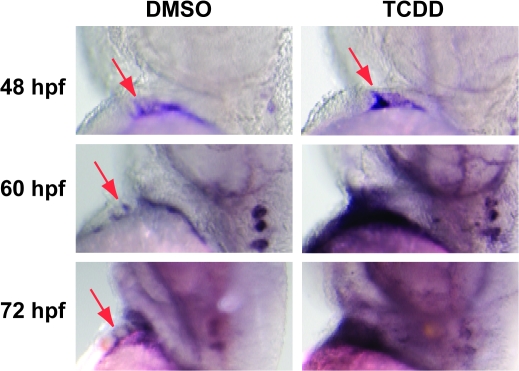
At 48 hpf, we consistently observed a small ring or dot of bmp4 staining, corresponding to the previously reported localized expression at the AV boundary myocardium in both vehicle and TCDD-exposed embryos. This is visible as a small region of staining at the AV boundary and another at the outflow tract junction (Fig. 7). By 60 hpf, a point at which the EMT process has normally started (Beis et al., 2005), we consistently saw bmp4 expressed in a broader, less restricted region in TCDD-treated embryos. By 72 hpf, the bmp4 signal was consistently more intense in the TCDD-treated hearts than in the controls, and covered a region that extended completely beyond the heart, making it quite evident that expression was no longer restricted to the AV boundary.
FIG. 7.
TCDD alters bmp4 mRNA localization in the developing heart. Newly fertilized zebrafish eggs were treated with TCDD or DMSO as a vehicle control as described in the “Materials and Methods.” Representative lateral view micrographs are shown for hearts stained by in situ hybridization with a probe specific for bmp4 mRNA. Arrows indicate bmp4 signal at the location of the AV boundary myocardium. The head is toward the top of each panel and the dorsal side is to the right.
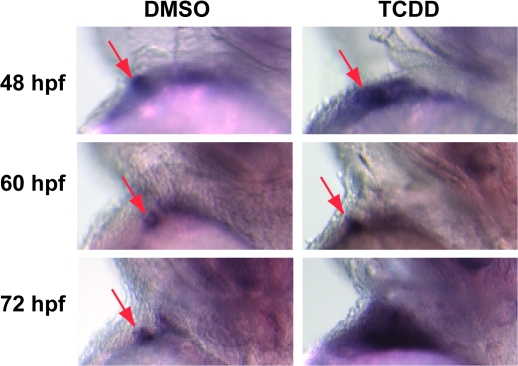
We observed very similar results with notch1b staining. As with bmp4, we initially observed a distinct spot of notch1b staining marking the AV junction endocardium in both control and TCDD-treated fish (Fig. 8). However, in the TCDD-treated fish this pattern of localized expression steadily broadened as the notch signal became progressively delocalized and stronger at 60 and 72 hpf.
FIG. 8.
TCDD alters notch1b mRNA localization in the developing heart. Newly fertilized zebrafish eggs were treated with TCDD or DMSO as a vehicle control as described in the “Materials and Methods.” Representative lateral view micrographs are shown for hearts stained by in situ hybridization with a probe specific for notch1b mRNA. Arrows indicate notch1b signal at the location of the AV boundary endocardium. The head is toward the top of each panel and the dorsal side is to the right.
These results demonstrate that TCDD alters processes specifying valve development at the AV boundary. The timing of the changes in bmp4 and notch1b localization corresponds to the loss of the localized pattern of endothelial cells at the AV and BV junctions depicted in Figure 6, and takes place at a stage in development during which critical processes affecting valve formation have been reported to occur. Thus, it is not surprising that TCDD prevented subsequent formation of valve structures, because TCDD leads to a loss of cellular conditions required for valve formation.
DISCUSSION
Our results demonstrate that TCDD exposure produces alterations in valve formation in the developing zebrafish heart. These effects were first observed as a failure of the AV junction to completely seal as the ventricle beats, allowing blood cells to slip backwards during ventricular systole. Although the observed blood regurgitation at the AV and BV valves is not the only defect caused by TCDD, the failure of a fraction of the blood cells to pass through the valves in the proper direction must contribute to the loss of peripheral circulation that TCDD exposure produces.
The changes in heart valve formation and function were associated with disruption of cellular patterns associated with normal valve formation. The localized clustering of endothelial cells that is normally observed at the AV and BV junctions as a precursor to valve development was disrupted by TCDD. Initially these localized clusters of endothelial cells formed normally despite the presence of TCDD, and were clearly present at 48 hpf. However, in the TCDD-exposed fish the endothelial cells do not remain localized to these junctions. This loss of endothelial cell localization coincided closely with delocalization of bmp4 and notch1b expression.
It is interesting to note that TCDD did not simply delay or halt the process of valve formation. At early time points the contractile process at the AV junction was normal, producing a functional barrier to retrograde blood flow despite the presence of TCDD. In addition, the localization of endothelial cells and the expression of notch1b and bmp4 appeared normal at early time points, despite the presence of TCDD. However, as time progressed, all of these normal features of early valve formation deteriorated, reversing whatever progress toward valve formation that had been made. Similarly, although TCDD did not prevent the initial formation of the BA, the size of the BA was greatly reduced in TCDD-exposed zebrafish. As with valve formation, TCDD did not appear to block the initial process of tissue specification, but appeared to prevent the development needed to complete the nascent structure.
TCDD and Endothelial Cells in the Heart
This work was initiated in part by casual observations that TCDD alters the distinct clusters of endothelial cells labeled with GFP at the sites of valve formation. Thereafter, we conducted numerous experiments directed at determining whether TCDD affects the different steps in the EMT process described in detail by Beis et al. (2005). As we were preparing this manuscript a report from Scherz et al. (2008) described work suggesting that there is no EMT process in the developing heart, and that instead the leaflets form directly from layers of endothelial cells. Some of our work, notably our observation of visible leaflets of GFP-positive endothelial cells in movies of hearts as early as 72 hpf, supports this recently proposed model. Beyond this, our work has little to contribute to the question of whether zebrafish valve formation goes through the classical EMT. Instead, our results demonstrate that the AHR agonist TCDD inhibits valve formation, entirely disrupting endothelial cell positioning at the presumptive valve site.
TCDD Alteration of bmp4 and notch1b Expression
Bmp4 and Notch1 are Wnt/β-catenin pathway components needed for normal valve development. Zebrafish mutants such as jekyll lack localization of bmp4 and notch1b at the AV boundary. These mutants fail to form ECs at 96 hpf and have retrograde blood flow (Stainier et al., 1996, 2002). Experiments in mice with a hypomorphic bmp4 allele indicate that bmp4 is specifically required for proper AV septation after the cushions have formed (Jiao et al., 2003). Mutation in the notch 1 receptor in mice or the RBPJk effector results in abnormal endocardial adhesion complexes and abortive endocardial EMT in vivo and in vitro (Grego-Bessa et al., 2004). Thus, the effects of TCDD on bmp4 and notch1b could be upstream of the morphological defects that we observed.
However, increased bmp4 and notch1b expression has been associated with promoting valve formation. Expression of mouse Notch1 (N1IC) in zebrafish embryos produces hypercellular ECs and enlarged AV valves, whereas suppression of N1IC causes atrophic ECs (Timmerman et al., 2004). Activation of the Wnt/β-catenin pathway leads to excessive development of valve cushions. This is associated with an upregulation of bmp4 and notch1b (Hurlstone et al., 2003). One might therefore expect the increased bmp4 and notch1b expression produced by TCDD would lead to increased formation of valve structures, rather than what we observed.
It is likely though that EC formation is regulated by a more complex signal than the simple levels of notch1b and bmp4 mRNAs. For example, zebrafish cloche mutants fail to develop endocardium and lack notch1b mRNA. This failure to express notch1b is associated with a loss of bmp4 localization at the AV junction. Thus, the absence of one layer abolishes processes needed to specify the site of the AV ring (Milan et al., 2006). It is possible that the elevated bmp4 and notch1b signals produced by TCDD result from a blockade of a process downstream in the Wnt signaling pathway needed for endothelial cell localization and valve formation. If this were the case, feedback mechanisms might upregulate bmp4 and notch1b in an attempt to restore normal Wnt signaling.
Blood Regurgitation, Valve Function, and Circulation
Because TCDD affects stroke volume and blood flow (Carney et al. 2006), it is possible that the effect on valve formation is secondary to an emerging loss of contractility and blood movement through the heart. The flow of blood through the heart is thought to generate signals that play an important role in valve formation (Hove et al., 2003). Silent heart (sih) mutants, with no blood flow, exhibit no EC formation. Similarly, cardiofunk mutants with no early blood flow, have defective valve cushion formation (Bartman et al., 2004; Sehnert et al., 2002).
It is noteworthy however that although neither the silent heart nor cardiofunk mutations alters the restricted bmp4 expression at the AV boundary, indicating that loss of blood flow does not alter AV boundary specification (Bartman et al., 2004). The effect of TCDD on localization of endothelial cells and molecular markers at the AV boundary is far more similar to the jekyll mutant, which produces delocalized bmp4 and notch1b expression, pericardial edema, a loss of endothelial cell localization at the AV boundary, and blood regurgitation by 48 hpf (Walsh and Stainier, 2001). Thus, although TCDD could produce altered valve development by altering blood flow, it is also possible that TCDD interferes with a developmental process that is independent of blood flow.
Environmental Factors and Valve Development
Over the past decade numerous studies have identified and characterized zebrafish mutants in an attempt to understand the key genes needed for normal heart development and function (Chen et al., 1996; Lee et al., 1994; Stainier et al., 1993, 1996). Although these studies have been useful in identifying genetic factors contributing to pathological conditions involving the cardiovascular system, there is little doubt that environmental causes also lead to heart abnormalities. By using the zebrafish, a well-established developmental model system, to study the effects of TCDD on heart valve development, we can combine our results with a wealth of knowledge obtained using genetic methods. This is a step toward the long-term goal of understanding how environmental and genetic factors combine to cause heart abnormalities and disease.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
National Institutes of Health grant (R01 ES012716), from the National Institute of Environmental Health Sciences, to W.H. and R.E.P.; the University of Wisconsin Sea Grant Institute; National Sea Grant College Program; National Oceanic and Atmospheric Administration; U.S. Department of Commerce grant number (NA 16RG2257); and Sea Grant Project numbers (R/BT-17, R/BT-20, and R/BT-22) to W.H. and R.E.P.
Supplementary Material
Acknowledgments
We thank Chad Vezina, Dagmara Antkiewicz, Adrian Grimes, Thomas Bartman, and Mary Halloran for useful discussions. We thank Randall Peterson for providing the bmp4 and notch1b plasmids, Beth Roman and Jau-Nian Chen for the Tg(flk1:GFP) line of zebrafish and Jackie Holquist, Dorothy Nesbit, C. Crows, and N. Jones for technical assistance. The contents are solely the responsibility of the authors and do not necessarily represent the official view of the National Institute of Environmental Health Sciences, National Institutes of Health.
References
- Antkiewicz DS, Burns CG, Carney SA, Peterson RE, Heideman W. Heart malformation is an early response to TCDD in embryonic zebrafish. Toxicol. Sci. 2005;84:368–377. doi: 10.1093/toxsci/kfi073. [DOI] [PubMed] [Google Scholar]
- Armstrong EJ, Bischoff J. Heart valve development: Endothelial cell signaling and differentiation. Circ. Res. 2004;95:459–470. doi: 10.1161/01.RES.0000141146.95728.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartman T, Walsh EC, Wen KK, McKane M, Ren J, Alexander J, Rubenstein PA, Stainier DY. Early myocardial function affects endocardial cushion development in zebrafish. PLoS Biol. 2004;2:E129. doi: 10.1371/journal.pbio.0020129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beis D, Bartman T, Jin SW, Scott IC, D'Amico LA, Ober EA, Verkade H, Frantsve J, Field HA, Wehman A, et al. Genetic and cellular analyses of zebrafish atrioventricular cushion and valve development. Development. 2005;132:4193–4204. doi: 10.1242/dev.01970. [DOI] [PubMed] [Google Scholar]
- Belair CD, Peterson RE, Heideman W. Disruption of erythropoiesis by dioxin in the zebrafish. Dev. Dyn. 2001;222:581–594. doi: 10.1002/dvdy.1213. [DOI] [PubMed] [Google Scholar]
- Bello SM, Heideman W, Peterson RE. 2,3,7,8-Tetrachlorodibenzo-p-dioxin inhibits regression of the common cardinal vein in developing zebrafish. Toxicol. Sci. 2004;78:258–266. doi: 10.1093/toxsci/kfh065. [DOI] [PubMed] [Google Scholar]
- Carney SA, Chen J, Burns CG, Xiong KM, Peterson RE, Heideman W. Aryl hydrocarbon receptor activation produces heart-specific transcriptional and toxic responses in developing zebrafish. Mol. Pharmacol. 2006;70:549–561. doi: 10.1124/mol.106.025304. [DOI] [PubMed] [Google Scholar]
- Chen JN, Haffter P, Odenthal J, Vogelsang E, Brand M, van Eeden FJ, Furutani-Seiki M, Granato M, Hammerschmidt M, Heisenberg CP, et al. Mutations affecting the cardiovascular system and other internal organs in zebrafish. Development. 1996;123:293–302. doi: 10.1242/dev.123.1.293. [DOI] [PubMed] [Google Scholar]
- Cross LM, Cook MA, Lin S, Chen JN, Rubinstein AL. Rapid analysis of angiogenesis drugs in a live fluorescent zebrafish assay. Arterioscler. Thromb. Vasc. Biol. 2003;23:911–912. doi: 10.1161/01.ATV.0000068685.72914.7E. [DOI] [PubMed] [Google Scholar]
- Emmanouilides GC, Allen HD, Riemenschneider TA, Gutgesell HP. Clinical Synopsis of Moss and Adams’ Heart Disease in Infants, Children, and Adolescents. Baltimore, MD: Williams and Wilkins; 1998. [Google Scholar]
- Enciso JM, Gratzinger D, Camenisch TD, Canosa S, Pinter E, Madri JA. Elevated glucose inhibits VEGF-A-mediated endocardial cushion formation: Modulation by PECAM-1 and MMP-2. J. Cell Biol. 2003;160:605–615. doi: 10.1083/jcb.200209014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan TC, Pandit A. Living artificial heart valve alternatives: A review. Eur. Cell. Mater. 2003;6:28–45. doi: 10.22203/ecm.v006a04. discussion 45. [DOI] [PubMed] [Google Scholar]
- Goldberg SJ, Lebowitz MD, Graver EJ, Hicks S. An association of human congenital cardiac malformations and drinking water contaminants. J. Am. Coll. Cardiol. 1990;16:155–164. doi: 10.1016/0735-1097(90)90473-3. [DOI] [PubMed] [Google Scholar]
- Grego-Bessa J, Diez J, Timmerman L, de la Pompa JL. Notch and epithelial-mesenchyme transition in development and tumor progression: Another turn of the screw. Cell Cycle. 2004;3:718–721. [PubMed] [Google Scholar]
- Grimes AC, Stadt HA, Shepherd IT, Kirby ML. Solving an enigma: Arterial pole development in the zebrafish heart. Dev. Biol. 2006;290:265–276. doi: 10.1016/j.ydbio.2005.11.042. [DOI] [PubMed] [Google Scholar]
- Heid SE, Walker MK, Swanson HI. Correlation of cardiotoxicity mediated by halogenated aromatic hydrocarbons to aryl hydrocarbon receptor activation. Toxicol. Sci. 2001;61:187–196. doi: 10.1093/toxsci/61.1.187. [DOI] [PubMed] [Google Scholar]
- Henry TR, Spitsbergen JM, Hornung MW, Abnet CC, Peterson RE. Early life stage toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in zebrafish (Danio rerio) Toxicol. Appl. Pharmacol. 1997;142:56–68. doi: 10.1006/taap.1996.8024. [DOI] [PubMed] [Google Scholar]
- Hove JR, Koster RW, Forouhar AS, Acevedo-Bolton G, Fraser SE, Gharib M. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature. 2003;421:172–177. doi: 10.1038/nature01282. [DOI] [PubMed] [Google Scholar]
- Hu N, Sedmera D, Yost HJ, Clark EB. Structure and function of the developing zebrafish heart. Anat. Rec. 2000;260:148–157. doi: 10.1002/1097-0185(20001001)260:2<148::AID-AR50>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Hurlstone AF, Haramis AP, Wienholds E, Begthel H, Korving J, Van Eeden F, Cuppen E, Zivkovic D, Plasterk RH, Clevers H. The Wnt/beta-catenin pathway regulates cardiac valve formation. Nature. 2003;425:633–637. doi: 10.1038/nature02028. [DOI] [PubMed] [Google Scholar]
- Ivnitski I, Elmaoued R, Walker MK. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) inhibition of coronary development is preceded by a decrease in myocyte proliferation and an increase in cardiac apoptosis. Teratology. 2001;64:201–212. doi: 10.1002/tera.1065. [DOI] [PubMed] [Google Scholar]
- Ivnitski-Steele ID, Walker MK. Vascular endothelial growth factor rescues 2,3,7,8-tetrachlorodibenzo-p-dioxin inhibition of coronary vasculogenesis. Birth Defects Res. A Clin. Mol. Teratol. 2003;67:496–503. doi: 10.1002/bdra.10074. [DOI] [PubMed] [Google Scholar]
- Jiao K, Kulessa H, Tompkins K, Zhou Y, Batts L, Baldwin HS, Hogan BL. An essential role of Bmp4 in the atrioventricular septation of the mouse heart. Genes Dev. 2003;17:2362–2367. doi: 10.1101/gad.1124803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehl KS, Loffredo CA. A cluster of hypoplastic left heart malformation in Baltimore, Maryland. Pediatr. Cardiol. 2006;27:25–31. doi: 10.1007/s00246-005-0859-x. [DOI] [PubMed] [Google Scholar]
- Lee RK, Stainier DY, Weinstein BM, Fishman MC. Cardiovascular development in the zebrafish. II. Endocardial progenitors are sequestered within the heart field. Development. 1994;120:3361–3366. doi: 10.1242/dev.120.12.3361. [DOI] [PubMed] [Google Scholar]
- Milan DJ, Giokas AC, Serluca FC, Peterson RT, MacRae CA. Notch1b and neuregulin are required for specification of central cardiac conduction tissue. Development. 2006;133:1125–1132. doi: 10.1242/dev.02279. [DOI] [PubMed] [Google Scholar]
- Rottbauer W, Wessels G, Dahme T, Just S, Trano N, Hassel D, Burns CG, Katus HA, Fishman MC. Cardiac myosin light chain-2: A novel essential component of thick-myofilament assembly and contractility of the heart. Circ. Res. 2006;99:323–331. doi: 10.1161/01.RES.0000234807.16034.fe. [DOI] [PubMed] [Google Scholar]
- Scherz PJ, Huisken J, Sahai-Hernandez P, Stainier DY. High-speed imaging of developing heart valves reveals interplay of morphogenesis and function. Development. 2008;135:1179–1187. doi: 10.1242/dev.010694. [DOI] [PubMed] [Google Scholar]
- Sehnert AJ, Huq A, Weinstein BM, Walker C, Fishman M, Stainier DY. Cardiac troponin T is essential in sarcomere assembly and cardiac contractility. Nat. Genet. 2002;31:106–110. doi: 10.1038/ng875. [DOI] [PubMed] [Google Scholar]
- Stainier DY, Beis D, Jungblut B, Bartman T. Endocardial cushion formation in zebrafish. Cold Spring Harb. Symp. Quant. Biol. 2002;67:49–56. doi: 10.1101/sqb.2002.67.49. [DOI] [PubMed] [Google Scholar]
- Stainier DY, Fouquet B, Chen JN, Warren KS, Weinstein BM, Meiler SE, Mohideen MA, Neuhauss SC, Solnica-Krezel L, Schier AF, et al. Mutations affecting the formation and function of the cardiovascular system in the zebrafish embryo. Development. 1996;123:285–292. doi: 10.1242/dev.123.1.285. [DOI] [PubMed] [Google Scholar]
- Stainier DY, Lee RK, Fishman MC. Cardiovascular development in the zebrafish. I. Myocardial fate map and heart tube formation. Development. 1993;119:31–40. doi: 10.1242/dev.119.1.31. [DOI] [PubMed] [Google Scholar]
- Thackaberry EA, Jiang Z, Johnson CD, Ramos KS, Walker MK. Toxicogenomic profile of 2,3,7,8-tetrachlorodibenzo-p-dioxin in the murine fetal heart: Modulation of cell cycle and extracellular matrix genes. Toxicol. Sci. 2005a;88:231–241. doi: 10.1093/toxsci/kfi301. [DOI] [PubMed] [Google Scholar]
- Thackaberry EA, Nunez BA, Ivnitski-Steele ID, Friggins M, Walker MK. Effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin on murine heart development: Alteration in fetal and postnatal cardiac growth, and postnatal cardiac chronotropy. Toxicol. Sci. 2005b;88:242–249. doi: 10.1093/toxsci/kfi302. [DOI] [PubMed] [Google Scholar]
- Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, Zheng ZJ, Flegal K, O'Donnell C, Kittner S, et al. Heart disease and stroke statistics–2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–e151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- Timmerman LA, Grego-Bessa J, Raya A, Bertran E, Perez-Pomares JM, Diez J, Aranda S, Palomo S, McCormick F, Izpisua-Belmonte JC, et al. Notch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev. 2004;18:99–115. doi: 10.1101/gad.276304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesely I. Heart valve tissue engineering. Circ. Res. 2005;97:743–755. doi: 10.1161/01.RES.0000185326.04010.9f. [DOI] [PubMed] [Google Scholar]
- Walsh EC, Stainier DY. UDP-glucose dehydrogenase required for cardiac valve formation in zebrafish. Science. 2001;293:1670–1673. doi: 10.1126/science.293.5535.1670. [DOI] [PubMed] [Google Scholar]
- Wang WD, Huang CJ, Lu YF, Hsin JP, Prabhakar VR, Cheng CF, Hwang SP. Heart-targeted overexpression of Nip3a in zebrafish embryos causes abnormal heart development and cardiac dysfunction. Biochem. Biophys. Res. Commun. 2006;347:979–987. doi: 10.1016/j.bbrc.2006.06.174. [DOI] [PubMed] [Google Scholar]
- Yelon D. Cardiac patterning and morphogenesis in zebrafish. Dev. Dyn. 2001;222:552–563. doi: 10.1002/dvdy.1243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.