Abstract
Background
At the time of transition from hospital to home, many patients are challenged by multi‐drug regimens. The authors' standard patient education tool is a personalised Medication Discharge Worksheet (MDW) that includes a list of medications and administration times. Nonetheless, patient understanding, satisfaction, and safety remain suboptimal. Therefore, the authors designed a new tool: Durable Display at Discharge (3D). Unlike MDW, 3D features (1) space in which a tablet or pill is to be affixed and displayed, (2) trade name (if apt), (3) unit strength, (4) number (and/or fraction) of units to be taken, (5) purpose (indication), (6) comment/caution, (7) larger font, (8) card stock durability and (9) a reconciliation feature.
Methods
The authors conducted an exploratory, randomised trial (n = 138) to determine whether 3D, relative to MDW, improves patient satisfaction, improves patient understanding and reduces self‐reported medication errors. Trained survey research personnel, blinded to hypotheses, interviewed patients by telephone 7–14 days after discharge.
Results
Both tools were similarly associated with high satisfaction and few self‐reported errors. However, 3D subjects demonstrated greater understanding of their medications.
Conclusions
Although both tools are associated with similarly high levels of patient satisfaction and low rates of self‐reported medication error, 3D appears to promote patient understanding of the medications, and warrants further study.
Improving patient safety in the use of medications, especially after hospital discharge, is a national concern.1 Medication errors can result from numerous systems failures or patient factors. We chose to focus on patient knowledge, which promotes competence and self‐efficacy.2 At hospital discharge, patients with numerous medications present unique challenges for effective medication education.3,4,5
Despite improvements in personalised education materials, shortfalls in medication discharge education (MDE) are well documented.2,3,4,5,6,7,8 Moreover, in certain subgroups such as elderly heart failure patients, with an average of eight medications,9,10 the adequacy of MDE mitigates hospital readmission.11,12
Individualised reminder cards,13 charts14 or schedules15 are reportedly effective. More comprehensively, a Medicine Record Form is recommended by the US Department of Health and Human Services, Agency for Healthcare Research and Quality, and the National Council on Patient Information and Education.16 Elements include: unit strength, indication, appearance (colour), and cautions. Such tools improve patient knowledge13,14,17 and reduce medication errors.15,17 Interestingly, Whyte found that patients frequently refer to their medication by physical description (colour, size, or shape), rather than by name even when the medication name is known.17
In 1990–2001 the author (DMM) in a urban solo practice in Pittsburgh, Pennsylvania (USA) developed a durable card‐stock MDE tool on which he wrote medication name, Reason (Indication), Caution, and provided space for (the patients) affixing a tablet or capsule. Thus the tool became a three dimensional, visual display of medications. Patients registered favorable anecdotal comments and often employed the low‐tech tool for months.
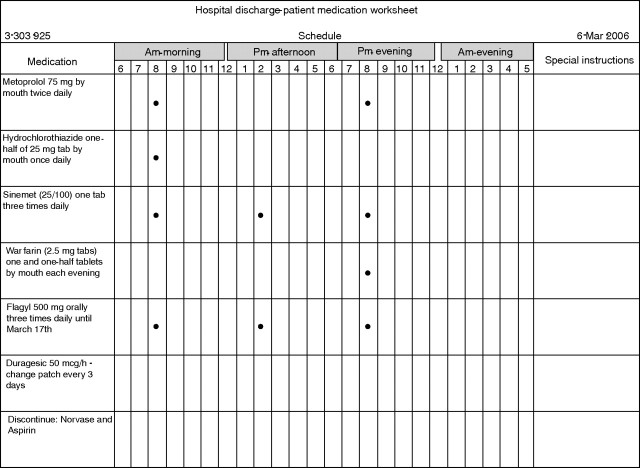
At Mayo Clinic Rochester, since 1997, a different tool has been employed—Medication Discharge Worksheet (MDW) (fig 1). MDW is paper medication list and schedule generated electronically by the nurse from the Hospital Discharge Summary (usually completed immediately prior to discharge) and given to the patient by the nurse as part of standard MDE.
Figure 1 Fictitious patient example of the standard Mayo Medication Discharge Worksheet (MDW), 1997–present.
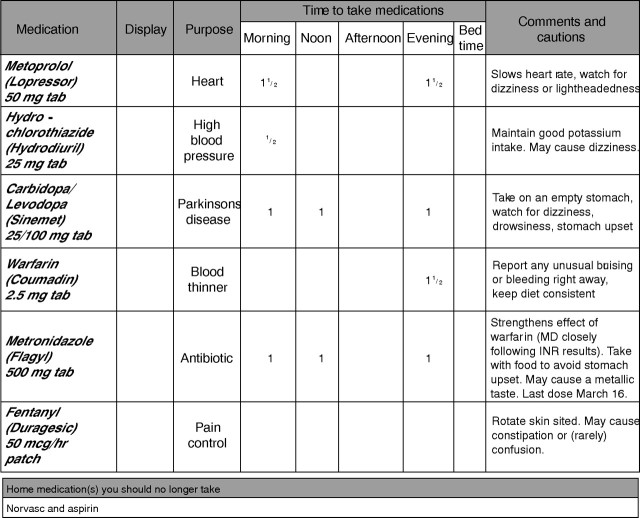
Because of our desire to incorporate both recent expert opinion1,16 and the durable display feature, the authors (all at Mayo Clinic College of Medicine since 2001) designed 3D (fig 2). We designed custom software (enabling semi‐automatic 3D form generation) including the following features: (1) 900 medication database with corresponding “Comments and Cautions” and “Purpose” (indication) written at sixth grade reading level; (2) enlarged font for the patient (12 point for medication name, 11 for comments—rather than 10 point font used in MDW); (4) reconciliation prompt (“Home Medications You Should No Longer Take…”); and (5) space for durable display. The result is a patient‐facing MDE instrument that we believe is concise, intuitive, and incorporating best evidence and recent national guidelines.16 But, will it be worth additional resources to develop and deploy (eventually) in day‐to‐day production? We lacked head‐to‐head comparison data to help begin to determine the relative value of 3D. Our question was: in terms of patient satisfaction, understanding and safety, is 3D better than MDW?
Figure 2 Fictitious patient example of the Durable Display at Discharge (3D) Model 2006.
Methods
We designed this exploratory, randomised clinical trial to test 3D versus MDW in patient satisfaction, knowledge, and self‐reported medication errors. The study protocol and all forms were reviewed and approved by the Mayo Clinic Institutional Review Board.
Setting
Study was conducted at Saint Marys Hospital, a 917‐bed hospital, part of an academic medical center (Mayo Clinic College of Medicine in Rochester, Minnesota), in the Midwest US.
Function: MDW
Study subjects were patients on one of four participating medical units during weekdays from 4 May 2004, through 28 January 2005. The standard MDE tool employed by the primary nurse is MDW printed on paper after the Hospital Summary is finalised.
Intervention: 3D
A secure custom web‐based information system was designed for the study. The system provided two major functionalities, one was to maintain an electronic medication database and the other was to process the study data. The medication database consisted of 40 000 FDA‐approved medication listings; from which the author (JGO'M) developed a customised 900 medication database of Indications and Comments/Cautions for the 3D tool.
Default entries for medication “purpose” and “comments and cautions” were developed, reviewed by a patient education specialist to meet a sixth grade reading level, and agreed upon by a three‐person panel of study investigators (PharmD, Clinical Nurse Specialist, Physician). For medications not listed in the database, a free‐text option allowed for addition of new 3D medication information into the display.
Measures: study design, implementation, and collection of data
Consenting patients over age 20, with more than three discharge medications, and returning to self‐care at home (or to care of a relative) were eligible for inclusion in this exploratory trial. Patients deemed ineligible were: discharged to nursing home, hospital, or assisted living facility; unable to speak or read English; unable to hear over the telephone to participate in the follow‐up telephone interview 7–14 days post‐hospital discharge; or pregnant.
Subject information was obtained on the highest level of education, whether the subject is or was employed as a healthcare professional (yes or no), present or past diagnosis of depression (yes or no) or of dementia, and assistance with taking medications (yes or no).
A discharge medication list from the electronic Hospital Summary was used as the source to populate the MDW or 3D medication sheets. MDW was generated by direct‐care unit nurses as per usual procedure. 3D medication sheets were generated by a study recruiter. Each 3D display was printed on a colour printer using cardstock paper. Unlike MDW, 3D is not electronically linked to the Hospital Summary. Therefore an additional quality control step was required: every 3D was reviewed and approved by either the principal investigator or the pharmacist co‐investigator before being given to the patient's nurse for review with the patient. Subjects randomised to 3D upon returning home and after filling any new prescriptions were encouraged to affix (with clear adhesive tape) a tablet or capsule of each medications onto the 3D adjacent to the medication name, and under the column labeled Display.
Before hospital dismissal, the primary nurse conducted her/his usual patient education session including usage of either MDW or 3D (per randomisation), and recorded her/his personal (total) time required for MDE.
Subjects received a follow‐up telephone call 7–14 days after hospital discharge by a trained telephone survey research assistant at the Mayo Clinic Survey Research Center. This research assistant was blinded to both the study hypotheses and the subject randomisation. Using a standardised script and questionnaire, subjects were asked questions regarding: (1) level of satisfaction with the medication worksheet they received; (2) assessment of knowledge of their medications (indication, appropriate dosing frequency, and special comments or cautions); and (3) self‐reported safety (Did the medication worksheet assist in preventing medication administration errors?).
Study design: 3D medication affixing issues
We did not affix the medications in hospital for several reasons: (1) hospital policy precludes dispensing going‐home medications, (2) we could not be certain of having all prior medications on hand at discharge, (3) new prescriptions had yet to be obtained by the patient, and (4) generic medications were best affixed by patient because supplier differences could result in different medication appearance.
Although patient compliance with the affixing medications to 3D is uncertain, we chose not to include this issue in the present study. In ignoring the issue we have analysed data (3D v MDW) on an intention‐to‐influence basis with knowledge that any non‐compliance might diminish the apparent 3D benefit (measuring effectiveness rather than efficacy) of the new tool.
Accrual of study subjects
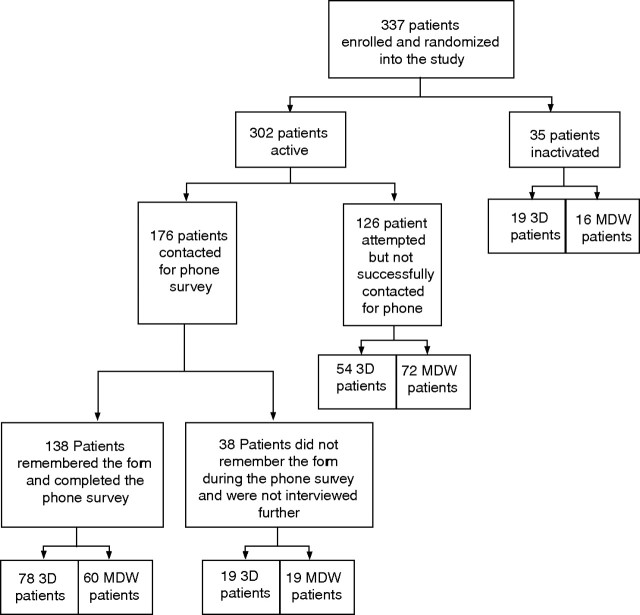
A total of 1151 patients were evaluated; 671 met one or more of the exclusion criteria. Of 480 eligible patients, 120 declined to participate (before they were aware of the appearance of either the MDW or the 3D tool). Twenty three were enrolled but discharged from hospital before receiving necessary study material. Therefore, 337 patients were enrolled as subjects. The accrual of study subjects is illustrated in fig 3. All 337 eligible study subjects received and signed an institutional review board approved consent form, and were randomly assigned to one of two study groups using a computer‐generated random number algorithm. Thirty five patients were inactivated when it was determined that one of the exclusion criteria were, in fact, met upon discharge. Of the 302 patients remaining, 126 did not complete the telephone survey for the following reasons:
Figure 3 Accrual of study subjects.
could not be reached during the 7–14 day post‐discharge contact period (93)
exclusion criteria met post‐discharge (12)
could not hear during the telephone contact (4)
telephone number incorrect (5)
did not receive the MDW (2)
too ill (5)
refused (4)
could not speak English (1).
Of the 176 subjects remaining, 38 patients (evenly split between the MDW and 3D groups) were excluded since they could not remember the discharge tool. This left 138 patients in the study for final analysis—78 in the 3D group and 60 in the MDW group.
Analytical methods
Patient satisfaction was measured on a five‐point Likert scale: from 1 (low) to 5 (high). Telephone surveyors queried subjects using the following script: “How satisfied were you with the form? This is the form you received from the nurse when she/he was talking to you about your taking medications. This form had a list of your medications and columns and rows. Please give the form a grade [A (best), B, C, D, F (worst)].” A t test was done between the means of the 3D and MDW groups.18
To measure patient understanding of medications, telephone surveyors asked:
1. “How many times per day do you take <first medication>?”
2. “Regarding your <second medication> what special instructions, cautions or comments were given to you?”
3. “What is the reason for which you take the <third medication>?”
Each question could be scored as correct or incorrect. Total scores of patients could range from 0 (no correct responses) to 3 (all correct responses). Answers that the surveyors could not characterise were transcribed verbatim and scored by a consensus of the three senior investigators (DMM, JOM and ARW) who remained blinded to each subject's randomisation group. A Mann–Whitney U test was done between the groups.19
Patients were asked to identify the number of medication errors made since discharge: “Does this form help you avoid making mistakes with your medications?” and “Since discharge, how many mistakes have you made taking your medications? (wrong time, wrong pill, and missed pill)”. Values on the answer to the second question ranged from 0–4. A t test was done between the means of the 3D and MDW groups.
The t tests were done using SAS version 9.1 for Windows.20 The Mann–Whitney test was done using StatXact version six.21 A conventional level of significance of p<0.05 was used in hypothesis testing.
Results
The ages of the 138 subjects remaining for study analysis ranged from 24–100 years with a mean age of 68 years. The number of medications prescribed at discharge ranged from 4–31; the mean number was 9.4. There were no statistically significant differences in demographics (gender, age, education, number of medications) between the experimental and control groups (table 1).
Table 1 Demographic comparisons between 3D and MDW subjects.
| Variable | n | 3D (n = 78) | MDW (n = 60) | t value | p value |
|---|---|---|---|---|---|
| Age (in years) | 138 | 68.1 (5.65 | 67.6 (13.06) | 0.21 | 0.83 |
| Gender (% male) | 138 | 0.51 (0.50 | 0.38 (0.49) | 1.52 | 0.13 |
| Medications (n) | 138 | 10.0 (4.42) | 8.7 (3.93) | 1.85 | 0.07 |
| Level of education* | 137 | 2.8 (1.53) | 2.8 (1.72) | 0.14 | 0.89 |
All values are expressed as mean (SD).
*0, 8th grade or less; 1, some high school; 2, high school completed; 3, some college or vocational; 4, completed associates; 5, completed college; 6, postgraduate; one level of education was unreported.
The number of patients successfully contacted during the 7–14 day post‐discharge telephone survey was 176 (52%) of the 337 enrolled. As suggested in figure 3, an appreciable loss of potential subjects occurred. Demographic comparisons also were done between subjects in each of the 3D and MDW groups at each level of patient loss, and no statistically significant differences were found.
Also obtained from queries of each of the primary nurses was the number of total minutes involved in doing MDE. The time estimates of the nurses (data not shown) indicated no statistically significant differences when either the 3D or the MDW was used.
This study did not find a statistically significant difference in patient satisfaction (table 2), or any difference in self‐reported medication errors with use of the 3D. The 3D, however, was associated with greater understanding of prescribed medications (Mann–Whitney test: U = 1792, p<0.0282; γ = 0.32).
Table 2 Three study hypotheses related to satisfaction, knowledge of medications, and self‐reported medication errors between the 3D (treatment group) and MDW (control group).
| Hypothesis | Measure | Mean (SD) | Test score | p Value | n |
|---|---|---|---|---|---|
| H1 | Satisfaction 3D | 4.24 (0.6986) | t = 0.64 | 0.5204 | 74 |
| Satisfaction MDW | 4.26 (0.8768) | 57 | |||
| H2 | Knowledge 3D | 1.96 (0.7561) | U = 1792 | 0.0282 | 76 |
| Knowledge MDW | 1.66 (0.6851) | 59 | |||
| H3 | Med errors 3D | 0.78 (0.4187) | t = 0.16 | 0.8760 | 72 |
| Med errors MDW | 0.79 (0.4113) | 57 |
Discussion
Summary
This study suggests that improved design of a discharge medication education tool can enhance patient knowledge. This tool incorporated expert guidelines espoused by the US Institute of Medicine,1 Agency for Healthcare Research and Quality16 and the National Council on Patient Information and Education. Although 3D use was not found to be associated with improved patient satisfaction or self‐reported reductions in medication errors this study may be biased toward non‐significant findings for the following reasons.
First, as to patient satisfaction, the institution receives very high scores on patient satisfaction in regularly completed patient surveys. In this study, measures of satisfaction with either the 3D or MDW exceeded means of 4.00 on a scale of 0–5. Finding statistically significant differences when such levels are attained is very difficult.
Second, patients in both the 3D and MDW groups failed to report substantial numbers of medication errors. Over 80% of the patients in either group indicated that the instrument received at discharge helped them “avoid making mistakes with medications.” The self‐report of medication errors by telephone interview is likely an insensitive method of assessing real medication errors. But, in safety studies, all attempts to measure medication errors have been difficult. Clearly, attention needs to be given as to how such errors and, most importantly, potential or actual harm to patients can be reliably and validly measured.
Context
Little is presently known about the relative merits of types of Medication Discharge Education tools. Many authors attest to the difficulty in patients comprehending complex medical regimes.1,2,3,4,5,6,7,8,9 Progress has been made11,12,13,14,15 but additional trials are still needed. A definitive solution is not is sight, and the number of medications (per patient) continues to increase.9,10
Study limitations
There are several limitations to this study. (1) Of the many possible MDE tools, only two were chosen for study. (2) Subjects tended to be elderly and were only from medical units; therefore, they may not be representative of a wider spectrum of age and care units. (3) Dropout rate was high, in part due to a narrow window of follow‐up. (4) Thirty eight (22%) of 176 patients could not recall the discharge tool. This was surprising. We do not know if this affected or skewed the results. (5) Compliance with instructions for medication cessation (Home Medications You Should No Longer Take …) was not studied. We assumed, perhaps with naiveté, that 3D subjects with such notation would comply. The fact that 19 of 97 (22%) 3D subjects could not remember the form throws this assumption into doubt.
Conclusion
Both tools are associated with high levels of patient satisfaction and self‐reported safety. 3D appears to offer an advantage in patient medication knowledge. This is an important characteristic of competence and self‐efficacy.2 If that is true, a larger trial, using more sensitive instruments of patient satisfaction and safety, and comparing 3D to other tools, may be warranted. Importantly, 3D did not add time to the nurse's patient education routine.
Whether subgroups with low health literacy and/or with more daunting regimens (eight medications or more, for example) would uniquely benefit from 3D is also an open question.
Changing of information systems to incorporate the 3D tool may be costly in terms of hardware, software and staff training time. Whether real safety benefits accrue from incremental medication understanding, and whether such benefits offset the costs, is also an important issue for further investigation.
Acknowledgements
We thank Kristen Vickers‐Douglas, PhD for health literacy analysis of 3D language elements, Kathleen K Raffel, MSW for the for Patient Education review, Roger Resar, MD for medication reconciliation language used in 3D, Mark Liebow, MD for advice regarding telephone surveyor blinding to hypothesis, and Peter Elkin, MD for Protocol review.
Abbreviations
3D - Durable Display at Discharge
MDE - medication discharge education
MDW - Medication Discharge Worksheet
Footnotes
Funding: Mayo Clinic Rochester, Department of Medicine, MIDAS Grant. Mayo Foundation for Education and Research, Small Grants Program
Accepted 14 October 2006
References
- 1.Kohn L T, Corrigan J M, Donaldson M S.To err is human: building a safer health system. Washington DC. 2000196–197. [PubMed]
- 2.Beckman A G K, Parker M G, Thorslund M. Can elderly people take their medications? Patient Educ Couns 200559186–191. [DOI] [PubMed] [Google Scholar]
- 3.Martens K H. An ethnographic study of the process of medication discharge education (MDE). J Adv Nurs 199827341–348. [DOI] [PubMed] [Google Scholar]
- 4.Gurwitz J H, Field T S, Harrold L R.et al Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA 20032891107–1116. [DOI] [PubMed] [Google Scholar]
- 5.Cleary P D. A hospitalization from hell: a patient's perspective on quality. Ann Intern Med 200313833–39. [DOI] [PubMed] [Google Scholar]
- 6.Holloway A. Patient knowledge and information concerning medication on discharge from hospital. J Adv Nurs 1996241169–1174. [DOI] [PubMed] [Google Scholar]
- 7.Moore C, Wisnivesky J, Williams S.et al Medical errors related to discontinuity of care from an inpatient to an outpatient setting. J Gen Intern Med 200318646–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forster A J, Murff H J, Peterson J F.et al The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med 2003138161–167. [DOI] [PubMed] [Google Scholar]
- 9.Masoudi F A, Baillie C A, Wang Y.et al The complexity and cost of drug regimens of older patients hospitalized with heart failure in the United States, 1998–2001. Arch Intern Med 20051652069–2076. [DOI] [PubMed] [Google Scholar]
- 10.Demers C, McMurray J J, Swedberg K.et al Impact of candesartan on nonfatal myocardial infarction and cardiovascular death in patients with heart failure. JAMA 20052941794–1798. [DOI] [PubMed] [Google Scholar]
- 11.Markey B T, Igou J F. Medication discharge planning for the elderly. Patient Educ Couns 19879241–249. [DOI] [PubMed] [Google Scholar]
- 12.Schneider J K, Hornberger S, Booker J.et al A medication discharge planning program: measuring the effect on readmissions. Clin Nurs Res 1993241–53. [DOI] [PubMed] [Google Scholar]
- 13.Grymonpre R, Sabiston C, Johns B. The development of a medication reminder card for elderly persons. Can J Hosp Pharm 19914455–62. [PubMed] [Google Scholar]
- 14.Raynor D K, Booth T G, Blenkinsopp A. Effects of computer generated reminder charts on patients' compliance with drug regimens. BMJ 19933061158–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esposito L. The effects of medication education on adherence to medication regimens in an elderly population. J Adv Nurs 199521935–943. [DOI] [PubMed] [Google Scholar]
- 16.Your Medicine: Play It Safe Patient guide. AHRQ Publication No. 03–0019, February 2003. Agency for Healthcare Research and Quality, Rockville, MD, and the National Council on Patient Information and Education, Bethesda, MD. Available at http://www.ahrq.gov/consumer/safemeds/safemeds.htm (accessed August 2006)
- 17.Whyte L A. Medication cards for elderly people: a study. Nurs Stand 1994825–28. [DOI] [PubMed] [Google Scholar]
- 18.Snedecor G W, Cochran W G.Statistical methods. Seventh edition. Ames, IA: Iowa State University, 1980
- 19.Moses L E, Emerson J D, Hosseini H. Analyzing data from ordered categories. In: Bailar JC III, Mosteller FM (eds). Medical uses of statistics. Waltham, MA: NEJM Books, 1992259–279.
- 20.SAS Institute SAS version 9.1 for Windows. Cary, NC: SAS Institute, 2003
- 21.Cytel Software Corporation StatXact version 6. Cambridge, MA: Cytel, Inc, 2004