Abstract
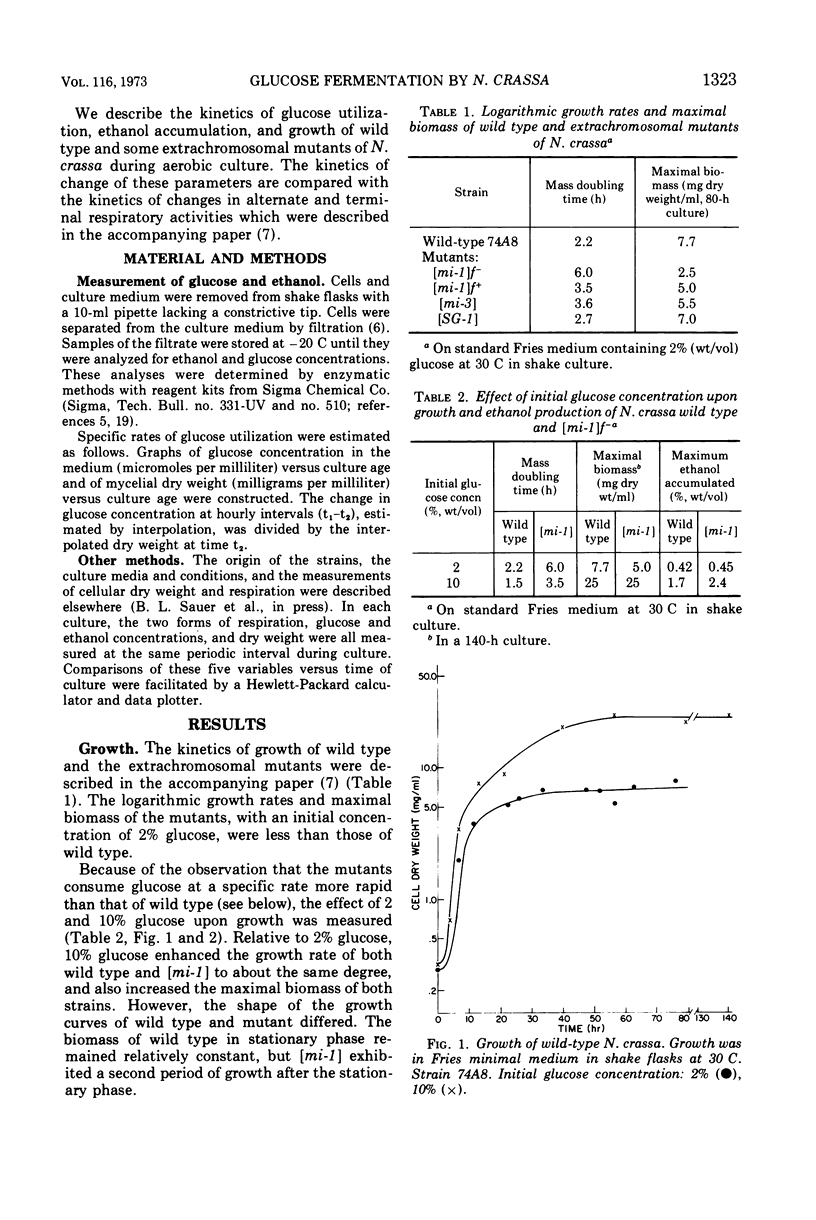
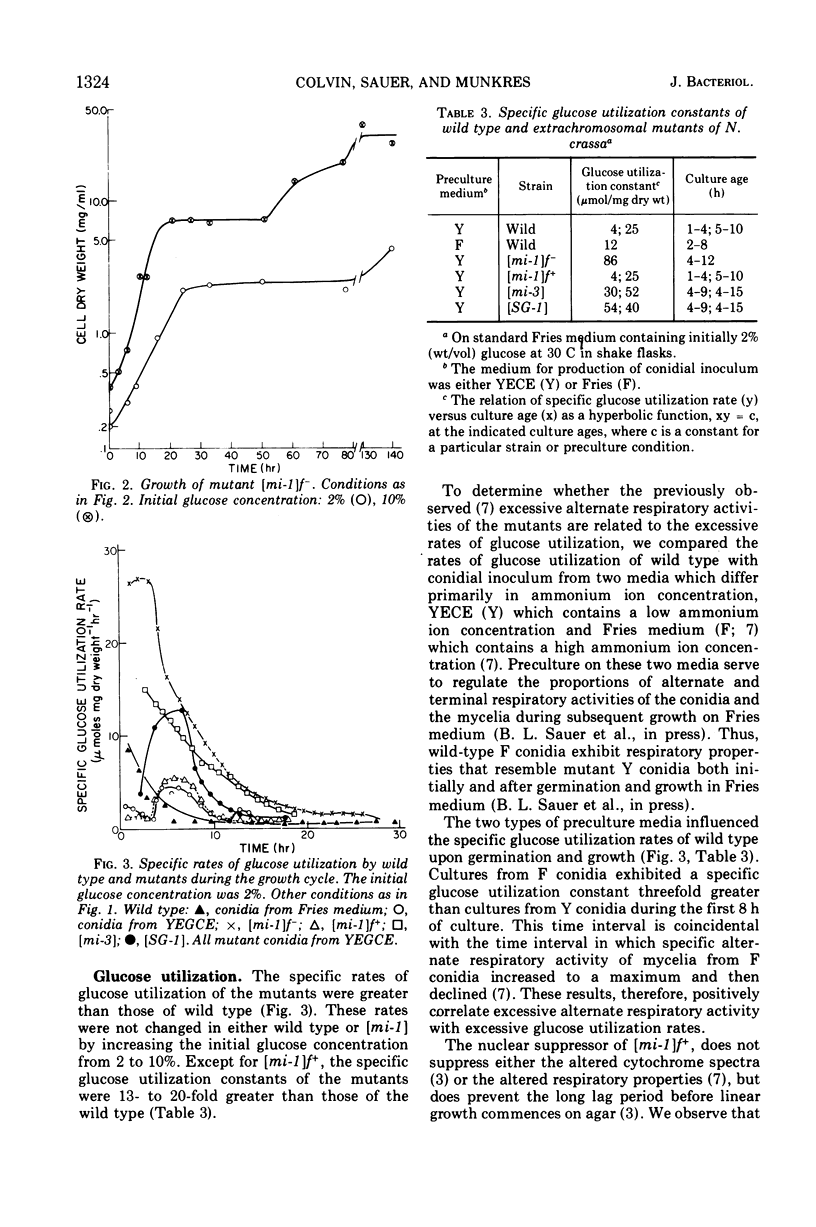
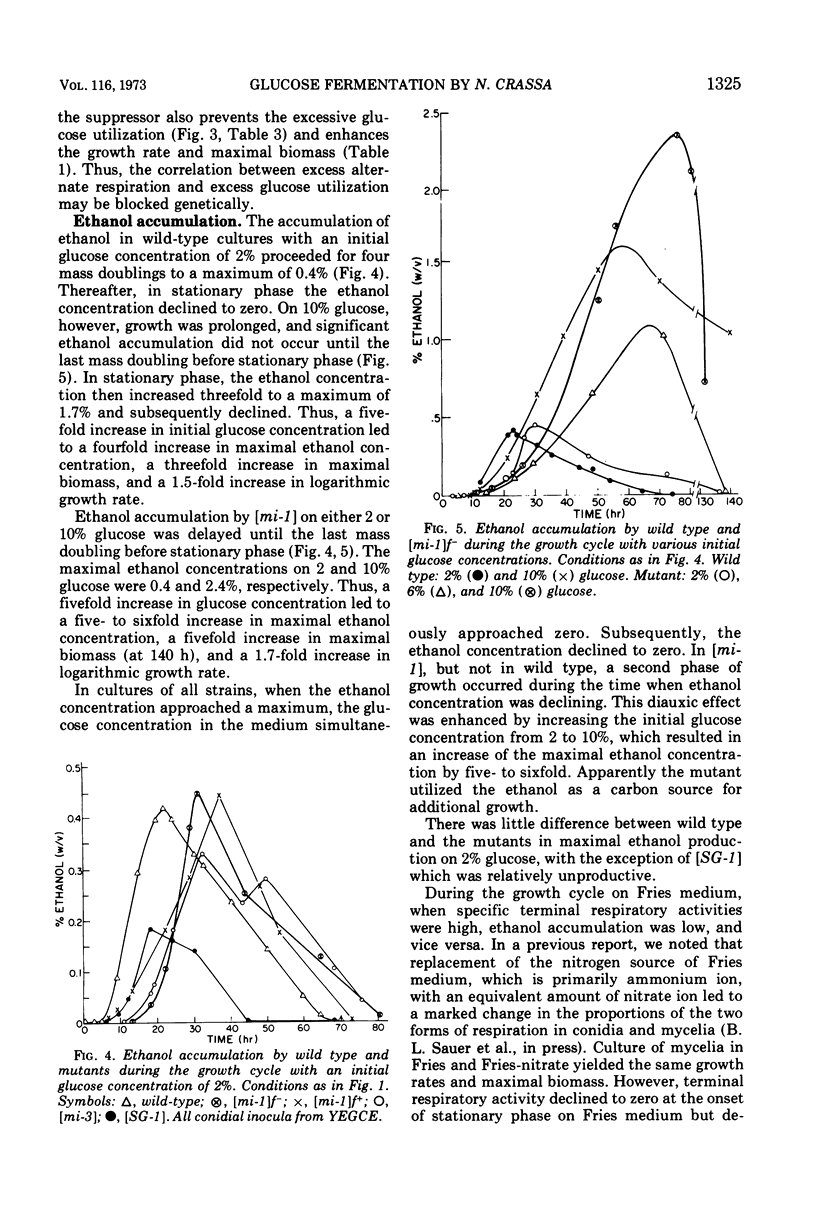
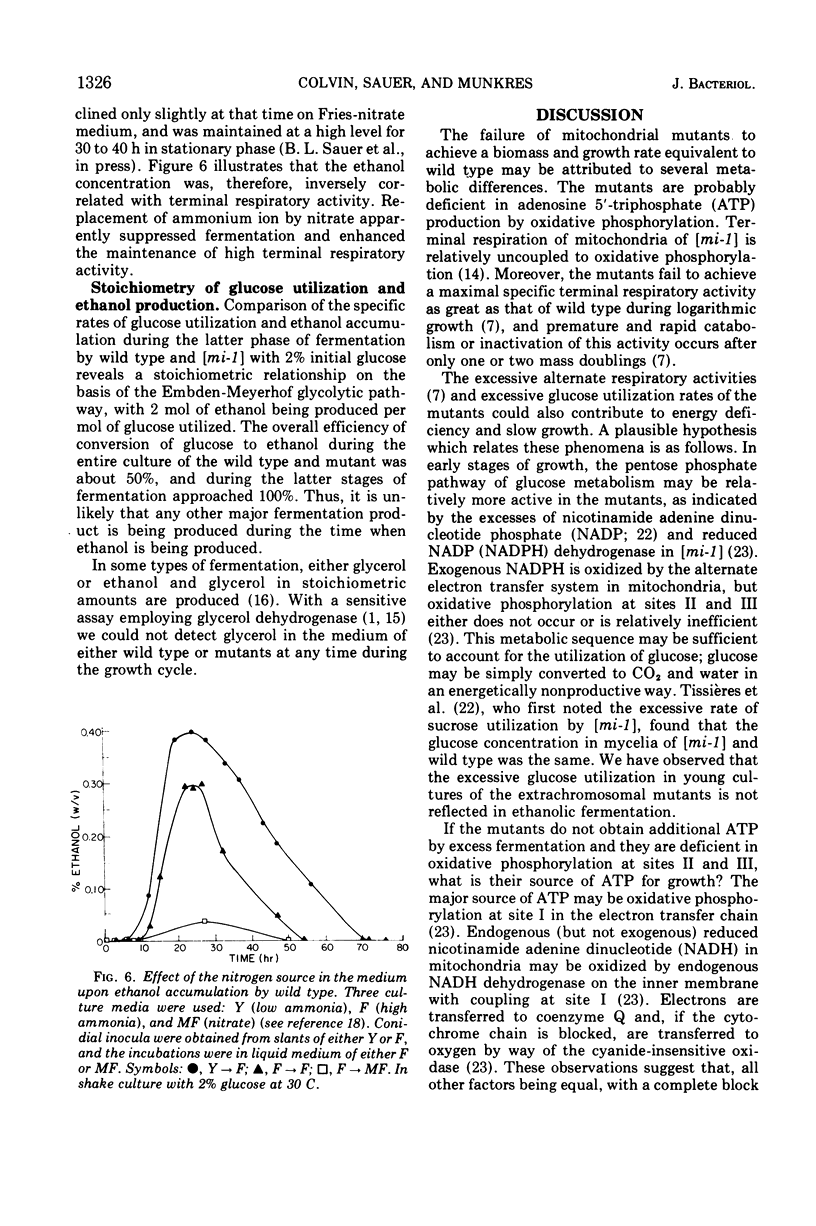
Logarithmic growth rates, maximal biomass, specific glucose utilization rates, and ethanol accumulation were measured in aerobic cultures of wild type and extrachromosomal mutants of Neurospora crassa. Maximal biomass and ethanol accumulation of wild type and [mi-1] were proportional to the initial glucose concentration in the range of 2 to 10%. The specific rates of glucose utilization by the mutants were 13- to 20-fold greater than those of wild type in young cultures. The specific rates of glucose utilization by wild type, however, were increased threefold by increasing the ammonium ion concentration in the preculture medium. The suppressor gene f+ suppressed the excessive glucose utilization and enhanced the growth rate and maximal biomass of [mi-1]. When the mutants were utilizing glucose at excessive rates, ethanol did not appear in the culture medium. Ethanol accumulation was maximum at stationary phase or thereafter, but there was little difference between the maxima of the mutants and wild type. The molar efficiency of the conversion of glucose to ethanol during the entire culture period of wild type and mutants was about 50% and, in the latter stages of fermentation, approached 100%. Replacement of ammonium ion by nitrate in the culture medium suppressed ethanol accumulation by wild type. The relationship of these results to previous observations on respiratory adaptation are discussed. We suggest that the Pasteur effect, the inhibition of fermentation by respiration, may be operative in N. crassa. Factors such as nitrogen source and concentration and oxygen tension, which may serve primarily to regulate the amount and form of respiration would, therefore, indirectly regulate fermentation. The mutants, although transiently deficient in terminal respiratory activity, do not accumulate more ethanol than wild type and, therefore, apparently do not ferment in excess to obtain additional adenosine 5′-triphosphate. We suggest that the excess activity of the alternate form of respiration of the mutants may be related to their excessive rate of glucose utilization by way of the pentose phosphate pathway and the oxidation of excess reduced nicotinamide adenine dinucleotide.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BONNICHSEN R. K., THEORELL H. An enzymatic method for the microdetermination of ethanol. Scand J Clin Lab Invest. 1951;3(1):58–62. doi: 10.3109/00365515109060572. [DOI] [PubMed] [Google Scholar]
- Benveniste K., Munkres K. D. Cytoplasmic and mitochondrial malate dehydrogenases of Neurospora. Regulatory and enzymic properties. Biochim Biophys Acta. 1970 Nov 11;220(2):161–177. doi: 10.1016/0005-2744(70)90003-3. [DOI] [PubMed] [Google Scholar]
- Bertrand H., Pittenger T. H. Isolation and classification of extranuclear mutants of Neurospora crassa. Genetics. 1972 Aug;71(4):521–533. doi: 10.1093/genetics/71.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin H. J., Sauer B. L., Munkres K. D. Respiration of wild type and extrachromosomal mutants of Neurospora crassa. J Bacteriol. 1973 Dec;116(3):1314–1321. doi: 10.1128/jb.116.3.1314-1321.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell R. B., Fincham J. R. Acetate-nonutilizing mutants of Neurospora rassa. II. Biochemical deficiencies and the roles of certain enzymes. J Bacteriol. 1968 Mar;95(3):1063–1068. doi: 10.1128/jb.95.3.1063-1068.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell N., Zuiches C. A., Munkres K. D. Mitochondrial biogenesis in Neurospora crassa. I. An ultrastructural and biochemical investigation of the effects of anaerobiosis and chloramphenicol inhibition. J Cell Biol. 1971 Sep;50(3):721–736. doi: 10.1083/jcb.50.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A. The Pasteur effect and the relations between respiration and fermentation. Essays Biochem. 1972;8:1–34. [PubMed] [Google Scholar]
- LIN E. C., MAGASANIK B. The activation of glycerol dehydrogenase from Aerobacter aerogenes by monovalent cations. J Biol Chem. 1960 Jun;235:1820–1823. [PubMed] [Google Scholar]
- Lambowitz A. M., Slayman C. W. Cyanide-resistant respiration in Neurospora crassa. J Bacteriol. 1971 Dec;108(3):1087–1096. doi: 10.1128/jb.108.3.1087-1096.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambowitz A. M., Slayman C. W., Slayman C. L., Bonner W. D., Jr The electron transport components of wild type and poky strains of Neurospora crassa. J Biol Chem. 1972 Mar 10;247(5):1536–1545. [PubMed] [Google Scholar]
- Lambowitz A. M., Smith E. W., Slayman C. W. Oxidative phosphorylation in Neurospora mitochondria. Studies on wild type, poky, and chloramphenicol-induced wild type. J Biol Chem. 1972 Aug 10;247(15):4859–4865. [PubMed] [Google Scholar]
- RAABO E., TERKILDSEN T. C. On the enzymatic determination of blood glucose. Scand J Clin Lab Invest. 1960;12(4):402–407. doi: 10.3109/00365516009065404. [DOI] [PubMed] [Google Scholar]
- STRAUSS B. S., PIEROG S. Gene interactions; the mode of action of the suppressor of acetate-requiring mutants of Neurospora crassa. J Gen Microbiol. 1954 Apr;10(2):221–235. doi: 10.1099/00221287-10-2-221. [DOI] [PubMed] [Google Scholar]
- Sussman A. S., Distler J. R., Krakow J. S. Metabolic Aspects of Neurospora Activation and Germination. Plant Physiol. 1956 Mar;31(2):126–135. doi: 10.1104/pp.31.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TISSIERES A., MITCHELL H. K., HASKINS F. A. Studies on the respiratory system of the poky strain of Neurospora. J Biol Chem. 1953 Nov;205(1):423–433. [PubMed] [Google Scholar]
- Weiss B., Turian G. A study of conidiation in Neurospora crassa. J Gen Microbiol. 1966 Sep;44(3):407–418. doi: 10.1099/00221287-44-3-407. [DOI] [PubMed] [Google Scholar]
- Zink M. W. Regulation of NAD-specific alcohol dehydrogenases in Neurospora crassa. Can J Microbiol. 1969 Mar;15(3):265–271. doi: 10.1139/m69-049. [DOI] [PubMed] [Google Scholar]
- von Jagow G., Weiss H., Klingenberg M. Comparison of the respiratory chain of Neurospora crassa wild type and the mi-mutants mi-1 and mi-3. Eur J Biochem. 1973 Feb 15;33(1):140–157. doi: 10.1111/j.1432-1033.1973.tb02665.x. [DOI] [PubMed] [Google Scholar]



