Abstract
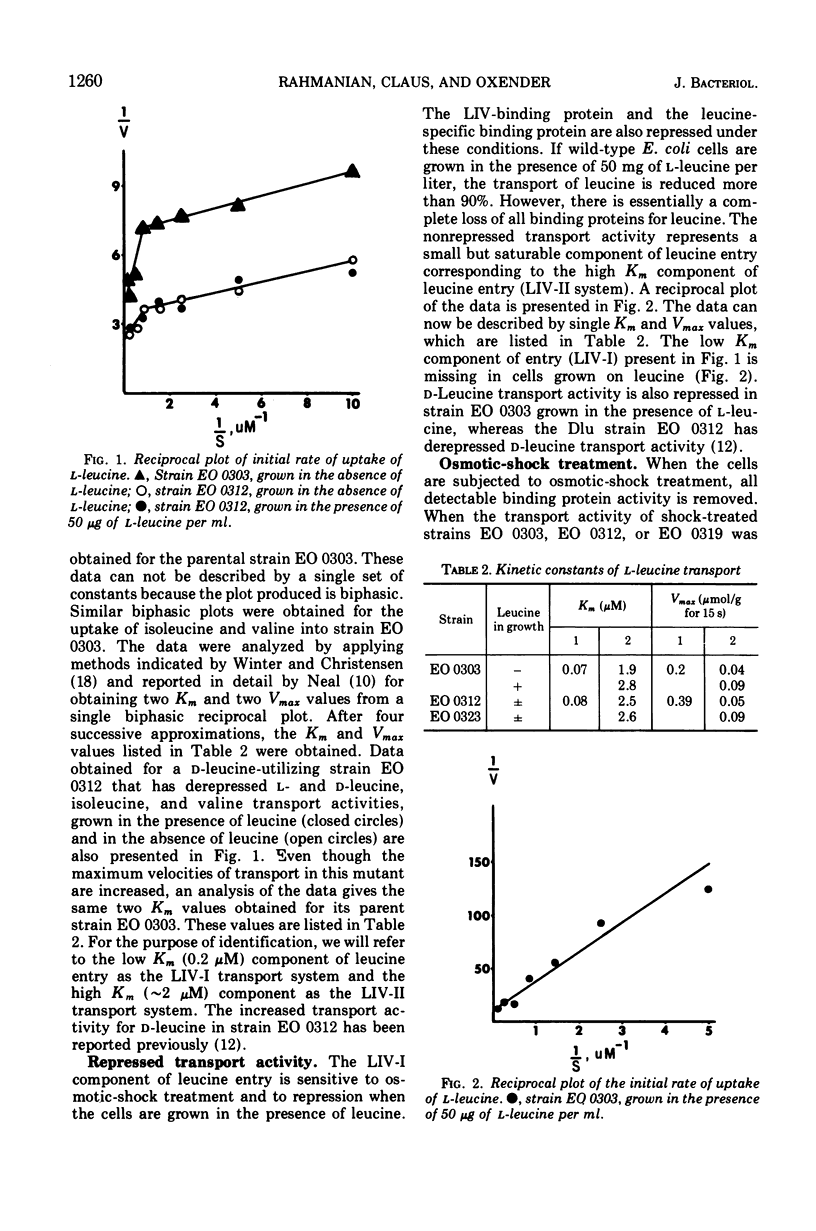
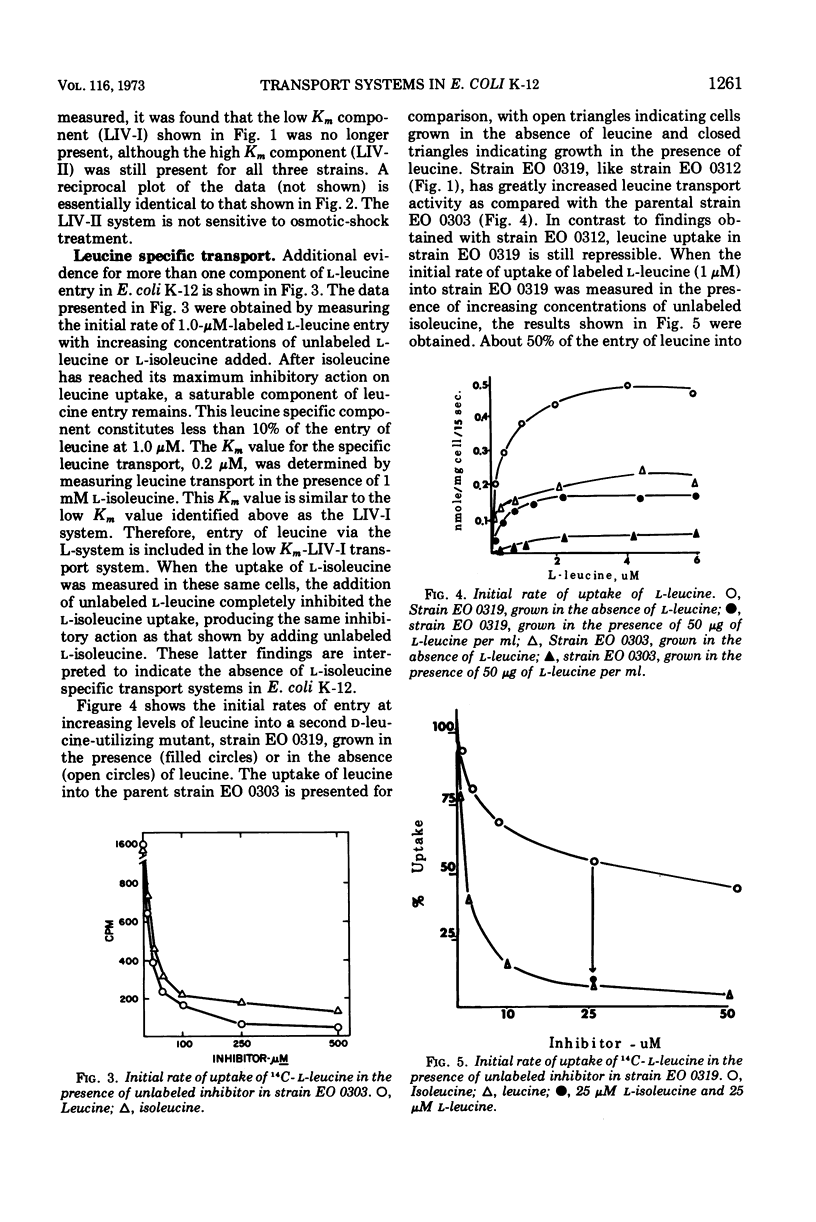
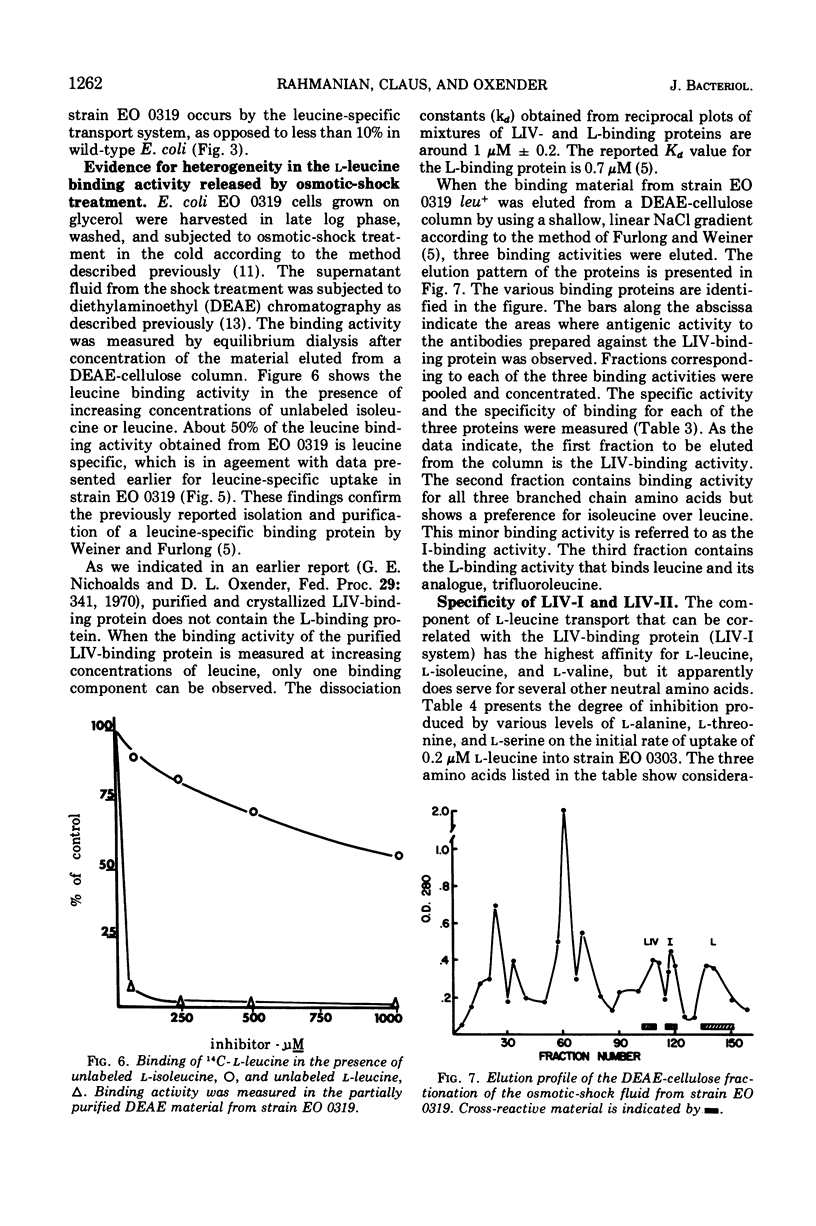
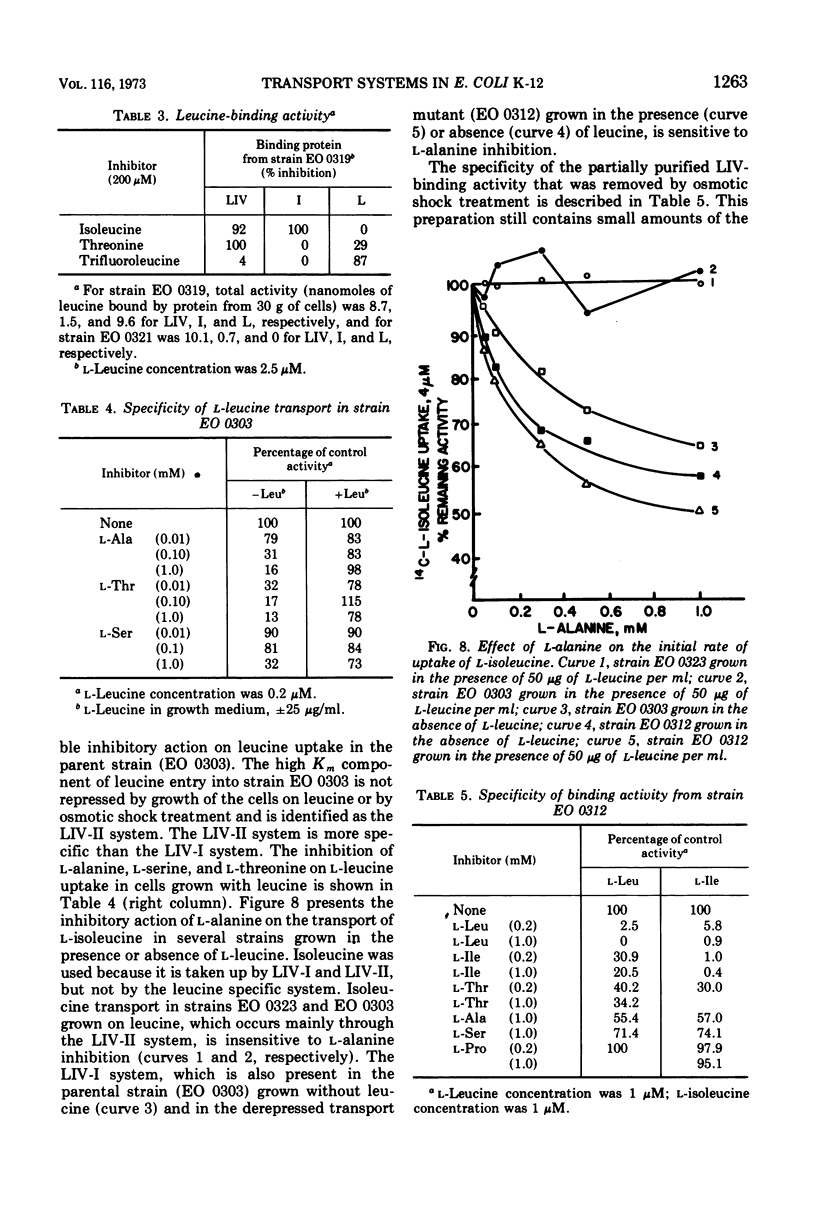
The major component of leucine uptake in Escherichia coli K-12 is a common system for l-leucine, l-isoleucine, and l-valine (LIV-I) with a Michaelis constant (Km) value of 0.2 μM (LIV-I system). The LIV-binding protein appears to be associated with this system. It now appears that the LIV-I transport system and LIV-binding protein also serve for the entry of l-alanine, l-threonine, and possibly l-serine. A minor component of l-leucine entry occurs by a leucine-specific system (L-system) for which a specific leucine-binding protein has been isolated. A mutant has been obtained that shows increased levels of the LIV-I transport activity and increased levels of both of the binding proteins. Another mutant has been isolated that shows only a major increase in the levels of the leucine-specific transport system and the leucine-specific binding protein. A third binding protein that binds all three branched-chain amino acids but binds isoleucine preferentially has been identified. The relationship of the binding proteins to each other and to transport activity is discussed. A second general transport system (LIV-II system) with a Km value of 2 μM and a relatively low Vmax can be observed in E. coli. The LIV-II system is not sensitive to osmotic shock treatment nor to growth of cells in the presence of leucine. This high Km system, which is specific for the branched-chain amino acids, can be observed in membrane vesicle preparations.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Lever J. Components of histidine transport: histidine-binding proteins and hisP protein. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1096–1103. doi: 10.1073/pnas.66.4.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRITTEN R. J., McCLURE F. T. The amino acid pool in Escherichia coli. Bacteriol Rev. 1962 Sep;26:292–335. doi: 10.1128/br.26.3.292-335.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN G. N., RICKENBERG H. V. Concentration spécifique réversible des amino acides chez Escherichia coli. Ann Inst Pasteur (Paris) 1956 Nov;91(5):693–720. [PubMed] [Google Scholar]
- Furlong C. E., Cirakoglu C., Willis R. C., Santy P. A. A simple preparative polyacrylamide disc gel electrophoresis apparatus: purification of three branched-chain amino acid binding proteins from Escherichia coli. Anal Biochem. 1973 Jan;51(1):297–311. doi: 10.1016/0003-2697(73)90478-8. [DOI] [PubMed] [Google Scholar]
- Furlong C. E., Weiner J. H. Purification of a leucine-specific binding protein from Escherichia coli. Biochem Biophys Res Commun. 1970 Mar 27;38(6):1076–1083. doi: 10.1016/0006-291x(70)90349-9. [DOI] [PubMed] [Google Scholar]
- Krajewska-Grynkiewicz K., Walczak W., Klopotowski T. Mutants of Salmonella typhimurium able to utilize D-histidine as a source of L-histidine. J Bacteriol. 1971 Jan;105(1):28–37. doi: 10.1128/jb.105.1.28-37.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn J., Somerville R. L. Mutant strains of Escherichia coli K12 that use D-amino acids. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2484–2487. doi: 10.1073/pnas.68.10.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi F. J., Kaback H. R. Mechanisms of active transport in isolated bacterial membrane vesicles. 8. The transport of amino acids by membranes prepared from Escherichia coli. J Biol Chem. 1972 Dec 25;247(24):7844–7857. [PubMed] [Google Scholar]
- Neal J. L. Analysis of Michaelis kinetics for two independent, saturable membrane transport functions. J Theor Biol. 1972 Apr;35(1):113–118. doi: 10.1016/0022-5193(72)90196-8. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Penrose W. R., Nichoalds G. E., Piperno J. R., Oxender D. L. Purification and properties of a leucine-binding protein from Escherichia coli. J Biol Chem. 1968 Nov 25;243(22):5921–5928. [PubMed] [Google Scholar]
- Piperno J. R., Oxender D. L. Amino acid transport systems in Escherichia coli K-12. J Biol Chem. 1968 Nov 25;243(22):5914–5920. [PubMed] [Google Scholar]
- Rahmanian M., Oxender D. L. Derepressed leucine transport activity in Escherichia coli. J Supramol Struct. 1972;1(1):55–59. doi: 10.1002/jss.400010108. [DOI] [PubMed] [Google Scholar]
- Robbins J. C., Oxender D. L. Transport systems for alanine, serine, and glycine in Escherichia coli K-12. J Bacteriol. 1973 Oct;116(1):12–18. doi: 10.1128/jb.116.1.12-18.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J. R. UGA nonsense mutations in Salmonella typhimurium. J Bacteriol. 1970 May;102(2):467–475. doi: 10.1128/jb.102.2.467-475.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter C. G., Christensen H. N. Contrasts in neutral amino acid transport by rabbit erythrocytes and reticulocytes. J Biol Chem. 1965 Sep;240(9):3594–3600. [PubMed] [Google Scholar]



