Abstract
Objective
To systematically review the literature regarding how statistical process control—with control charts as a core tool—has been applied to healthcare quality improvement, and to examine the benefits, limitations, barriers and facilitating factors related to such application.
Data sources
Original articles found in relevant databases, including Web of Science and Medline, covering the period 1966 to June 2004.
Study selection
From 311 articles, 57 empirical studies, published between 1990 and 2004, met the inclusion criteria.
Methods
A standardised data abstraction form was used for extracting data relevant to the review questions, and the data were analysed thematically.
Results
Statistical process control was applied in a wide range of settings and specialties, at diverse levels of organisation and directly by patients, using 97 different variables. The review revealed 12 categories of benefits, 6 categories of limitations, 10 categories of barriers, and 23 factors that facilitate its application and all are fully referenced in this report. Statistical process control helped different actors manage change and improve healthcare processes. It also enabled patients with, for example asthma or diabetes mellitus, to manage their own health, and thus has therapeutic qualities. Its power hinges on correct and smart application, which is not necessarily a trivial task. This review catalogues 11 approaches to such smart application, including risk adjustment and data stratification.
Conclusion
Statistical process control is a versatile tool which can help diverse stakeholders to manage change in healthcare and improve patients' health.
Quality improvement (QI) practices represent a leading approach to the essential, and often challenging, task of managing organisational change.1 Statistical process control (SPC) is, in turn, a key approach to QI.2 SPC was developed in the 1920s by the physicist Walter Shewhart to improve industrial manufacturing. It migrated to healthcare, first in laboratory settings (eg, Fisher and Humphries3) and then into direct patient care applications, along with other approaches to QI. Before we report on our systematic review of the literature on how SPC has been applied to QI in healthcare, there is a need to define SPC and its role in QI.
“Statistical process control (SPC) is a philosophy, a strategy, and a set of methods for ongoing improvement of systems, processes, and outcomes. The SPC approach is based on learning through data and has its foundation in the theory of variation (understanding common and special causes). The SPC strategy incorporates the concepts of an analytic study, process thinking, prevention, stratification, stability, capability, and prediction. SPC incorporates measurement, data collection methods, and planned experimentation. Graphical methods, such as Shewhart charts (more commonly called ‘control charts'), run charts, frequency plots, histograms, Pareto analysis, scatter diagrams, and flow diagrams are the primary tools used in SPC.” (Carey4, p xviii)
The terms “statistical process control” and “statistical quality control” are often used interchangeably,5 although sometimes the latter is used to describe a broader organisational approach to quality management that evolved into the concept of total quality management.6
One of the tenets of QI is that to improve healthcare performance we must change our way of working.7 But change does not always mean improvement. To discriminate between changes that yield improvement and those that do not, relevant aspects of performance need to be measured. In addition, measurement guides decisions about where improvement efforts should be focused in the first place. SPC may facilitate such decision making. Control charts, central to SPC, are used to visualise and analyse the performance of a process—including biological processes such as blood pressure homoeostasis or organisational processes such as patient care in a hospital—over time, sometimes in real time. Statistically derived decision rules help users to determine whether the performance of a process is stable and predictable or whether there is variation in the performance that makes the process unstable and unpredictable. One source of such variation can be a successful intervention aimed at improvement that changes performance for the better. If the improvement is maintained, the process will stabilise again at its new level of performance. All of this can be easily determined by using SPC.4
Although there are theoretical propositions that SPC can facilitate decision making and QI in healthcare (eg, Berwick,8 Benneyan et al,9 Plsek10) it is not clear what empirical support there is in the literature for such a position11:
“The techniques of statistical process control, which have proved to be invaluable in other settings, appear not to have realised their potential in health care. ... Is this because they are, as yet, rarely used in this way in health care? Is it because they are unsuccessful when used in this way and thus not published (publication bias)? Or is it that they are being successfully used but not by people who have the inclination to share their experience in academic journals?” (p 200)
The present systematic review aimed to answer these questions. We examined the literature for how and where SPC has been applied in QI of clinical/patient care processes and the benefits, limitations, barriers and facilitating factors related to such application.
Materials and methods
Drawing on the principles and procedures for systematic review of QI interventions12 we searched for articles on the application of SPC in healthcare QI published between 1966 and June 2004 (see appendix A) in the following databases: Web of Science, Ovid Medline(R), EMBASE, CINAHL (Cumulative Index to Nursing and Allied Health Literature), PsycInfo, and the Centre for Reviews and Dissemination databases. We also included articles found by searching reference lists or from elsewhere which we were aware of, if they met our inclusion criteria: original empirical studies of SPC application in improvement of clinical/patient care processes in healthcare organisations, published in English. We excluded articles dealing with application of SPC in laboratory or technical processes (eg, film processing) and in surveillance/monitoring (unless they also contained empirical data about improvement efforts), as well as tutorials (unless they contained empirical case studies), letters, book reviews and dissertations.
We reviewed abstracts, when available, or else other information about the publication provided in the database (eg, publication type such as letters, book reviews or original articles). Articles that did not meet the inclusion criterion were excluded. We retrieved and read the full text of the remaining articles, again excluding the articles that did not meet the inclusion criterion.
We developed, pilot tested and modified a data abstraction form which we then used to consistently capture information of relevance to our review questions on reading the full text articles. The information recorded was: whether and how the article met the inclusion criterion; study objective(s); study design; whether the study empirically compared application of SPC with any other method for process data display and analysis; reported benefits, limitations, barriers and facilitating factors related to SPC; organisational setting; country where study was conducted; clinical specialty; unit of analysis; variables for SPC analysis; and other observations. Some questions in the form required a yes/no or brief response (eg, country where study was conducted) and others required answers in the form of direct quotes from the article or the a summary of the article written by the reviewer. Each article was read and data abstracted by one member of the review team (the coauthors of this review). Following this, all the data abstraction forms were reviewed by the first author, who solicited clarification and checked for any missing or incomplete data to ensure consistency in reporting across all articles reviewed. He also conducted the initial data synthesis, which was then reviewed by the entire team.
We determined the study design for each article and whether the investigators intended to test the utility of SPC application, alone or in combination with other interventions. In several articles, the study design or study objectives were not explicitly stated. Our determination of such intention in such cases was based on our reading of the full text papers.
Simple descriptive statistics—for example, the number of publications per year of publication or per country—were used to characterise the included studies. The qualitative nature of our research questions and of the abstracted data shaped our analysis and synthesis of findings regarding benefits, limitations, SPC variables, etc.13 The abstracted data was reviewed one question at a time and data from each article was classified into one or more thematic categories, each with a descriptive heading. Informed by our present understanding of QI and healthcare, we developed these categories as we reviewed the data, rather than using categories derived a priori from theory. For data that did not fit into an existing category, we developed a new one. Thus the categories emerged as we synthesised the data. We report the categorised data in tabular form, illustrated with examples, and give the references of all the source studies.
To strengthen our review through investigator triangulation,14 we sought feedback on an earlier version of this manuscript from two SPC experts: one was the most frequent coauthor in the included studies and the other was an expert on SPC application also in settings other than healthcare. Their comments helped us refine our data synthesis and distil our findings.
Results
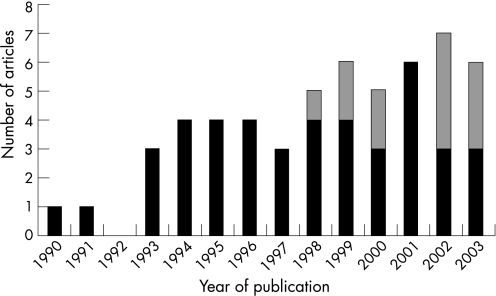
The database searches yielded 311 references. The initial review (abstracts etc.) yielded 100 articles which we read in full text form. Of these, 57 articles met the inclusion criteria and have been included in the review.15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71 To characterise the body of liferature, figure 1 shows the year of publication and whether the studies were conducted in USA or elsewhere (further specified below); table 1 gives the study designs and objectives—whether or not to test SPC utility.
Figure 1 The number of included articles by year of publication. (A total of 55 articles were published in 1990–2003; the two articles from 2004 are not included in this graph since the database searches were conducted in June 2004.) Black bars: studies conducted in the USA; grey bars: studies conducted outside the USA.
Table 1 Study design and objectives of the studies included in the systematic review*.
| Study design | Number of articles | References | Number of articles in each study design category intending to test utility of SPC | References |
|---|---|---|---|---|
| Case study | 28 | 16, 17, 19–21, 24, 26, 29, 33, 34, 36, 39, 41, 44–49, 53, 55, 57, 58, 61, 63–65, 67 | 16 | 16, 17, 20, 24, 26, 36, 41, 44, 47, 48, 57, 61, 63–65, 67 |
| Tutorial with illustrative case study | 23 | 15, 18, 22, 23, 25, 27, 30, 31, 35, 37, 38, 40, 42, 50–52, 54, 59, 62, 66, 69–71 | 3 | 22, 25, 30 |
| Observational study of change in clinical procedure(s) using historical controls | 2 | 43, 68 | 0 | |
| Controlled before‐and‐after intervention studies | 2 | 28, 56 | 1 | 56 |
| Patient case reports | 2 | 32, 60 | 2 | 32, 60 |
*The study designs are reported in order of frequency.
Most of the articles (45/57) concerned application of SPC in healthcare improvement in the USA.15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,37,38,39,40,42,43,45,47,49,50,51,52,53,54,55,56,59,60,63,67,68,69,70,71 While the first US‐based article was published in 1990, the non‐US articles were published between 1998 and 2003: three articles were from the UK,61,62,66 three were from Switzerland,36,41,46 and one each were from Australia,58 Finland,65 France,57 Indonesia,44 Norway64 and Spain.48 The intention to test the utility of SPC is exemplified by a study aiming to reduce the rate of acquisition of methicillin‐resistant Staphylococcus aureus (MRSA) on wards and units at Glasgow Royal Infirmary hospitals.61 Annotated control charts displaying data on MRSA acquisition were fed back monthly to medical staff, managers and hotel services. Sustained reductions in the rate of acquisition from the baseline, which could not otherwise be accounted for, started 2 months later. In contrast, investigators at a paediatric emergency department used SPC to demonstrate a decline in the rate of contamination following the introduction of a new approach to drawing blood for culture specimens,68 but the study had no intention to test the utility of SPC per se.
To characterise the content of the articles, we first present how and where SPC has been applied to healthcare QI. Tables 2–4 present the study settings (ie, hospital etc. where SPC was applied; table 2), the field of healthcare (ie, specialties or forms of care; table 3), and the units of analysis (table 4). Table 5 enlists the 97 distinct SPC variables that have been reported. Tables 6–9 convey our synthesis of the reported benefits, limitations, barriers and facilitating factors related to SPC application. For each category, we have given explanations or examples and references to the source articles.
Table 2 How and where SPC was applied: study settings*.
| Study setting | Number of articles | References |
|---|---|---|
| Hospital settings (total) | 40 | |
| Entire hospital | 14 | 18, 21, 24, 25, 27, 31, 33, 37, 42, 44, 54, 56, 61, 69 |
| Hospital department | 12 | 17, 22, 28, 30, 39, 45, 47, 53, 57, 64, 68, 71 |
| Hospital departments in collaboration | 5 | 36, 41, 43, 46, 48 |
| Hospital outpatient clinic | 5 | 29, 32, 49, 60, 67 |
| Hospital clinical chemistry laboratory | 2 | 19, 58 |
| Hospital outpatient clinics in collaboration | 1 | 55 |
| Hospital wards | 1 | 65 |
| Non‐hospital outpatient settings (total) | 12 | |
| Outpatient single specialty clinic | 5 | 35, 37, 40, 50, 70 |
| Primary care centre | 4 | 34, 52, 62, 66 |
| Outpatient multispecialty clinic | 2 | 15, 59 |
| Ambulatory nursing centre clinic | 1 | 23 |
| Other settings (total) | 6 | |
| Healthcare system | 2 | 26, 63 |
| Nursing home | 2 | 16, 51 |
| Mental health residential facility | 1 | 20 |
| Not specified | 1 | 38 |
*The study settings are divided in three groups, each consisting of several different settings. One article reported data from two settings, hence a total of 58 settings are reported.
Table 3 How and where SPC was applied: fields of healthcare*.
| Field of healthcare (specialty) | Number of articles | References |
|---|---|---|
| Anaesthesia—intensive care | 12 | 22, 25, 33, 36, 39, 41–43, 46, 48, 57, 64 |
| Family practice—primary care | 7 | 34, 52, 55, 59, 62, 63, 66 |
| Emergency medicine | 6 | 19, 45, 46, 48, 53, 68 |
| Cardiac (cardiothoracic) surgery | 5 | 22, 30, 42, 47, 56 |
| Cardiology | 5 | 22, 32, 46, 48, 56 |
| Internal medicine | 4 | 15, 32, 37, 50 |
| Surgery | 4 | 42, 43, 49, 59 |
| Asthma–allergology | 3 | 35, 40, 70 |
| Nursing | 3 | 23, 24, 65 |
| Clinical chemistry | 2 | 19, 58 |
| Nursing home | 2 | 16, 51 |
| Urology | 2 | 36, 41 |
| Endocrinology | 1 | 55 |
| Haematology | 1 | 67 |
| Mental health | 1 | 20 |
| Orthopaedics | 1 | 28 |
| Oncology | 1 | 67 |
| Otolaryngology | 1 | 29 |
| Paediatrics | 1 | 59 |
| Radiology | 1 | 17 |
| Rehabilitation medicine | 1 | 60 |
*The specialties or fields of healthcare (eg, nursing homes) where SPC was applied are rank ordered according to the number of articles in which such application was reported. Whereas 11 articles did not specify a specialty or field of healthcare, several articles included more than one specialty.
Table 4 How and where SPC was applied: units of analysis*.
| Unit of analysis | Number of articles | References |
|---|---|---|
| Single patients (including patients using control charts themselves) | 9 | 16, 22, 32, 35, 38, 40, 50, 60, 70 |
| Single clinicians | 4 | 37, 52, 62, 66 |
| Outpatient clinic | 3 | 29, 49, 59 |
| An ambulatory nursing centre clinic | 1 | 23 |
| Primary care/family medicine centre | 2 | 34, 63 |
| Population(s) of patients in nursing home(s) | 2 | 16, 51 |
| Staff at mental health residential facility living areas | 1 | 20 |
| Hospital wards | 5 | 26, 33, 56, 61, 65 |
| Department process level | 15 | 17, 19, 21, 25, 27, 30, 39, 42, 45, 53, 54, 57, 64, 68, 71 |
| Hospital department and community‐based orthopaedic surgeons | 1 | 28 |
| Microsystem(s) | 6 | 15, 37, 43, 47, 50, 67 |
| Process that involves more than one department | 6 | 36, 41, 46, 48, 55, 58 |
| Entire hospital | 6 | 18, 21, 24, 31, 44, 69 |
*The units of analysis—the levels at which SPC was applied—are ordered according to the degree of aggregation (from individual patients to entire organisations). Some articles reported data from more than one unit of analysis. A microsystem is defined as “a small group of people who work together on a regular basis to provide care to discrete subpopulations of patients”.72 The distinction between microsystems and other categories is not absolute but a matter of judgment.
Table 5 SPC variables*.
| I | Biomedical variables |
| Blood glucose and HbA1c measures | |
| 1 | HbA1c level in groups of diabetic patients52 |
| 2 | Blood glucose, as a daily group average, for intensive care patients on parenteral or enteral nutrition33 |
| 3 | Average blood glucose levels among intensive care unit patients on total parenteral nutrition per week25 |
| 4 | Standard deviation of blood glucose levels among intensive care unit patients on total parenteral nutrition per week25 |
| 5 | Capillary blood glucose measurements of individual patients56 |
| 6 | Individual patient blood glucose measurements38 |
| 7 | Individual patient fasting blood sugar measurements37,50 |
| 8 | Average of five consecutive HbA1c measurements in diabetic patients cared for by one clinician50 |
| Peak expiratory flow rates (PEFR) | |
| 9 | Daily am (ie, morning) pre‐bronchodilator PEFR in asthmatic patients (litres/min)35,40 |
| Pain | |
| 10 | Daily individual patient visual analogue pain scale recordings60 |
| Cardiovascular system measures | |
| 11 | Blood pressure22,23,32 |
| 12 | Heart rate22,32 |
| 13 | Central venous pressure22 |
| Urinary output | |
| 14 | Urinary output (during intensive care)22 |
| Oxygen saturation | |
| 15 | Oxygen saturation (during intensive care)22 |
| II | Biomedical measurement variable |
| 16 | Blood pressure measurement error (mm Hg)23 |
| III | Other variables related to patient health |
| 17 | Patient fall rate (number of patient falls/number of cases per month)51 |
| 18 | Days in between asthma attacks70 |
| 19 | Nausea on day 3 after administration of chemotherapy, as reported by patients on a scale, displayed as the average in each sample of four consecutive patients67 |
| 20 | Incontinence volume (of fluid) for individual incontinent patients (in “a change‐program”) on scheduled inspections16 |
| 21 | Volume of irrigation fluid absorbed during endoscopic renal pelvic surgery41 |
| IV | Clinical management variables |
| Time to complete (part of) a clinical process | |
| 22 | Time between patient check‐in and interpretation of a preoperative radiograph17 |
| 23 | Door‐to‐needle time: “the time span between hospital admission and the initiation of thrombolytic therapy in patients with acute myocardial infarction”46,48 |
| 24 | Average length of stay for inpatients with congestive heart failure54 |
| 25 | Length of stay after cardiac surgery (risk adjusted and transformed)71 |
| 26 | Average length of stay for total hip replacement patients28 |
| 27 | Intensive care unit admission time42 |
| 28 | Average postoperative (bowel surgery) length of stay (in days) per month42 |
| 29 | Admission time (from “sign in” to “patient in room”)18,31 |
| 30 | Duration of intravenous antibiotic treatment for patients with pneumonia37 |
| 31 | Time to administration of antibiotic treatment for patients with pneumonia37 |
| 32 | Average length of hospital stay for inpatients with pneumonia37 |
| 33 | Time to extubation after coronary artery bypass grafting (CABG) surgery (hours)47 |
| 34 | Length of stay in the intensive care unit after CABG (days)47 |
| 35 | Total hospital length of stay after CABG (days)47 |
| 36 | Laboratory turnaround time for blood tests19,27 |
| 37 | Average postoperative length of stay after non‐emergent CABG surgery, per quarter30 |
| 38 | Time from receiving a referral to first patient contact (days)49 |
| Time, or number of consecutive cases, between events | |
| 39 | Time between deaths in chronic obstructive pulmonary disease54 |
| 40 | Number of successful elective CABG surgeries between cases with mortality42 |
| 41 | Number of successful CABG surgeries between cases with complications42 |
| 42 | Number of successful bowel resection operations between cases with complications42 (see also variable 18 for another example) |
| Rate of events in a clinical process (“defect rate” or “success rate”) | |
| 43 | Percentage of chief complaints (of patients in an emergency department) charted in free text (as opposed to coded) in the medical record, per day53 |
| 44 | Percentage of anaesthesia sessions with at least one “significant anaesthetic event”57 |
| 45 | Mortality in patients with congestive heart failure54 |
| 46 | Proportion of low birthweight infants54 |
| 47 | Percentage of intravenous medication administration events associated with an error24 |
| 48 | Occurrence of four intraoperative adverse events, of relevance to anaesthetic quality and safety: inadequate analgesia during brachial plexus block, emergence from general anaesthesia, intubation problems and medication errors64 |
| 49 | Proportion of patients with excessive absorption of irrigation fluid during transurethral resection of the prostate36 |
| 50 | Mortality after CABG42 |
| 51 | Proportion of patients on enteral, or parenteral, nutrition with blood glucose levels outside a (clinical management) target range33 |
| 52 | Daily percentage of satisfied requests for medical records at a paediatric outpatient clinic18 |
| 53 | “Guideline non‐adherence rate”; proportion of chemotherapy administrations in which guideline recommendations to prevent nausea were not followed67 |
| 54 | Percentage of all blood cultures growing contaminants per month68 |
| 55 | Proportion of intensive care unit patients on total parenteral nutrition with blood glucose values that were higher than the given target (>11.1 mmol/l (>200 mg/dl)) per week25 |
| 56 | Percentage of all acute bronchitis visits during which a β‐agonist was prescribed, per month34 |
| 57 | Percentage of all acute bronchitis visits during which an antibiotic was prescribed, per month34 |
| 58 | Percentage of outpatient surgical procedures per month (of all procedures)43 |
| 59 | Proportion of cases where tests were delayed or not reported in a timely fashion15 |
| 60 | Proportion of incontinent patients (in “a toileting program”) who were wet on scheduled inspections16 |
| 61 | Monthly percentage of emergency department patients who leave without being seen45 |
| 62 | Percentage of inpatients on the ward in each of three acuity levels (low, medium and high) per day26 |
| 63 | The quarterly incidence rate of eight variables among cardiac surgery patients: perioperative death, perioperative myocardial infarction; cerebrovascular accident; re‐exploration for bleeding or tamponade; atrial fibrillation; leg wound; acute tubular necrosis; sternal infection; acute renal failure30 |
| 64 | Rates of total and major complications per patient after cardiac surgery30 |
| 65 | Proportion of first case starts each day in the operating theatres that are delayed due to the department of anaesthesia39 |
| 66 | Proportion of cases in which the turnaround time between cases exceeded the given performance standard39 |
| Number of defects/events or occurrences in a clinical process | |
| 67 | Number of medication errors, per month24 |
| 68 | Number of out‐of‐hours “stat” (blood test analyses) requests received each week58 |
| 69 | Number of MRSA cases per month61 |
| 70 | Number of diabetes patients (seen at office visits) with HbA1c measurements, per month34 |
| 71 | Number of patients (seen at office visits) at a department of family medicine with a recorded diagnosis of tobacco abuse, per month34 |
| 72 | Total number of surgical procedures per month43 |
| 73 | Number of patients with diarrhoea admitted to the hospital, per week44 |
| 74 | Number of referrals per month for patients with diabetes from primary care to endocrinology55 |
| 75 | Number of new referrals per work day49 |
| 76 | Net number of new patients ( = practice growth)63 |
| Clinical decision making | |
| 77 | (Square root of) the number of cases per general practitioner (GP) diagnosed as having tonsillitis versus number of cases diagnosed as having any throat infection62 |
| 78 | (Square root of) the number of GP patients diagnosed as having tonsillitis and non‐tonsillar throat infection, who receive antibiotics62 |
| 79 | (Square root of) the number of GP cases with any throat infection diagnosis who received antibiotics62 |
| 80 | (Square root of) the number of GP cases with a diagnosis of sore throat in which antibiotics were not prescribed versus the number of cases in which antibiotics were prescribed66 |
| V | Financial resources variables |
| Cost of care | |
| 81 | Average cost per procedure (total hip replacement)28 |
| 82 | Staffing expense per shift, depicted as variance in US$ from budget26 |
| 83 | Staffing expense per 24‐h period, depicted as variance in US$ from budget26 |
| Productivity and efficiency | |
| 84 | Relative value unit (RVU) production per provider FTE (full‐time equivalent) ( = provider productivity)63 |
| 85 | FTE support staff per FTE provider ( = practice efficiency)63 |
| Organisational financial performance | |
| 86 | Net patient revenue per relative value unit (a primary care physician‘s practice) ( = practice profitability)63 |
| 87 | Provider cost as a percent of net revenue ( = practice cost management)63 |
| 88 | Non‐provider cost as a percentage of net revenue ( = practice cost management)63 |
| VI | Variables relating to the experience of healthcare |
| Patient satisfaction indicators | |
| 89 | “Physician care scale score”, which is the average of patient survey responses to 10 questions59 |
| 90 | Patient satisfaction with (nursing) care65 |
| 91 | Percentage of patients (who responded to a patient satisfaction survey) whose response was that the overall visit was excellent29 |
| Other experience with care | |
| 92 | Patient rating of assistance in solving the patient's current health problem65 |
| 93 | Staff satisfaction with (nursing) care65 |
| 94 | Staff rating of assistance in solving patients' current health problems65 |
| VII | Variables related to clinical staff supervision |
| Clinical staff supervision | |
| 95 | Active treatment monitoring index, based on observing staff performance in clinical encounters and assessing nine criteria20 |
| Completion of mandatory staff training | |
| 96 | Percentage of employees who have completed mandatory safety training21 |
| VIII | Other variables |
| 97 | “A temperature signal” (of unspecified origin; it is probably not body temperature since it varies around 74°F or 23°C). “[T]he signal could just as easily have been bed occupancy, ED wait time, expense per equivalent discharge, or staffed care days.”69 |
*The variables are divided into eight categories and then further subdivided under subheadings in a category. Several articles reported more than one SPC variable.
Table 6 Benefits of using SPC to improve clinical processes*.
| Examples/explanations | ||
|---|---|---|
| SPC facilitated and documented healthcare process improvement | ||
| 1 | SPC application helped people assess the impact of changes to the process19,24,27,30,32,33,34,37,42,43,45,52,53,55,57,58,60,64,67,68 | Many articles reported a helpful role of SPC in determining the effects of interventions made to healthcare processes. Examples include successful effects, adverse effects or a lack of effect |
| 2 | SPC application contributed (led) to improvement of healthcare processes16,17,20,26,28,30,31,36,37,46,53,56,65 | Several articles reported improvement of a healthcare process and attributed that improvement, more or less explicitly, to improvement efforts in general and SPC application in particular (the foundations for such attribution were methodologically weak—for instance, case reports without comparisons). |
| For example, investigators evaluating the “door‐to‐needle‐time” (DTNT) for thrombolytic treatment of patients with acute myocardial infarction (AMI) using control charts found that “in the beginning there was considerable variability of DTNT signifying that the process of in‐hospital management of patients with AMI was out of statistical control. Shortly after the initiation of formal process analysis DTNTs were reduced significantly and remained within a narrow range, now showing a stable and statistically controlled pattern. Thus, process quality was significantly improved by our efforts” (Bonetti et al,46 p 315) | ||
| 3 | SPC application helped people identify areas for improvement21,26,40,42,47,49,58,70 | Several investigators identified process performance that was not satisfactory, and hence in need of improvement, using SPC. For instance, investigators applying SPC to the care of patients with asthma concluded that when the care process yields a range of breathing that “is clinically problematic, the care process should be redesigned and monitored to ensure that the problem has been corrected” (Boggs et al,40 p 175) |
| SPC was a useful tool for healthcare management | ||
| 4 | SPC application helped people distinguish special from common cause variation15,30,35,38,39,41,42,45,49,51,54,59,64,70,71 | Reflecting one of the tenets of SPC, many articles reported how SPC helped people identify common cause and special cause variation, and understand the difference. Investigators applying SPC to surgical care, for instance, concluded that “plotting data over time and using control chart techniques will tell us whether the variation in a surgical process is stable and predictable or whether variation signals a significant change in the process” (Levett and Carey,42 p 358) |
| 5 | SPC seen as a valuable QI tool which was easy to use18,19,25,29,30,38,40,42,47,48,50,56,62,69,70 | Many articles report the usefulness of SPC as a tool in healthcare process management and improvement. Investigators at a cardiac surgery department, for example, designed a system for automated data collection and generation of control chart for critical pathway adherence, and concluded that it was “a cost effective method for providing timely feedback. The benefits of timely feedback with the control chart method are strongly suggested by this study” (Ratcliffe et al,47 p 1821) |
| 6 | SPC enabled valuable prediction of future process performance30,35,40 | A few studies reported the value of being able to predict future performance of stable processes. A predictable outcome was beneficial to the patient, doctor and insurer.30 In asthma care, such prediction enabled clinicians and patients to decide whether to maintain the current care regimen or alter it to improve future outcomes35,40 |
| 7 | SPC helped describe and quantify process variability22,23,35 | SPC allowed characterisation of process variability, including variability present in blood pressure measurement23 |
| SPC helped process stakeholders | ||
| 8 | SPC enabled patients to be partners in clinical management (and research)35,38,40,50,60 | SPC enabled patients with chronic conditions to better understand and manage—alone or in partnership with clinicians—their condition, specifically, diabetes mellitus,38,50 hypertension,38 muscular pain60 and asthma.35 SPC has also been proposed as a research strategy whereby patients can be active partners in data collection and analysis.38 |
| In asthma care, for example, when patients control‐chart their breathing function the benefits include “knowing each patient's signals and their meaning; natural limits of the current care process; and having the ability to anticipate this patient's future level of function given no change in his/her care process[. This] enhances both the patient's and physician's ability to meaningfully engage in the process of continuous quality improvement of asthma. [It] elevates the level of communication and dialogue between patients and physicians exponentially beyond that generated by traditional run chart information” (Boggs et al,35 p 561) | ||
| 9 | SPC application improved communication between process actors35,36,52,63,70 | SPC provided a common language and facilitated communication between managers and clinicians, or between clinicians and patients. See the example above |
| 10 | SPC application enabled (better) informed decision making21,26,35,44,54,61,63,66 | SPC helped inform decision making in healthcare organisation governance and management21,26,44,54,61,63 and in clinical decision making regarding—or in partnership with—patients.35,66 |
| Reporting on efforts at the Intermountain Health Care system to improve inpatient acuity level assessment, Shaha concluded that “control charts have given information to delivery‐level decision makers which they have never before enjoyed. The system has helped nurse managers better understand the acuity on their units and plan more effectively for appropriate staffing to support patient care. Improved staffing has enhanced teamwork and the quality of work life on these units” (Shaha,26 p 30) | ||
| 11 | SPC empowered process stakeholders15,21,26,35,50,54,61 | SPC had an empowering effect among—for example, patients,35,50 nurse managers,26 a medical assistant,15 healthcare workers and managers,21,61 and board members21,54 |
| 12 | SPC application helped stakeholders learn about their processes40,63,68 | Stakeholder—or “organisational”—learning was facilitated by SPC application and involved patients,40 clinicians40,63,68 and managers.63 |
| “Both you and your patient are learners. Let the charts teach you and help you learn what is of practical value to your patients. You will find that learning together is a strong invitation of commitment to the [asthma care] process for both of you” (Boggs et al,40 p 175) | ||
*All articles reported benefits, and many reported several. The benefits formed 12 categories which are grouped into three broad areas.
Table 7 Limitations of SPC application in improvement of clinical processes*.
| Examples/explanations | ||
|---|---|---|
| Limitations of the ability to improve clinical processes using SPC | ||
| 1 | Sharing performance data in control chart format does not automatically lead to improvement in healthcare organisations15,67 | Even if control charts can help visualise the performance of a clinical process, and signal a need for improvement, there is no guarantee that such signals prompt improvement. For example, Mertens et al studied the effects of a number of interventions, including control chart format feedback, on oncologist prescribing patterns for the management of delayed chemotherapy‐induced nausea and emesis. “In our study, these [control] charts effectively measured the degree of compliance with guideline recommendations, but they were not effective in improving physician compliance ... . These findings indicate that statistically valid charting, although useful in measuring compliance, will not achieve improved compliance as a physician feedback tool. However, enhanced compliance may be achieved when adverse patient outcomes are coupled with evidence of poor compliance with evidence‐based guidelines” (Mertens et al,67 p 1377) |
| 2 | Statistical control does not necessarily equal clinical control nor desired performance27,35,63,64 | “It is important to note that a chart can be in statistical control, and [the patient can still] be at risk for severe asthma. So statistical control does not equate with clinical control. It simply reflects the absence of special cause variation” (Boggs et al,35 p 555) |
| 3 | Cause and effect relationships are not always obvious, even if a change is identified with statistical confidence45,60,61 | “Although control charts can help detect when the [MRSA] rate has increased or decreased, they typically will not identify the specific cause of the change. Once a change is detected, the infection control team must use its skills to assess the situation, identify possible causes, and promote improvement in practices” (Curran et al,61 p 16) |
| Limitations of the applicability of SPC to clinical processes | ||
| 4 | Differences between patients may limit the appropriateness of combining data about their care onto one control chart25,30,51,52 | “Unlike manufacturing, the health care industry deals with a variable input: Patients differ in their severity of illness on admission. This variability on admission affects care outcomes.[Ref] Therefore, it is imperative to adjust for patients' risk for adverse outcomes. ... Blindly applying methods of manufacturing to health care may be misleading” (Alemi and Oliver,51 p 2) |
| 5 | The ability of stakeholders to apply SPC correctly may be limited16,35 | Discussing an article which contains a methodological error—irrational subgrouping—Boggs et al emphasise that “the mathematics which defines the upper and lower control limits and the zone lines (from which signals are defined) is derived from the range between the values composing each subgroup. If the daily subgroupings are irrational—i.e., the AM pre‐ and postbronchodilator and PM pre‐ and postbronchodilator are included in the same subgrouping—the daily ranges are distorted, the mathematical calculations are erroneous, and the control charts these calculations produce are improperly constructed. An improperly constructed chart is by definition uninterpretable. This error of irrational subgrouping is fatal and invalidates both their analysis and conclusions” (Boggs et al,35 p 560). |
| Another aspect of this limitation is when staff members lack sufficient knowledge of QI in general and SPC in particular16 | ||
| 6 | Limitations regarding data for use in control charts25,26,38,44,45,54,63,64,65 | Several articles highlight limitations related to the data needed for control charting: |
| • The sample size needs to be “big enough”. A minimum amount of data are needed to produce reliable control charts.26,54 “If a control chart has too few data points, then it may appear that a special cause exists when it really does not” (Caron and Neuhauser,54 p 31) | ||
| • Common types of control charts are not well suited to analysing infrequent events. Such events require special types of control charts64 | ||
| • (Manual) data collection can be prohibitively demanding65 | ||
| • Oversampling of patients whose values are out of control pulls the statistic in the direction of derangements25 | ||
| • Choosing too long a sampling period may delay control chart signalling and delay decision making44 | ||
| • (Retrospective) control chart interpretation can be difficult if charts are not annotated with interventions or other influences on process performance45 | ||
| • Autocorrelation may limit the ability to analyse control chart data. “Autocorrelation is a phenomenon whereby the preceding observation predicts the next observation. ... If yesterday's blood pressure predicts today's blood pressure, there is autocorrelation” (Solodky et al,38 p AS14) | ||
| • System‐level aggregation of SPC data may prevent local sense making (if the data cannot be disaggregated). For example, performance measures of one primary care medical group practice “are computed on a group practice level. This level of aggregation means that the SPC charts cannot currently be used to evaluate the results of individual and team efforts within individual group practices” (Stewart and Greisler,63 pp 258–9) | ||
*We found statements regarding one or more limitations of SPC application in 22 articles. The limitations formed six categories which are grouped into two broad areas.
Table 8 Barriers to SPC application*.
| Examples/explanations | ||
|---|---|---|
| People related barriers | ||
| 1 | Lack of knowledge on how to apply SPC correctly16,35,37,51 | Even though control charts, constructed correctly, may be easy to use, not all healthcare professionals and managers have sufficient SPC knowledge |
| 2 | At first, SPC may represent a new and challenging way of thinking18,40 | “It is not at all surprising that many persons avoid involvement in statistical process control. The statistical procedures developed in industry to monitor production processes are somewhat different from those employed in biostatistics and social science applications. Many find it difficult to adjust to these procedures and to apply statistical process control techniques to situations in health care settings” (McKenzie,18 p 81) |
| 3 | If SPC application is not perceived as helpful, it may not succeed39,65 | “Whereas Finison et al.[Ref] claim that the mean and standard deviation are simple and easy for staff to understand, the staff involved in this study found them difficult to perceive. This is partly accounted for by the staff's resistance, as it seemed that they did not want to understand the results of the figures. The staff expected the investigators to tell them what to do and did not want to process the results and the issues underlying them” (Hyrkas and Lehti,65 p 186) |
| Data collection and chart construction related barriers | ||
| 4 | Collection and attribution of data to different hospital units for SPC application can be difficult61 | In the case of hospital acquired infections, investigators found that it was hard to assign new cases to the correct ward or unit's control chart. “The few disadvantages of this approach primarily concern implementation. The ability to assign a new MRSA case to a specific ward rarely can be completely accurate. We attempt to minimize this imprecision by encouraging the input of ward staff in any discussions or debates as to where a patient acquired the MRSA” (Curran et al,61 p 17) |
| 5 | Finding the right level of aggregation of data for SPC application can be difficult and require trade‐offs61 | Continuing with the example of hospital acquired MRSA infections, finding the best level of data aggregation was a barrier: “If the control chart includes too many wards, then staff may feel the responsibility lies elsewhere and they may not be inclined to use the feedback to alter their practices. Further, if the chart contains information from too many departments or units, it sometimes can mask local problems or out‐of‐control data. [Furthermore, monitoring] a large number of units on individual charts also can decrease the overall specificity and increase the false‐alarm rate, especially if [2 sigma] warning limits are used as control limits” (Curran et al,61 pp 16–8) |
| 6 | Data collection and analysis can be time consuming and costly17,19,37,39 | “Other real obstacles that challenge physicians are the time required for and the financial costs of data collection and analysis. ... Certainly, measurement may consume valuable resources” (Nelson et al,37 p. 465) |
| 7 | Constructing the most appropriate control chart can be difficult30 | “The most difficult task is deciding which probability distribution, and thus which control chart, is most appropriate for the data, and then choosing homogenous and rational subgroups to analyze” (Shahian et al,30 p 1356) |
| 8 | Lack of access to reliable data in a timely fashion can be a barrier to real‐time SPC application47 | Discussing care pathways in cardiac surgery, one group found that “accurate process data are difficult and expensive to obtain. Data obtained from hospital and medical records data systems are often inaccurate, temporally inefficient, and not focused on daily patient events[.] Among the difficulties with optimizing outcome‐based cardiac surgical care are the expense and problems with [multi‐institutional] databases, including lack of standardization, cost of dedicated data entry/delivery personnel, and lack of timely feedback[. Those] systems were not designed to provide rapid feedback of process data, which is necessary if individual practitioners are to effectively manage cardiac surgery pathways” (Ratcliffe et al,47 p 1820) |
| Information technology related barriers | ||
| 9 | Lack of computer power was a barrier to real‐time SPC application22 | “There are several reasons why control charts and other statistical process control techniques have to date not been introduced as tools to improve real‐time clinical decision making. These include lack of computer power to perform calculations in real time, inabilities to interface medical monitors with computers and to produce clean measurement with an acceptable signal to noise ratio, and lack of statistical process control training for clinical decision makers” (Laffel et al,22 pp 76–7) |
| 10 | Software problems can hamper SPC application26 | One study reported unspecified, and subsequently resolved, software problems as the only barrier to SPC application |
*We found reports of barriers in 15 articles. They formed 10 categories which are grouped into three broad areas.
Table 9 Factors or conditions facilitating application of SPC*.
| Examples/explanations | ||
|---|---|---|
| Information technology and other tools facilitated SPC application | ||
| 1 | Easy‐to‐use tools and resources exist to aid with data management and control chart construction22,30,31,35,45,47,69,70 | The evolution of information technology has brought many new possibilities for SPC data management, control chart construction and interpretation |
| 2 | Electronic clinical information systems with SPC capability facilitated data management and SPC application19,25,28,30,34,47,49 | When designing a system which produced control charts for measures reflecting performance relative to a critical pathway for cardiac surgery, investigators found that a facilitating factor “was the existing electronic medical record, which requires that nurses enter a computerized progress note. ... Integration of the data collection with the generation of a nursing progress note clearly provides the motivation for the bedside nurse to use the system. Moreover, we were able to couple our data collection system with the requirement for an electronic progress note efficiently, so that ... the intranet‐based system decreased by 50% the amount of time the nurses spent charting their progress note” (Ratcliffe et al,47 pp 1820–1) |
| Feedback, training and learning may facilitate SPC application | ||
| 3 | User feedback guided changes to facilitate SPC application40 | “In a focus group session with patients, we learned that many objected to plotting the exact PEFR values over time on a chart. The fear was misplotting – thereby ruining the chart. This insight led to a redesign of the chart in a manner that respected their concern yet did not compromise the chart's ability to provide the information needed” (Boggs et al,40 p 174) |
| 4 | Training users in SPC application may be helpful15,26,44,50 | Reflecting on efforts to apply SPC at a provincial hospital in Indonesia, the author suggests that “(i) the process of data management should be performed by the hospital staff under supervision and with training they should be able to do it without supervision; (ii) a training session for staff on the creation and interpretation of the control chart should be carried out” (Purba,44 p 147) |
| 5 | Gradual introduction of SPC can allow learning and improvement to support subsequent application and spread25,34,61 | At a family medicine clinic, the impact of QI including SPC application “had to be demonstrated through initial projects before there was more general support for the process by other residents and faculty within the department” (Ornstein et al,34 p 360) |
| 6 | Professionals in training led improvement efforts using SPC as part of a clinical scholars programme34 | “Many of the ideas for patient care improvement projects [using SPC] have evolved from the work and leadership of a group of family residents, pharmacy residents, and faculty that is involved in a unique educational experience known as the clinical scholars program. The program was developed ... as a mechanism to provide the family medicine residents with hands‐on opportunities to learn clinical research and quality improvement methods. ... Generally, the project leaders are residents in the clinical scholars program” (Ornstein et al,34 p 351) |
| 7 | SPC application to clinical topics captured the interest of clinicians34 | “Clinical topics interest clinicians, and recognition of the potential personal benefits of a project will increase individual commitment and participation” (Ornstein et al,34 p 360) |
| Patients can have a role in SPC application | ||
| 8 | The benefits of SPC application motivated and empowered patients to record and control chart the data35 | “Adherence in our clinic population is better, both short‐term and long‐term, with [asthma patient operated control charts of PEFR] than for any PEFR monitoring method we have used in the past. ... It is our assessment that the reasons for high patient adherence with our method include (1) the PEFR measurement per se is simple and can be performed using inexpensive equipment; (2) the charts our patients use are easy to keep and understand; (3) the concepts of signals and not being at‐risk are easily understood and appreciated by patients; (4) the chart plays a central role in each patient's follow‐up visit and in telephone calls between visits; (5) because of the latter, patients know we will be monitoring their adherence; (6) patients understand that their chart provides information we need and will use to make decisions that will impact their care; and (7) the quality of the communication with patients is more substantive” (Boggs et al,35 p 561) |
| 9 | Patients as partners may be better at collecting data for SPC analysis than “human subjects” are in traditional research38 | “For the control chart analysis used in the current study, the patient has been a partner (the first author of this article). Patients in RCTs usually are seen as ‘human subjects.' Psychological theory suggests that partners are better data collectors than subjects” (Solodky et al,38 pp AS18–9) |
| Literature and experts can guide SPC application | ||
| 10 | There is literature to guide SPC application15,35 | “The statistical methods to be used in calculating control charts are well described in the literature.[Ref]” (Re et al,15 p 395) |
| 11 | Local experts may provide technical assistance for SPC application18,47 | “At the Indiana University Medical Center several persons from different hospital departments have volunteered to form a cadre of internal statistical process control consultants. The group has come to be known by some as the statistical process control ‘think tank'. No doubt a small group of individuals who are interested in statistics, quality control, and computers can be found in most hospitals. While it is necessary that the large majority of managers and supervisors in any health care organization are knowledgeable about process management and control charts at the concept level of understanding, it takes a relatively few to understand and use such control charts. As internal consultants, these few persons can provide technical assistance when such aid is needed” (McKenzie,18 p 81) |
| 12 | Collaboration with SPC experts may facilitate application23,34 | Collaboration between scholars from nursing and experts in industrial measurement error research “has facilitated the conception and completion of a study [of blood pressure measurement error in a nursing clinic] that could not have been accomplished by either discipline alone” (Nelson et al,23 p 27) |
| Smart application of SPC can be helpful | ||
| 13 | Freezing control limits and the centre line at pre‐intervention levels helped decipher the impact of interventions59,70 | “In [a figure], note that we have separated the plot into two periods: before and after the intervention. For calculating the [control limits], one should use only the data from before the intervention. In this fashion, the patient and the clinicians can decipher if the postintervention data exceeds their expectations from historical patterns” (Alemi and Neuhauser,70 p 99) |
| 14 | Data stratification or disaggregation helped disentangle performance problems and focus improvement efforts37,59 | “The physicians at Site A needed to develop a theory about why their care was rated lower than at other sites. They theorized that the results might be different for the three service areas: family practice, pediatrics, and ambulatory surgery. Therefore, they developed a control chart to disaggregate the data by service. The results ... for each service were juxtaposed on the same control chart using a technique called rational ordering” (Carey,59 p 80) |
| 15 | Risk adjustment of SPC data revealed process changes that previously were hidden51,52,71 | “Before risk adjustment, the process improvement was hidden by the variations in patient risk. Taking the patient risks into account by risk adjustment revealed the significant decrease in [length of stay] during the final 3 months of the 18‐month period” (Hart et al,71 p 115) |
| 16 | Annotations and feedback helped users interpret control charts54,61 | “For anyone not familiar with the information presented in a control chart, annotations are essential. Annotations will aid in explaining observed variation and explaining changes in the underlying process” (Caron and Neuhauser,54 p 30) |
| 17 | Keeping control charts up to date increased their utility61 | “Because the infection control team updates the [control] charts frequently, they can respond more immediately to an increased rate and interact with the ward in a more timely manner” (Curran et al,61 p 15) |
| 18 | Providing SPC information as locally as possible increased its accuracy and utility26,61 | “A major reason for the system's success is that these data charts exist on the PCs at the patient care areas. The information is available to the caregivers and their manager. ... In the [Intermountain Health Care] model, the information is seen first by those who should care the most about its accuracy and reliability. They immediately seek legitimate and operational explanations for anomalies, genuinely showing a high degree of ownership and desire for data integrity” (Shaha,26 p 30) |
| 19 | Making SPC charts readily available to stakeholders may facilitate SPC application34,65 | “Feedback to physicians and other providers is an important component of all the ongoing improvement projects. To facilitate this communication, monthly updates to the control charts used to monitor ongoing CQI projects are posted on a large bulletin board [and also distributed at meetings, and sent out by e‐mail]” (Ornstein et al,34 p 353) |
| 20 | Knowing the clinical context helped in the interpretation of control charts25 | Discussing the issue that blood glucose tests are taken more frequently from patients whose blood glucose level is deranged, investigators argue that they “interpret the charts in context, aware that points are weighted toward derangements” (Oniki et al,25 p 589) |
| 21 | Evidence of autocorrelation can be detected easily38 | “Evidence for [autocorrelation—that the preceding observation predicts the next observation] is detected easily by correlating the value of the variable at time t with the value of the variable at time t + 1” (Solodky et al,38 p AS14) |
| 22 | Concurrent controls can help interpret variation detected using SPC38 | “Control charts focus on change over time. Adding concurrent control patients can help monitor the effects of unknown exogenous factors that may influence outcomes” (Solodky et al,38 p AS17) |
| 23 | A key quality indicator helped reflect the functioning of a complex set of processes in a simple way46 | “Overall process quality cannot be measured directly; we must resort to the use of quality indicators as easy to assess surrogate markers reflecting overall quality. DTNT [door‐to‐needle‐time for patients with acute myocardial infarction who receive a thrombolytic drug] in our example, is a prototype of such an indicator, because it represents the result of a very complex set of processes but nevertheless is simple to measure” (Bonetti et al,46 p 314) |
*We found reports of facilitating conditions or factors in 28 articles. The factors formed 23 categories which are grouped into five broad areas.
Discussion
SPC has been applied to healthcare improvement in a wide range of settings and specialties, at diverse levels of organisations and directly by patients, using many types of variables (fig 1, tables 2–5). We found reports of substantial benefits of SPC application, as well as important limitations of, barriers to and factors that facilitate SPC application (tables 6–9). These findings indicate that SPC can indeed be a powerful and versatile tool for managing changes in healthcare through QI. Besides helping diverse stakeholders manage and improve healthcare processes, SPC can also help clinicians and patients understand and improve patients' health when applied directly to health indicators such as PEFR in asthma or blood sugar concentrations in diabetes. In healthcare, the “study subject” can thus also be an active agent in the process, as when patients apply SPC to their own health. Several studies indicated the empowering effects this may have on patients.35,38,40,50 SPC application thus has therapeutic potential as it can help patients manage their own health. We agree with Alemi and Neuhauser70 that this potential merits further investigation.
Most of the included articles concerned application of SPC in healthcare improvement in the USA. Articles from other countries appeared only towards the end of the study period (fig 1). We have no explanation for this finding, but we speculate that it is related to differences between US and other healthcare systems with regard to QI awareness and implementation.73
Only 22 studies included in the review were intended to test the utility of SPC (table 1). Of the four controlled studies, only one included a control chart in the intervention (as a minor component which did not fully exploit the features of SPC). In 35 articles we did not find an intention to test the utility of SPC application. In those cases, SPC was applied for other reasons (ie, to evaluate the impact of other interventions). Even though many articles thus did not address the utility of SPC, all studies offered information—to varying degrees—relevant to our review's question of how SPC has been applied to healthcare. The utility of SPC is reflected in benefits reported regarding SPC application (table 6).
SPC has been applied in over 20 specialties or fields of healthcare, at a wide range of levels (tables 3 and 4), suggesting that SPC has broad applicability in healthcare. The dominance of anaesthesia and intensive care can be explained in large part by the fact that many studies included their services in conjunction with other specialties. This reflects the way in which anaesthesia has a vital supporting role in many clinical care processes. The 97 SPC variables reported (table 5) demonstrate a diversity of situations in which SPC has been applied, ranging from process indicators of patients' health to health outcomes and many aspects of healthcare processes and organisational performance. This indicates that SPC is a versatile QI tool.
The benefits of SPC application (table 6) mirror those given in books and tutorials on SPC (exemplified by the quote in the Introduction to this review). As noted in a report from a top‐ranked healthcare system which has applied SPC widely:
“Among the most powerful quality management tools that IHC [Intermountain Health Care, USA] has applied is statistical process control, SPC. Most notable among those tools are control charts. Under optimal conditions, these graphical depictions of process performance allow participants to know what is happening within their processes as ‘real time' data enable them to make appropriate decisions. The capability of truly understanding processes and variation in a timely manner has resulted in the most dramatic, immediate, and ongoing improvements of any management technique applied at IHC.” (Shaha,26 p 22)
The limitations of SPC application (table 7) identified by this review are important, and yet perhaps less emphasised than the benefits in books and tutorials on SPC. SPC cannot solve all problems and must be applied wisely. There are many opportunities to “go wrong”, as illustrated by the case where incorrect application was highlighted by other authors (limitation number 5 in table 7). In several cases, our own understanding of SPC suggested that investigators had not used it correctly or fully (eg, standard decision rules to detect special causes were not applied to identify process changes). In the worst case scenario, incorrect application of SPC could lead to erroneous conclusions about process performance and waste time, effort and spirit and even contribute to patient harm. In the more authoritative studies we reviewed, co‐investigators included experts in industrial engineering or statistics or authors who otherwise had developed considerable expertise in SPC methodology. On the basis of these observations, we conclude that although SPC charts may be easy to use even for patients, clinicians or managers without extensive SPC training, they may not be equally simple to construct correctly. To apply SPC is, paradoxically, both simple and difficult at the same time. Its power hinges on correct and smart application, which is not necessarily a trivial task. The key, then, is to develop or recruit the expertise necessary to use SPC correctly and fully and to make SPC easy for non‐experts to use, before using it widely.
Autocorrelation is another limitation of SPC highlighted by this review. Our review, and published books, offer limited advice on how to manage it:
“There is no single acceptable way of dealing with autocorrelation. Some would say simply to ignore it. [Others] would disagree and suggest various measures to deal with the phenomenon. One way is to avoid the autocorrelation by sampling less frequently. ... Others argue against plotting autocorrelated data on control charts and recommend that the data be plotted on a line chart (without any centerline or control limits).” (Carey,4 p 68)
Just over a quarter of the articles reported barriers to SPC application (table 8). The three broad divisions of barriers—people, data and chart construction, and IT—indicate where extra care should be taken when introducing SPC in a healthcare organisation. Ideas on how to manage the limitations of and barriers to SPC application can be found among the factors reported to facilitate SPC application (table 9). They deal with, and go beyond, the areas of barriers we found. We noted the prominence of learning and also of focusing on topics of interest to clinicians and patients. The 11 categories under the heading “Smart application of SPC can be helpful” contain valuable approaches that can be used to improve SPC application. Examples include risk adjustment51,52,71 and stratification30,37,59 to enable correct SPC analysis of data from heterogeneous populations of patients (or organisational units). Basic understanding of SPC must be taught to stakeholders and substantial skill and experience is required to set up successful SPC application. Experts, or facilitators, in healthcare organisations can help, as indicated in table 9, and as we have described for other QI methods.74
We found more information on SPC benefits and facilitating factors than on limitations and barriers, and this may represent a form of publication bias, as indicated by the quote in the Introduction.11 We did not find any study that reported failed SPC application. We can speculate that there have been situations when SPC application failed, just as there must be many cases of successful SPC application that have not been reported in the literature. Studies of failed SPC application efforts, as well as studies designed to identify successful ways to apply SPC to manage change, would help inform future SPC application efforts. On the basis of this review, we agree with the argument that “medical quality improvement will not reach its full potential unless accurate and transparent reports of improvement work are published frequently and widely (p 319),”75 and also that the way forward is to strengthen QI research rather than to lower the bar for publication.76
Methodological considerations regarding the included studies
None of the studies we found was designed to evaluate the effectiveness quantitatively—that is, the magnitude of benefits—of SPC application. This would have required other study designs such as cluster randomised trials or quasi‐experimental studies.12 Although the “methods of evaluating complex interventions such as quality improvement interventions are less well described [than those to evaluate less complex interventions such as drugs]”, Eccles et al argue that the “general principle underlying the choice of evaluative design is ... simple—those conducting such evaluations should use the most robust design possible to minimise bias and maximise generalisability. [The] design and conduct of quantitative evaluative studies should build upon the findings of other quality improvement research (p 47).”77 This review can provide such a foundation for future evaluative studies.
An important distinction is warranted here: we believe that SPC rests on a solid theoretical, statistical foundation and is a highly robust method for analysing process performance. The designs of the studies included in this systematic review were, however, not particularly robust with regard to evaluating the effectiveness of SPC application, and that was not their objective. This does not mean that SPC is not a useful tool for QI in healthcare, only that the studies reviewed here were more vulnerable to bias than more robust study designs, even if they do indicate many clear benefits of SPC application (table 6). Despite the studies not being designed to evaluate the effectiveness of SPC, many used SPC to effectively show the impact of QI or other change initiatives. In this way, SPC analysis can be just as powerful and robust as study designs often deemed superior, such as randomised controlled trials (RCTs).77 The key to this power is the statistical and practical ability to detect significant changes over time in process performance when applying SPC.9 On the basis of a theoretical comparison between control charts and RCTs, Solodky et al38 argue that control charts can complement RCTs, and sometimes even be preferable to RCTs, since they are so robust and enable replication—“the gold standard” for research quality—at much lower cost than do RCTs. These points have been further elaborated in subsequent work.78,79
A curious methodological wrinkle in our review is: can you evaluate the application of a method (eg, SPC) using that same method for the evaluation? Several of the included studies used SPC both as (part of) an intervention and as a method to evaluate the impact of that intervention. For example, Curran et al used annotated control charts to feed information on MRSA acquisition rates back to stakeholders and used these same control charts to show the effectiveness of the feedback programme.61
Relationship between monitoring and improvement
When SPC is applied for monitoring, rather than for managing change, the aims are different—for example, to detect even small but clinically important deviations in performance—as are the methodological challenges.80,81 This review focused on the latter. Thus although studies on SPC application for monitoring healthcare performance were excluded from this review, we recognise the importance of such monitoring. The demarcation between monitoring and improvement is not absolute. Indeed, there are important connections between measurement, monitoring and improvement, even if improvement does not follow automatically from indications of dissatisfactory performance. “To improve performance, organizations and individuals need the capability to control, improve, and design processes, and then to monitor the effects of this improvement work on the results. Measurement alone will not suffice (pp 1–35).”82
Monitoring performance by way of control charts has been suggested as a better approach to clinical governance in the British National Health Service. Through six case studies, Mohammed et al demonstrate how control chart monitoring of performance can distinguish normal performance from performance that is either substandard or better than usual care. “These case studies illustrate an important role for Shewhart's approach to understanding and reducing variation. They demonstrate the simplicity and power of control charts at guiding their users towards appropriate action for improvement (p 466).”83
Comments on the review methodology
No search strategy is perfect, and we may well have missed some studies where SPC was applied to healthcare QI. There are no SPC specific keywords (eg, Medical Subject Headings, MeSH) so we had to rely on text words. Studies not containing our search terms in the title or abstract could still be of potential interest although presumably we found most of the articles where SPC application was a central element. We believe the risk that we systematically missed relevant studies to be small. Therefore, our findings would probably not have changed much due to such studies that we might have missed.
The review draws on our reading, interpretation and selection of predominantly qualitative data—in the form of text and figures—in the included articles to answer the questions in our data abstraction form. The questions we addressed, the answers we derived from the studies, and the ways we synthesised the findings are not the only ways to approach this dataset. Furthermore, each member of the review team brought different knowledge and experiences of relevance to the review, potentially challenging the reliability of our analysis. An attempt was made to reduce that risk by having one investigator read all data abstraction forms, and obtain clarifications or additional data from the original articles when needed. That investigator also conducted the initial data synthesis, which was then reviewed by the entire team and the two outside experts. Although other interpretations and syntheses of these data are possible, we believe that ours are plausible and hope they are useful.
The methods for reviewing studies based primarily on qualitative data in healthcare are less well developed than the more established methods for quantitative systematic reviews, and they are in a phase of development and diversification.13,84,85 Among the different methods for synthesising evidence, our approach is best characterised as an interpretive (rather than integrative) review applying thematic analysis—it “involves the identification of prominent or recurrent themes in the literature, and summarising the findings of different studies under thematic headings”.86 There is no gold standard for how to conduct reviews of primarily qualitative studies. Our response to this uncertainty has been to use the best ideas we could find, and to be explicit about our approach to allow readers to assess the findings and their provenance.
The main limitation of this review is the uncertainty regarding the methodological quality of many of the primary studies. Assessment of quality of qualitative studies is still under debate, and there is no consensus on whether at all, or, if so, how to conduct such assessments.84 We reviewed all the studies that satisfied our inclusion criteria and made no further quality assessment. Therefore our findings should be considered as tentative indications of benefits, limitations, etc to be corroborated, or rejected, by future research. The main strength of this review is our systematic and explicit approach to searching and including studies for review, and to data abstraction using a standardised form. It has helped generate an overview of how SPC has been applied to healthcare QI with both breadth and depth—similar to the benefits of thematic analysis reported by investigators reviewing young people's views on health and health behaviour.87
In conclusion, this review indicates how SPC has been applied to healthcare QI with substantial benefits to diverse stakeholders. Although there are important limitations and barriers regarding its application, when applied correctly SPC is a versatile tool which can enable stakeholders to manage change in healthcare and improve patients' health.
Acknowledgements
We thank Ms Christine Wickman, Information Specialist at the Karolinska Institutet Library, for expert assistance in conducting the database searches. We also acknowledge the pilot work conducted by Ms Miia Maunuaho as a student project at Helsinki University, supervised by Professor Brommels, which provided a starting point for this study. We thank Professor Duncan Neuhauser, Case Western Reserve University, Cleveland, Ohio, USA, and Professor Bo Bergman, Chalmers University of Technology, Gothenburg, Sweden, for their helpful comments on an earlier version of this manuscript. We thank Dr Rebecca Popenoe for her editorial assistance.
Abbreviations
MRSA - methicillin resistant Staphylococcus aureus
PEFR - peak expiratory flow rate
QI - quality improvement
RCT - randomised controlled trial
SPC - statistical process control
Appendix A
Database search strategy
Web of Science (1986 – 11 June 2004)
TS [topic search] = ((statistical process control or statistical quality control or control chart* or (design of experiment and doe)) and (medical or nurs* or patient* or clinic* or healthcare or health care))
We limited the search to articles in English only which reduced the number of hits from 167 to 159. We saved these 159 titles with abstracts in an EndNote library. Using a similar strategy, we searched the following databases through Ovid:
Ovid MEDLINE(R) (1966 to week 1, June 2004)
EMBASE (1988 to week 24, 2004)
CINAHL (1982 to week 1, June 2004)
PsycINFO (1985 to week 5, May 2004)
This yielded 287 hits, including many duplicates, which we saved in the same EndNote library as above.
Centre for Reviews and Dissemination (CRD)
We searched all CRD databases and found two articles which we also added to our EndNote library.
Footnotes
Funding: No dedicated funding was received for this study. All coauthors were supported by their respective employers in conducting this research as part of their work.
Competing interests: None.
References
- 1.Burnes B.Managing change: a strategic approach to organisational dynamics, 3rd edn. London: Financial Times/Prentice Hall, 2000
- 2.Wheeler D J, Chambers D S.Understanding statistical process control, 2nd edn. Knoxville, TN: SPC Press, 1992
- 3.Fisher L M, Humphries B L. Statistical quality control of rabbit brain thromboplastin for clinical use in the prothrombin time determination. Am J Clin Pathol 196645148–152. [DOI] [PubMed] [Google Scholar]
- 4.Carey R G.Improving healthcare with control charts: basic and advanced SPC methods and case studies. Milwaukee: ASQ Quality Press, 2003
- 5.Daniels S, Johnson K, Johnson C. Quality glossary. 2006 [cited 28 June 2006], http://www.asq.org/glossary/s.html
- 6.Blumenthal D. Applying industrial quality management science to physicians' clinical decisions. In: Blumenthal D, Scheck A, eds. Improving clinical practice: total quality management and the physician. San Francisco: Jossey‐Bass Publishers, 199525–49.
- 7.Berwick D M. A primer on leading the improvement of systems. BMJ 1996312619–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berwick D M. Controlling variation in health care: a consultation from Walter Shewhart. Med Care 1991291212–1225. [DOI] [PubMed] [Google Scholar]
- 9.Benneyan J C, Lloyd R C, Plsek P E. Statistical process control as a tool for research and healthcare improvement. Qual Saf Health Care 200312458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plsek P E. Quality improvement methods in clinical medicine. Pediatrics 1999103(1 Suppl E)203–214. [PubMed] [Google Scholar]
- 11.Wilcock P M, Thomson R G. Modern measurement for a modern health service. Qual Health Care 20009199–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grimshaw J, McAuley L M, Bero L A.et al Systematic reviews of the effectiveness of quality improvement strategies and programmes. Qual Saf Health Care 200312298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mays N, Roberts E, Popay J. Synthesising research evidence. In: Fulop N, Allen P, Clarke A, et al, eds. Studying the organisation and delivery of health services: research methods. London: Routledge, 2001188–220.
- 14.Fulop N, Allen P, Clarke A.et al Issues in studying the organisation and delivery of health services. In: Fulop N, Allen P, Clarke A, et al, eds. Studying the organisation and delivery of health services: research methods. London: Routledge, 20011–23.
- 15.Re R N, Krousel‐Wood M A. How to use continuous quality improvement theory and statistical quality control tools in a multispecialty clinic [see comment]. Qrb Qual Rev Bull 199016391–397. [DOI] [PubMed] [Google Scholar]
- 16.Schnelle J F, Newman D R, Fogarty T E.et al Assessment and quality‐control of incontinence care in long‐term nursing facilities. J Am Geriatr Soc 199139165–171. [DOI] [PubMed] [Google Scholar]
- 17.Bluth E I, Havrilla M, Blakeman C. Quality improvement techniques: value to improve the timeliness of preoperative chest radiographic reports. AJR Am J Roentgenol 1993160995–998. [DOI] [PubMed] [Google Scholar]
- 18.McKenzie L. Process management: two control charts. Health Care Superv 19931270–81. [DOI] [PubMed] [Google Scholar]
- 19.Rollo J L, Fauser B A. Computers in total quality management. Statistical process control to expedite stats. Arch Pathol Lab Med 1993117900–905. [PubMed] [Google Scholar]
- 20.Dey M L, Sluyter G V, Keating J E. Statistical process control and direct care staff performance. J Ment Health Adm 199421201–209. [DOI] [PubMed] [Google Scholar]
- 21.Guinane C S, Sikes J I, Wilson R K. Using the PDSA cycle to standardize a quality assurance program in a quality improvement‐driven environment. Jt Comm J Qual Improv 199420696–705. [DOI] [PubMed] [Google Scholar]
- 22.Laffel G, Luttman R, Zimmerman S. Using control charts to analyze serial patient‐related data. Qual Manag Health Care 1994370–77. [PubMed] [Google Scholar]
- 23.Nelson F E, Hart M K, Hart R F. Application of control chart statistics to blood pressure measurement variability in the primary care setting. J Am Acad Nurse Pract 1994617–28. [DOI] [PubMed] [Google Scholar]
- 24.Carey R G, Teeters J L. CQI case‐study—reducing medication errors. Jt Comm J Qual Improv 199521232–237. [DOI] [PubMed] [Google Scholar]
- 25.Oniki T A, Clemmer T P, Arthur L K.et al Using statistical quality control techniques to monitor blood glucose levels. Proc Annu Symp Comput Appl Med Care 1995586–590. [PMC free article] [PubMed]
- 26.Shaha S H. Acuity systems and control charting. Qual Manag Health Care 1995322–30. [DOI] [PubMed] [Google Scholar]
- 27.Ziegenfuss J T, Jr, McKenna C K. Ten tools of continuous quality improvement: a review and case example of hospital discharge. Am J Med Qual 199510213–220. [DOI] [PubMed] [Google Scholar]
- 28.Johnson C C, Martin M. Effectiveness of a physician education program in reducing consumption of hospital resources in elective total hip replacement. South Med J 199689282–289. [DOI] [PubMed] [Google Scholar]
- 29.Piccirillo J F. The use of patient satisfaction data to assess the impact of continuous quality improvement efforts. Arch Otolaryngol Head Neck Surg 19961221045–1048. [DOI] [PubMed] [Google Scholar]
- 30.Shahian D M, Williamson W A, Svensson L G.et al Applications of statistical quality control to cardiac surgery. Ann Thorac Surg 1996621351–8 discussion 89. [DOI] [PubMed] [Google Scholar]
- 31.Tsacle E G, Aly N A. An expert system model for implementing statistical process control in the health care industry. Computers & Industrial Engineering 199631447–450. [Google Scholar]
- 32.Cornelissen G, Halberg F, Hawkins D.et al Individual assessment of antihypertensive response by self‐starting cumulative sums. J Med Eng Technol 199721111–120. [DOI] [PubMed] [Google Scholar]
- 33.Linford L H, Clemmer T P, Oniki T A. Development of a blood glucose protocol using statistical quality control techniques. Nutr Clin Pract 19971238–41. [DOI] [PubMed] [Google Scholar]
- 34.Ornstein S M, Jenkins R G, Lee F W.et al The computer‐based patient record as a CQI tool in a family medicine center. Jt Comm J Qual Improv 199723347–361. [DOI] [PubMed] [Google Scholar]
- 35.Boggs P B, Wheeler D, Washburne W F.et al Peak expiratory flow rate control chart in asthma care: chart construction and use in asthma care. Ann Allergy Asthma Immunol 199881552–562. [DOI] [PubMed] [Google Scholar]
- 36.Konrad C, Gerber H R, Schuepfer G.et al Transurethral resection syndrome: effect of the introduction into clinical practice of a new method for monitoring fluid absorption. J Clin Anesth 199810360–365. [DOI] [PubMed] [Google Scholar]
- 37.Nelson E C, Splaine M E, Batalden P B.et al Building measurement and data collection into medical practice. Ann Intern Med 1998128460–466. [DOI] [PubMed] [Google Scholar]
- 38.Solodky C, Chen H G, Jones P K.et al Patients as partners in clinical research—a proposal for applying quality improvement methods to patient care. Med Care 199836AS13–AS20. [DOI] [PubMed] [Google Scholar]
- 39.Vitez T S, Macario A. Setting performance standards for an anesthesia department. J Clin Anesth 199810166–175. [DOI] [PubMed] [Google Scholar]
- 40.Boggs P B, Hayati F, Washburne W F.et al Using statistical process control charts for the continual improvement of asthma care. Jt Comm J Qual Improv 199925163–181. [DOI] [PubMed] [Google Scholar]
- 41.Konrad C, Gerber H, Schupfer G.et al Detection of fluid volume absorption by end‐tidal alcohol monitoring in patients undergoing endoscopic renal pelvic surgery. J Clin Anesth 199911386–390. [DOI] [PubMed] [Google Scholar]
- 42.Levett J M, Carey R G. Measuring for improvement: from Toyota to thoracic surgery. Ann Thorac Surg 199968353–358. [DOI] [PubMed] [Google Scholar]
- 43.Pollard J B, Garnerin P. Outpatient preoperative evaluation clinic can lead to a rapid shift from inpatient to outpatient surgery: a retrospective review of perioperative setting and outcome. J Clin Anesth 19991139–45. [DOI] [PubMed] [Google Scholar]
- 44.Purba M. Use of a control chart to monitor diarrhoea admissions: a quality improvement exercise in West Kalimantan Provincial Hospital, Pontianak, Indonesia. J Qual Clin Pract 199919145–147. [DOI] [PubMed] [Google Scholar]
- 45.Schwab R A, DelSorbo S M, Cunningham M R.et al Using statistical process control to demonstrate the effect of operational interventions on quality indicators in the emergency department. J Healthc Qual 19992138–41. [DOI] [PubMed] [Google Scholar]
- 46.Bonetti P O, Waeckerlin A, Schuepfer G.et al Improving time‐sensitive processes in the intensive care unit: the example of “door‐to‐needle time” in acute myocardial infarction. Int J Qual Health Care 200012311–317. [DOI] [PubMed] [Google Scholar]
- 47.Ratcliffe M B, Khan J H, Magee K M.et al Collection of process data after cardiac surgery: Initial implementation with a Java‐based intranet applet. Ann Thorac Surg 2000691817–1821. [DOI] [PubMed] [Google Scholar]
- 48.Saturno P J, Felices F, Segura J.et al Reducing time delay in the thrombolysis of myocardial infarction: an internal quality improvement project. Am J Med Qual 20001585–93. [DOI] [PubMed] [Google Scholar]
- 49.Sinanan M, Wicks K, Peccoud M.et al Formula for surgical practice resuscitation in an academic medical center. Am J Surg 2000179417–421. [DOI] [PubMed] [Google Scholar]
- 50.Staker L V. Changing clinical practice by improving systems: the pursuit of clinical excellence through practice‐based measurement for learning and improvement. Qual Manag Health Care 200091–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alemi F, Oliver D W. Tutorial on risk‐adjusted P‐charts. Qual Manag Health Care 2001101–9. [PubMed] [Google Scholar]
- 52.Alemi F, Sullivan T. Tutorial on risk adjusted X‐bar charts: applications to measurement of diabetes control. Qual Manag Health Care 2001957–65. [DOI] [PubMed] [Google Scholar]
- 53.Aronsky D, Kendall D, Merkley K.et al A comprehensive set of coded chief complaints for the emergency department. Acad Emerg Med 20018980–989. [DOI] [PubMed] [Google Scholar]
- 54.Caron A, Neuhauser D V. Health care organization improvement reports using control charts for key quality characteristics: ORYX measures as examples. Qual Manag Health Care 2001928–39. [DOI] [PubMed] [Google Scholar]
- 55.Quinn D C, Graber A L, Elasy T A.et al Overcoming turf battles: developing a pragmatic, collaborative model to improve glycemic control in patients with diabetes. Jt Comm J Qual Improv 200127255–264. [DOI] [PubMed] [Google Scholar]
- 56.Roman S H, Chassin M R. Windows of opportunity to improve diabetes care when patients with diabetes are hospitalized for other conditions. Diabetes Care 2001241371–1376. [DOI] [PubMed] [Google Scholar]
- 57.Boelle P Y, Bonnet F, Valleron A J. An integrated system for significant anaesthetic events monitoring. J Am Med Inform Assoc 20029S23–S27. [PMC free article] [PubMed] [Google Scholar]
- 58.Burnett L, Chesher D, Burnett J R. Optimizing the availability of “stat” laboratory tests using Shewhart “C” control charts. Ann Clin Biochem 200239140–144. [DOI] [PubMed] [Google Scholar]
- 59.Carey R G. Improving patient satisfaction: a control chart case study. J Ambul Care Manage 20022578–83. [DOI] [PubMed] [Google Scholar]
- 60.Chu J, Neuhauser D V, Schwartz I.et al The efficacy of automated/electrical twitch obtaining intramuscular stimulation (atoims/etoims) for chronic pain control: evaluation with statistical process control methods. Electromyogr Clin Neurophysiol 200242393–401. [PubMed] [Google Scholar]
- 61.Curran E T, Benneyan J C, Hood J. Controlling methicillin‐resistant Staphylococcus aureus: A feedback approach using annotated statistical process control charts. Infect Control Hosp Epidemiol 20022313–18. [DOI] [PubMed] [Google Scholar]
- 62.Marshall T, Mohammed M A, Lim H T. Understanding variation for clinical governance: an illustration using the diagnosis and treatment of sore throat. Br J Gen Pract 200252277–283. [PMC free article] [PubMed] [Google Scholar]
- 63.Stewart L J, Greisler D. Measuring primary care practice performance within an integrated delivery system: a case study. J Healthc Manag 200247250–261. [PubMed] [Google Scholar]
- 64.Fasting S, Gisvold S E. Statistical process control methods allow the analysis and improvement of anesthesia care. Can J Anaesth 200350767–774. [DOI] [PubMed] [Google Scholar]
- 65.Hyrkas K, Lehti K. Continuous quality improvement through team supervision supported by continuous self‐monitoring of work and systematic patient feedback. J Nurs Manag 200311177–188. [DOI] [PubMed] [Google Scholar]
- 66.Marshall T, Mohammed M A. Understanding variation in quality improvement: the treatment of sore throats in primary care. Fam Pract 20032069–73. [DOI] [PubMed] [Google Scholar]
- 67.Mertens W C, Higby D J, Brown D.et al Improving the care of patients with regard to chemotherapy‐induced nausea and emesis: the effect of feedback to clinicians on adherence to antiemetic prescribing guidelines. J Clin Oncol 2003211373–1378. [DOI] [PubMed] [Google Scholar]
- 68.Norberg A, Christopher N C, Ramundo M L.et al Contamination rates of blood cultures obtained by dedicated phlebotomy vs intravenous catheter. JAMA 2003289726–729. [DOI] [PubMed] [Google Scholar]
- 69.Rosow E, Adam J, Coulombe K.et al Virtual instrumentation and real‐time executive dashboards. Solutions for health care systems. Nurs Adm Q 20032758–76. [DOI] [PubMed] [Google Scholar]
- 70.Alemi F, Neuhauser D. Time‐between control charts for monitoring asthma attacks. Jt Comm J Qual Saf 20043095–102. [DOI] [PubMed] [Google Scholar]
- 71.Hart M K, Robertson J W, Hart R F.et al Application of variables control charts to risk‐adjusted time‐ordered healthcare data .[comment]. Qual Manag Health Care 20041399–119. [DOI] [PubMed] [Google Scholar]
- 72.Nelson E C, Batalden P B, Huber T P.et al Microsystems in health care: Part 1. Learning from high‐performing front‐line clinical units. Jt Comm J Qual Improv 200228472–493. [DOI] [PubMed] [Google Scholar]
- 73.Olsson J, Elg M, Molfenter T. Reflections on transnational transferability of improvement technologies—a comparison of factors for successful change in the United States and northern Europe. Qual Manag Health Care 200312259–269. [DOI] [PubMed] [Google Scholar]
- 74.Thor J, Wittlov K, Herrlin B.et al Learning helpers: how they facilitated improvement and improved facilitation—lessons from a hospital‐wide quality improvement initiative. Qual Manag Health Care 20041360–74. [DOI] [PubMed] [Google Scholar]
- 75.Davidoff F, Batalden P. Toward stronger evidence on quality improvement. Draft publication guidelines: the beginning of a consensus project. Qual Saf Health Care 200514319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pronovost P, Wachter R. Proposed standards for quality improvement research and publication: one step forward and two steps back. Qual Saf Health Care 200615152–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Eccles M, Grimshaw J, Campbell M.et al Research designs for studies evaluating the effectiveness of change and improvement strategies. Qual Saf Health Care 20031247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Diaz M, Neuhauser D. Pasteur and parachutes: when statistical process control is better than a randomized controlled trial. Qual Saf Health Care 200514140–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Neuhauser D, Diaz M. Quality improvement research: are randomised trials necessary? Qual Saf Health Care 20071677–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Benneyan J C, Borgman A D. Risk‐adjusted sequential probability ratio tests and longitudinal surveillance methods [see comment] [comment]. Int J Qual Health Care 2003155–6. [DOI] [PubMed] [Google Scholar]
- 81.Lim T O. Statistical process control tools for monitoring clinical performance. Int J Qual Health Care 2003153–4. [DOI] [PubMed] [Google Scholar]
- 82.Berwick D M, James B, Coye M J. Connections between quality measurement and improvement. Med Care 200341(1 Suppl)I30–I38. [DOI] [PubMed] [Google Scholar]
- 83.Mohammed M A, Cheng K K, Rouse A.et al Bristol, Shipman, and clinical governance: Shewhart's forgotten lessons. Lancet 2001357463–467. [DOI] [PubMed] [Google Scholar]
- 84.Mays N, Pope C, Popay J. Systematically reviewing qualitative and quantitative evidence to inform management and policy‐making in the health field. J Health Serv Res Policy 200510(Suppl 1)6–20. [DOI] [PubMed] [Google Scholar]
- 85.Pawson R, Greenhalgh T, Harvey G.et al Realist review—a new method of systematic review designed for complex policy interventions. J Health Serv Res Policy 200510(Suppl 1)21–34. [DOI] [PubMed] [Google Scholar]
- 86.Dixon‐Woods M, Agarwal S, Jones D.et al Synthesising qualitative and quantitative evidence: a review of possible methods. J Health Serv Res Policy 20051045–53. [DOI] [PubMed] [Google Scholar]
- 87.Harden A, Garcia J, Oliver S.et al Applying systematic review methods to studies of people's views: an example from public health research. J Epidemiol Community Health 200458794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]