Abstract
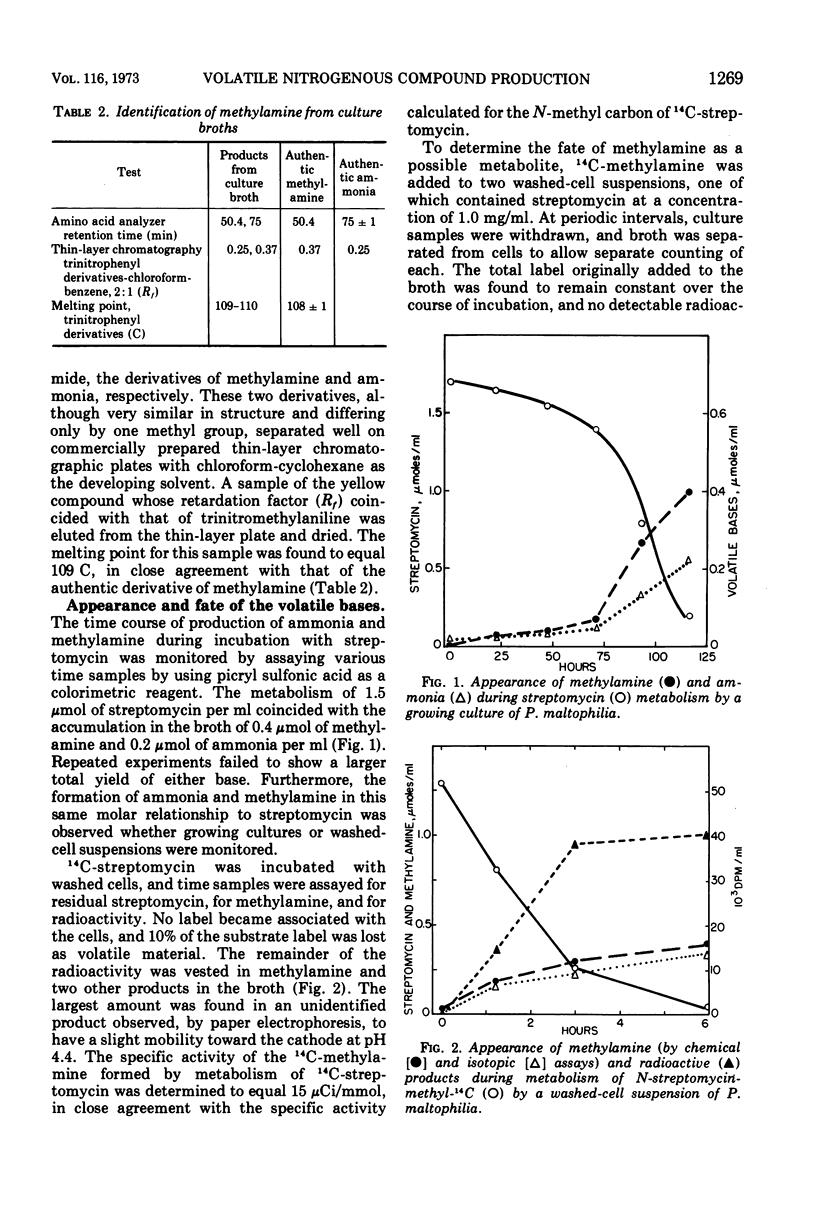
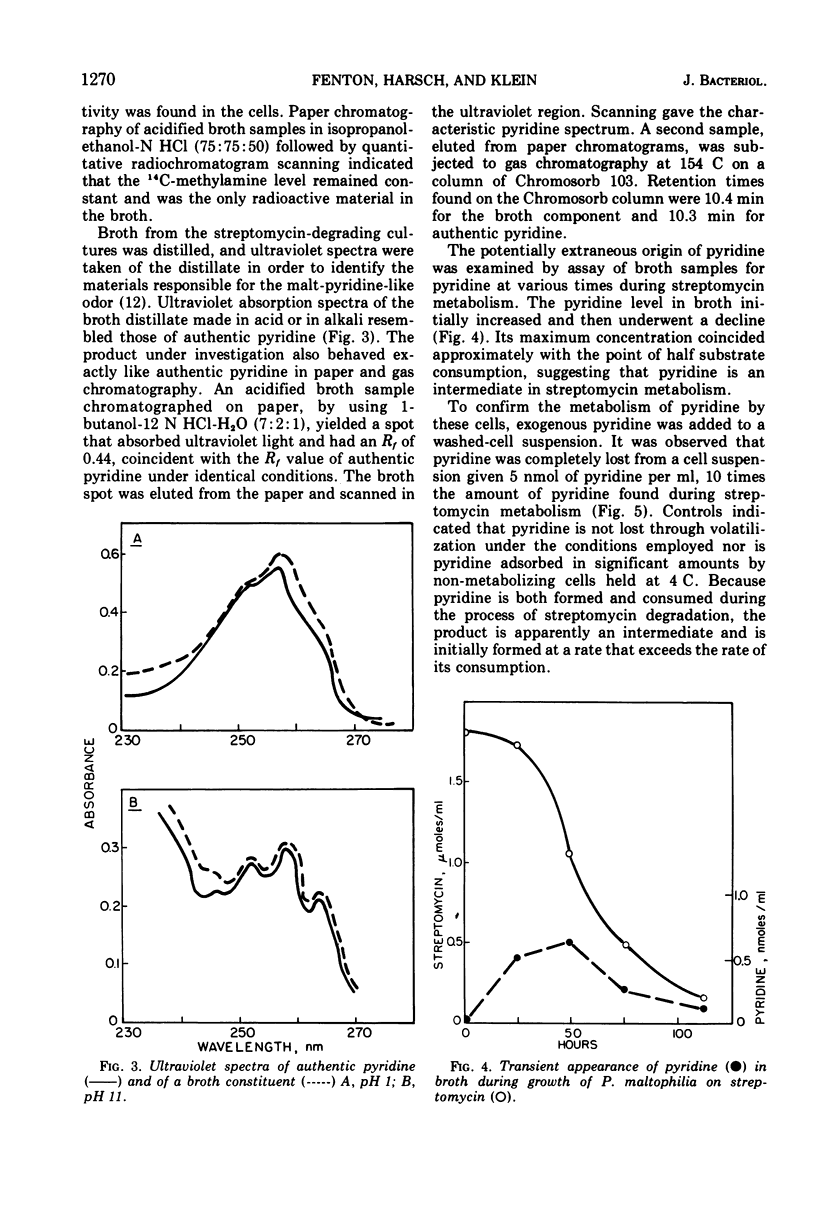
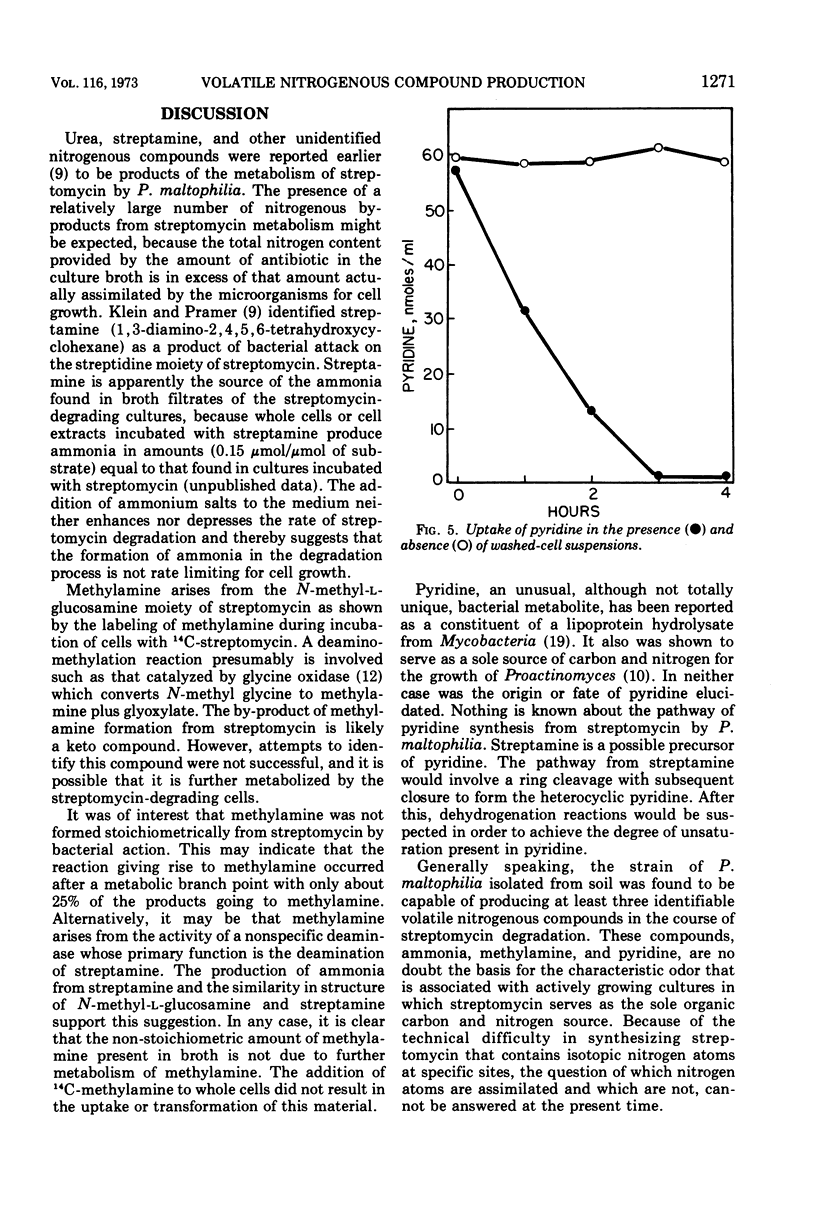
Ammonia, methylamine, and pyridine were detected in broth filtrates of a streptomycin-degrading strain of Pseudomonas maltophilia during growth on streptomycin as a sole carbon and nitrogen source. Ammonia and methylamine, quantitatively measured by conversion to chromophores with picryl sulfonic acid, were found to accumulate in broth, whereas pyridine concentration increased in the early stages of streptomycin degradation and then decreased as the degradation of the antibiotic neared completion. Exogenous pyridine was metabolized by washed-cell suspensions. Use of N-streptomycin-methyl-14C showed that the methylamine arose from the N-l-glucosamine-methyl moiety of streptomycin. Methylamine was an end product and was not further metabolized by cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruton J., Horner W. H., Russ G. A. Biosynthesis of streptomycin. IV. Further studies on the biosynthesis of streptidine and N-methyl-L-glucosamine. J Biol Chem. 1967 Mar 10;242(5):813–818. [PubMed] [Google Scholar]
- Candy D. J., Blumsom N. L., Baddiley J. The biosynthesis of streptomycin. Incorporation of 14C-labelled compounds into streptose and N-methyl-L-glucosamine. Biochem J. 1964 Apr;91(1):31–35. doi: 10.1042/bj0910031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilardi G. L. Characterization of Pseudomonas species isolated from clinical specimens. Appl Microbiol. 1971 Mar;21(3):414–419. doi: 10.1128/am.21.3.414-419.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALLIDAY W. J. A new colour reaction of streptomycin. Nature. 1952 Feb 23;169(4295):335–336. doi: 10.1038/169335b0. [DOI] [PubMed] [Google Scholar]
- HUNTER G. D., HERBERT M., HOCKENHULL D. J. Actinomycete metabolism: origin of the guanidine groups in streptomycin. Biochem J. 1954 Oct;58(2):249–254. doi: 10.1042/bj0580249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEIN D., PRAMER D. Bacterial dissimilation of streptomycin. J Bacteriol. 1961 Oct;82:505–510. doi: 10.1128/jb.82.4.505-510.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEIN D., PRAMER D. Some products of the bacterial dissimilation of streptomycin. J Bacteriol. 1962 Feb;83:309–313. doi: 10.1128/jb.83.2.309-313.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto S., Suzuki Y. Chloramphenicol-, dihydrostreptomycin-, and kanamycin-inactivating enzymes from multiple drug-resistant Escherichia coli carrying episome 'R'. Nature. 1965 Dec 25;208(5017):1301–1303. doi: 10.1038/2081301a0. [DOI] [PubMed] [Google Scholar]
- Pramer D., Starkey R. L. Decomposition of Streptomycin. Science. 1951 Feb 2;113(2927):127–127. doi: 10.1126/science.113.2927.127. [DOI] [PubMed] [Google Scholar]
- ROCHE J., THOAI N. V., HATT J. L., TRAN-THI A. N. Sur les déguanidases de Streptomyces griseus (Waksman). C R Seances Soc Biol Fil. 1956 May 28;150(1):112–114. [PubMed] [Google Scholar]
- SAKAKIBARA E. Studies on the streptomycin-resistant variants in bacteria. 7. On streptomycin-decomposing enzyme (streptomycinase). Acta Sch Med Univ Kioto. 1951;29(2):116–124. [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Yamada T., Tipper D., Davies J. Enzymatic inactivation of streptomycin by R factor-resistant Escherichia coli. Nature. 1968 Jul 20;219(5151):288–291. doi: 10.1038/219288a0. [DOI] [PubMed] [Google Scholar]



