Abstract
Background
Although intensivist physician staffing is associated with improved outcomes in critical care, little is known about the mechanism leading to this observation.
Objective
To determine the relationship between intensivist staffing and select process‐based quality indicators in the intensive care unit.
Research design
Retrospective cohort study in 29 academic hospitals participating in the University HealthSystem Consortium Mechanically Ventilated Patient Bundle Benchmarking Project.
Patients
861 adult patients receiving prolonged mechanical ventilation in an intensive care unit.
Results
Patient‐level information on physician staffing and process‐of‐care quality indicators were collected on day 4 of mechanical ventilation. By day 4, 668 patients received care under a high intensity staffing model (primary intensivist care or mandatory consult) and 193 patients received care under a low intensity staffing model (optional consultation or no intensivist). Among eligible patients, those receiving care under a high intensity staffing model were more likely to receive prophylaxis for deep vein thrombosis (risk ratio 1.08, 95% CI 1.00 to 1.17), stress ulcer prophylaxis (risk ratio 1.10, 95% CI 1.03 to 1.18), a spontaneous breathing trial (risk ratio 1.37, 95% CI 0.97 to 1.94), interruption of sedation (risk ratio 1.64, 95% CI 1.13 to 2.38) and intensive insulin treatment (risk ratio 1.40, 95% CI 1.18 to 1.79) on day 4 of mechanical ventilation. Models accounting for clustering by hospital produced similar estimates of the staffing effect, except for prophylaxis against thrombosis and stress ulcers.
Conclusions
High intensity physician staffing is associated with increased use of evidence‐based quality indictors in patients receiving mechanical ventilation.
Considerable observational evidence suggests that staffing the intensive care unit (ICU) with a doctor trained in critical care medicine is associated with improved outcomes, including lower mortality and shorter lengths of stay.1 This finding has led some payer groups and professional societies to call for expanded use of the intensivist‐led model of care for critically ill patients.2,3,4 Despite the large body of literature associating intensivists with outcome, there is little information exactly how intensivists achieve this improvement.5,6 Specifically, there are few studies showing that patients under the care of an intensivist receive higher quality care in the form of evidence‐based care practices. Given the potential expense of adopting the intensivist model,7 the predicted shortage of trained intensivists,8 and the current low rate of use of an all‐intensivist model of care among hospitals,9 it is important to better understand the ways in which staffing is related to the process of care in the ICU. A demonstration of a link between intensivist staffing and process‐of‐care quality indicators would strengthen the argument for universal intensivist staffing and also give insight into other care strategies in situations in which the intensivist model is not feasible.10
This study aimed to determine the relationship between intensivist staffing and process‐based quality indicators. We analysed data from the 2005 University HealthSystem Consortium (UHC) Mechanically Ventilated Patient Bundle Benchmarking Project, a multicentre study evaluating the delivery of standardised quality measures to critically ill patients in academic ICUs.
Methods
Study design and patients
We conducted a retrospective cohort study using data from the UHC Mechanically Ventilated Patient Bundle Benchmarking Project, a multicentre study of process‐based quality indicators in the ICU designed as a quality improvement initiative. This work used a previously collected dataset with no personal identifiers and therefore received exempt status from the University of Washington institutional review board.
A total of 29 US academic hospitals participated in the project. Each hospital collected detailed patient‐level data on 30 consecutive ICU patients receiving mechanical ventilation for greater than 96 h, who were discharged or who expired before 1 April 2005. Subjects were identified using the International Classification of Disease‐Clinical Modification, 9th Revision code 96.72 (continuous ventilator support for at least 96 h). We excluded patients younger than 18 years of age, those with a long‐term tracheotomy on admission and those receiving only non‐invasive ventilation. Local staff collected the data at each site. To ensure reliability in coding and data entry, each chart abstracter was given standardised training via teleconference. They also received centralised support from UHC staff as needed, and used an uniform online data submission tool.
Variables
Data on demographic information, the presence of select comorbidities and the primary diagnosis were collected for each patient on day 4 of mechanical ventilation. Day 4 was chosen because it is after the initial period of resuscitation and stabilisation, yet by our case definition would still be a day of mechanical ventilation for all patients. The primary exposure was the highest level of intensivist involvement by day 4 of mechanical ventilation at the patient level, as determined from the medical record. An intensivist was defined as a US board‐certified doctor with specialty training or certification in the subspecialty of critical care.11 The intensivist's participation was assigned into one of six categories:
primary responsibility as mandated by ICU policy;
primary responsibility by request of the attending doctor;
consultation as mandated by ICU policy;
consultation by request of the attending doctor;
no role by day 4;
no intensivist available.
For the analysis, staffing was further categorised into high intensity (primary responsibility or mandatory consult; categories 1–3) or low intensity (optional consult or no intensivist; categories 4–6).12
The primary outcome was whether the patient received select quality indicators on ventilator day 4, as determined from the medical record. These included deep vein thrombosis (DVT) prophylaxis,13 stress ulcer prophylaxis,14 a spontaneous breathing trial,15 interruption of sedative infusion,16 and intensive insulin treatment for hyperglycaemia.17,18 These quality indicators were chosen because of their strength of association with outcome and the ability to validly abstract these variables from the medical record.19 All except for intensive insulin are advocated by the Institute for Healthcare Improvement as important care processes for patients on mechanical ventilation.20 Table 1 shows the complete definitions of the quality indictors as well as eligibility requirements.
Table 1 Definitions and eligibility requirements for process measures on day 4 of mechanical ventilation.
| Measure | Definition | Exclusion criteria |
|---|---|---|
| DVT prophylaxis | Received either low dose unfractionated heparin, low molecular weight heparin or an intermittent pneumatic compression device | Primary diagnosis: DVT or PE |
| DVT or PE documented as complication or comorbidity | ||
| Fully anticoagulated with heparin or warfarin | ||
| Coagulopathy or chronic liver disease | ||
| Documented contraindication to both pharmacological anticoagulants and non‐pharmacological prophylactic devices | ||
| Stress ulcer prophylaxis | Received either an H2 receptor blocker, a proton pump inhibitor, or sucralfate | Primary diagnosis gastrointestinal bleeding |
| Documented contraindication to stress ulcer prophylaxis | ||
| Spontaneous breathing trial | T‐piece, CPAP or inspiratory pressure support ⩽5 cm H2O to test ability to breath spontaneously | FiO2 >0.50 |
| Plateau pressure >30 cm H2O | ||
| PEEP >8 cm H2O | ||
| Arterial pH <7.25 | ||
| Receiving a vasopressor other than dopamine ⩽3 μg/kg/min or dobutamine ⩽5 μg/kg/min | ||
| Sedation interruption | Continuous infusion held or the dose reduced by 50% | Not receiving continuous intravenous sedation |
| Chart evidence that patient is to be maintained in deep sedation | ||
| Intensive insulin treatment | Continuous intravenous insulin infusion used to maintain blood glucose <8.3 mmol/l (<150 mg/dl) | Highest blood glucose value <8.3 mmol/l (<150 mg/dl) without insulin |
CPAP, continuous positive airway pressure; DVT, deep vein thrombosis; FiO2, fraction of inspired oxygen; PE, pulmonary embolus; PEEP, positive end expiratory pressure.
Analysis
Demographic variables are expressed as means or proportions. We compared groups with an unpaired t test or χ2 test, as appropriate. Quality indicators are expressed as the ratio of patients receiving the indicator (numerator) to patients eligible for the indicator (denominator). The effect of staffing on the proportion of patients receiving the quality indictors is expressed as a risk ratio. Relative risk regression with generalised estimating equations (GEE) and robust variance estimators were used to account for clustering by hospital.21,22 Analyses were performed with STATA version 9.1. Since this project used previously collected data, we did not perform a formal power calculation.
Results
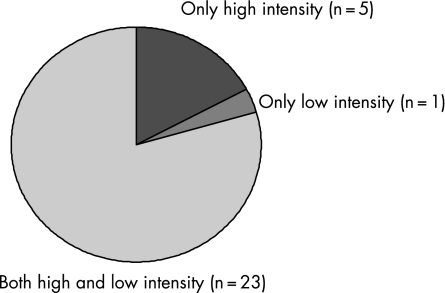
The hospitals were located in diverse regions throughout the USA (table 2). Most hospitals had multiple ICUs; most had specialty medical and surgical ICUs, and many had specialty trauma, neurological and coronary care units. Most hospitals collected data on the requested 30 patients; no hospital collected data on fewer than 25 patients. In total, 861 patients were included in the study. Most patients had some intensivist staffing presence by day 4 of mechanical ventilation. With regard to our categorisation of staffing, 668 patients received care under a high intensity model and 193 under a low intensity model. Figure 1 shows the distribution of staffing models by hospital, 23 (79%) hospitals had more than one type of staffing within the hospital, ensuring that our comparisons of staffing models were not simply comparisons of hospitals.
Table 2 Key characteristics of the hospitals participating in the study.
| Variable | Value |
|---|---|
| Region, n (%) | |
| Northeast | 6 (21) |
| South | 10 (35) |
| Midwest | 8 (28) |
| West | 5 (17) |
| ICUs per hospital, mean (SD), range | 4.3 (2.1), 1–8 |
| Total hospital beds, mean (SD) | 504 (265) |
| Total ICU beds, mean (SD) | 46 (23) |
| Specialty ICU types per hospital, n (%) | |
| Medical ICU | 19 (70) |
| Surgical ICU | 20 (74) |
| Coronary care unit | 13 (48) |
| Trauma/burn unit | 13 (48) |
| Neurological ICU | 12 (44) |
| Subjects per hospital, mean (SD), range | 30 (3), 25–41 |
ICU, intensive care unit.
Figure 1 Variation in staffing patterns by hospital (n = 29).
Table 3 shows the patient demographics categorised by staffing model. Patients cared for under a high intensity staffing model were more likely to have respiratory failure and acute lung injury as the primary diagnosis and less likely to have stroke or intracranial haemorrhage as the primary diagnosis. Patients cared for under the high intensity model were also more likely to have a protocol for ventilator weaning.
Table 3 Patient characteristics by intensivist staffing role.
| Variable | Low intensity (n = 193) | High intensity (n = 668) | p Value |
|---|---|---|---|
| Age, mean (SD) | 61 (16) | 58 (18) | 0.01 |
| Gender (% female) | 74 (38) | 273 (41) | 0.53 |
| Primary diagnosis,* n (%) | |||
| Trauma/burn | 25 (13) | 90 (14) | 0.001 |
| Respiratory failure/ALI | 12 (6) | 86 (13) | |
| CVA/ICH | 30 (16) | 47 (7) | |
| Surgical/vascular | 11 (6) | 50 (8) | |
| Malignancy | 13 (7) | 44 (7) | |
| Sepsis | 5 (3) | 51 (8) | |
| Gastrointestinal/liver disease | 14 (7) | 41 (6) | |
| Neurosurgical | 17 (9) | 35 (5) | |
| Congestive heart failure | 14 (7) | 36 (5) | |
| Pneumonia | 9 (5) | 40 (6) | |
| Acute coronary syndrome | 14 (7) | 26 (4) | |
| COPD/asthma | 2 (1) | 16 (12) | |
| Other infection | 7 (4) | 20 (3) | |
| Other neurologic | 2 (1) | 18 (3) | |
| Other general | 18 (9) | 68 (10) | |
| Hospital admission source, n (%) | |||
| Emergency department | 110 (57) | 409 (61) | 0.53 |
| Routine direct | 32 (17) | 92 (14) | |
| Transfer from outside <~!?show=[sr]?>centre | 48 (25) | 161 (24) | |
| Other | 3 (2) | 6 (1) | |
| Comorbidities, n (%) | |||
| COPD | 34 (18) | 174 (26) | 0.02 |
| Diabetes | 59 (31) | 194 (29) | 0.68 |
| Tobacco | 50 (26) | 221 (33) | 0.06 |
ALI, acute lung injury; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; ICH, intracranial haemorrhage.
*From principal International Classification of Disease (ninth revision) diagnosis code during current admission.
Among patients eligible for each care process, those under the high intensity model were more likely to receive DVT prophylaxis, stress ulcer prophylaxis, a spontaneous breathing trial, interruption of continuous sedation and intensive insulin treatment on day 4 of mechanical ventilation (table 4). The effect of an intensivist was significant (p⩽0.05) for all of these except spontaneous breathing trial, for which a strong trend was present (p = 0.06). Relative risk regression, in which GEE was used to account for clustering by hospital, produced similar estimates (table 5) except for stress ulcer prophylaxis and DVT prophylaxis, in which the effect of an intensivist was no longer significant.
Table 4 Intensivist staffing role and process‐based quality measures on day 4 of mechanical ventilation.
| Care process | Low intensity (n = 193) | High intensity (n = 668) |
|---|---|---|
| Deep vein thrombosis prophylaxis | ||
| Eligible (n) | 156 | 541 |
| Received (n) | 128 | 478 |
| Proportion received | 0.82 | 0.88 |
| Relative risk (95% CI) | 1.00 (ref) | 1.08 (1.00 to 1.17)* |
| Stress ulcer prophylaxis | ||
| Eligible (n) | 186 | 658 |
| Received (n) | 157 | 612 |
| Proportion received | 0.84 | 0.93 |
| Relative risk (95% CI) | 1.00 (ref) | 1.10 (1.03 to 1.18)*** |
| Spontaneous breathing trial | ||
| Eligible (n) | 81 | 336 |
| Received (n) | 25 | 142 |
| Proportion received | 0.31 | 0.42 |
| Relative risk (95% CI) | 1.00 (ref) | 1.37 (0.97 to 1.94) |
| Sedation interruption | ||
| Eligible (n) | 87 | 329 |
| Received (n) | 23 | 143 |
| Proportion received | 0.26 | 0.44 |
| Relative risk (95% CI) | 1.00 (ref) | 1.64 (1.13 to 2.38)** |
| Intensive insulin treatment | ||
| Eligible (n) | 149 | 482 |
| Received (n) | 50 | 227 |
| Proportion received | 0.34 | 0.47 |
| Relative risk (95% CI) | 1.00 (ref) | 1.40 (1.18 to 1.79)** |
*p = 0.05; **p<0.01; ***p<0.001.
Table 5 Effect of an intensivist on process‐based quality indicators in the regression analysis accounting for clustering by hospital.
| Care process | Risk ratio | |
|---|---|---|
| Baseline | GEE† | |
| Deep vein thrombosis prophylaxis | 1.08 (1.00 to 1.17)* | 1.04 (0.98 to 1.10) |
| Stress ulcer prophylaxis | 1.10 (1.03 to 1.18)* | 1.02 (0.95 to 1.10) |
| Spontaneous breathing trial | 1.37 (0.97 to 1.94) | 1.24 (0.87 to 1.76) |
| Sedation interruption | 1.64 (1.13 to 2.38)* | 1.44 (1.03 to 2.03)* |
| Intensive insulin treatment | 1.40 (1.18 to 1.79)* | 1.31 (1.01 to 1.72)* |
GEE, generalized estimating equation.
*p<0.05.
†Risk ratios account for clustering by centre using relative risk regression with GEEs.
Discussion
Our study showed that in a cohort of patients receiving prolonged mechanical ventilation in academic hospitals, those receiving care under a high intensity staffing model were more likely to receive standardised quality measures on day 4 of mechanical ventilation, including DVT and stress ulcer prophylaxis, a spontaneous breathing trial, interruption of continuous sedation and intensive insulin treatment for glycaemic control. The effect of staffing on use of sedation interruption and intensive insulin treatment was particularly strong: high intensity staffing was associated with a 64% increase in the use of sedation interruption and a 40% increase in the use of intensive insulin treatment. The effect of staffing on the use of DVT and stress ulcer prophylaxis was less strong, and after accounting for clustering by centre, no longer significant. These treatments had a very high proportion of patients receiving appropriate care, making it more difficult to detect an association.
Our study indicates that intensivist physician staffing is associated with higher quality of care in ICU as defined by the use of these practices. This is an important corollary to the relatively consistent evidence that intensivist staffing is associated with improved risk adjusted mortality.12 In a traditional framework of quality measurement, healthcare quality has three interrelated domains: the structure of care, the process of care and the outcome of care.23 Past studies of staffing and quality in the ICU have mainly focused on the link between structure and outcome; few have shown the link between structure and process. This link is important because it indicates a potential mechanism by which intensivists improve outcome, lending further support to the recent calls for an increased intensivist presence in the ICU. Both the Leapfrog group, a consortium of healthcare purchasers that advocates for improved quality in healthcare, and the Society of Critical Care Medicine have actively supported universal adoption of the intensivist‐led model of care.2,3 The need for intensivists was also highlighted in the Framing Options for Critical Care in the United States (FOCCUS) report, a multidisciplinary consensus statement addressing the shortage of trained intensive care providers.24 Knowledge that intensivist‐led care is associated with improved quality as defined by standardised process measures helps support these calls for expanded use of intensivists where feasible. Overall, these data indicate that hospitals may be able to improve the quality of care in ICUs by adopting a high intensity model of care.
This study also addresses ways to improve the outcome of critically ill patients in the absence of intensivists staffing. Given the barriers to adopting the intensivist model of care25 and the shortage of trained intensivists,8 novel strategies are needed to improve care for patients who do not have access to intensivist‐led care. If part of the reason intensivists improve outcome is by enhanced use of evidence‐based care practices, perhaps the same outcomes can be achieved by expanding the use of these practices through other means, such as pharmacy and respiratory treatment driven care protocols.26,27,28 Other potential targets include nurse staffing,29 multidisciplinary care,30 the organisation of the critical care team,31 the presence of a pharmacist on daily rounds32 and overall unit culture.33
Limitations of the study
Our study has several limitations. We did not formally control for potential confounders such as severity of illness, differences in case mix or ICU type. Instead, we used the method of restriction to deal with potential confounding. By restricting each analysis to only those patients eligible for each care measure, we eliminated bias that might have arisen if patient eligibility differed across staffing groups or ICUs. In addition, process‐based quality measures are typically much less sensitive to confounding than outcome‐based quality measures, supporting the validity of these findings.34 Our results may also be affected by poor reliability of our chosen measures.35 If reliability is low, there could be misclassification of outcome, making it more difficult to detect an association between ICU organisation and process of care.36 Because the UHC hospitals used trained chart abstracters, standardised data entry, and have extensive experience in abstracting quality measures from the medical record,37 we expect misclassification to be minimal. There is also no reason to suspect that misclassification was different among patients with different staffing patterns, making bias improbable. The hospitals in this study were also self‐selected to participate in a quality improvement initiative, and may not be representative of academic medical centres as a whole. However, any selection bias would be expected to attenuate the observed relationship between staffing and quality, since hospitals motivated to improve quality might be more likely to use evidence‐based quality measures independent of intensivist staffing. Finally, we examined only one day of mechanical ventilation in patients ventilated for 4 days or more, as opposed to examining all days for all patients. This was done to simplify data collection and to narrow the population to those most likely to benefit from the care processes. Although this may have affected the estimates of prevalence for quality indicators, there are probably no systematic differences between the groups that would result in meaningful bias.
Conclusions
High intensity physician staffing is associated with improved quality of care for patients receiving mechanical ventilation, as defined by standardised process measures. This finding supports the need for expanded use of intensivists and highlights ways to improve the quality of care for critically ill patients in settings where intensivist staffing is not available. Future research should be directed at the relationship between intensivist staffing and other organisational attributes of ICUs to determine the best way to increase use of evidence‐based care practices for critically ill patients.
Acknowledgements
We acknowledge Mark Sloane, Judith Jacobi, and Susan Pingleton for invaluable advice regarding study design and manuscript critique, as well as the nurses and staff at the University HealthSystem Consortium Mechanically Ventilated Patient Bundle Benchmarking Project sites.
Abbreviations
DVT - deep vein thrombosis
GEE - generalised estimating equation
ICU - intensive care unit
UHC - University HealthSystem Consortium
Footnotes
Funding: This study was funded by the University HealthSystem Consortium and by the individual participating hospitals. JMK is supported by a career development award from the National Institutes of Health (K23HL082650).
Competing interests: None.
The study sponsors had no role in study design, collection, analysis and interpretation of the data, writing of the report, and the decision to submit the paper for publication.
References
- 1.Young M P, Birkmeyer J D. Potential reduction in mortality rates using an intensivist model to manage intensive care units. Eff Clin Pract 20003284–289. [PubMed] [Google Scholar]
- 2.Milstein A, Galvin R S, Delbanco S F.et al Improving the safety of health care: the Leapfrog initiative. Eff Clin Pract 20003313–316. [PubMed] [Google Scholar]
- 3.Durbin C G., Jr Team model: advocating for the optimal method of care delivery in the intensive care unit. Crit Care Med 200634S12–S17. [DOI] [PubMed] [Google Scholar]
- 4.Ewart G W, Marcus L, Gaba M M.et al The critical care medicine crisis: a call for federal action: a white paper from the critical care professional societies. Chest 20041251518–1521. [DOI] [PubMed] [Google Scholar]
- 5.Hall J B. Advertisements for ourselves—let's be cautious about interpreting outcome studies of critical care services. Crit Care Med 199927229–230. [DOI] [PubMed] [Google Scholar]
- 6.Rubenfeld G D, Kahn J. Critical care's great leap forward? Crit Care Med 2006341262–1263. [DOI] [PubMed] [Google Scholar]
- 7.Pronovost P J, Needham D M, Waters H.et al Intensive care unit physician staffing: financial modeling of the Leapfrog standard. Crit Care Med 2004321247–1253. [DOI] [PubMed] [Google Scholar]
- 8.Angus D C, Kelley M A, Schmitz R J.et al Current and projected workforce requirements for care of the critically ill and patients with pulmonary disease: can we meet the requirements of an aging population? JAMA 20002842762–2770. [DOI] [PubMed] [Google Scholar]
- 9.Angus D C, Shorr A F, White A.et al Critical care delivery in the United States: Distribution of services and compliance with Leapfrog recommendations. Crit Care Med 2006341016–1024. [DOI] [PubMed] [Google Scholar]
- 10.Angus D C, Black N. Improving care of the critically ill: institutional and health‐care system approaches. Lancet 20043631314–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The Leapfrog Group Hospital quality and safety survey, version 3.1. Washington DC: The Leapfrog Group, 2005
- 12.Pronovost P J, Angus D C, Dorman T.et al Physician staffing patterns and clinical outcomes in critically ill patients: a systematic review. JAMA 20022882151–2162. [DOI] [PubMed] [Google Scholar]
- 13.Samama M M, Cohen A T, Darmon J Y.et al A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group. N Engl J Med 1999341793–800. [DOI] [PubMed] [Google Scholar]
- 14.Messori A, Trippoli S, Vaiani M.et al Bleeding and pneumonia in intensive care patients given ranitidine and sucralfate for prevention of stress ulcer: meta‐analysis of randomised controlled trials. BMJ 20003211103–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ely E W, Baker A M, Dunagan D P.et al Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med 19963351864–1869. [DOI] [PubMed] [Google Scholar]
- 16.Kress J P, Pohlman A S, O'Connor M F.et al Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med 20003421471–1477. [DOI] [PubMed] [Google Scholar]
- 17.van den Berghe G, Wouters P, Weekers F.et al Intensive insulin therapy in the critically ill patients. N Engl J Med 20013451359–1367. [DOI] [PubMed] [Google Scholar]
- 18.Van den Berghe G, Wilmer A, Hermans G.et al Intensive insulin therapy in the medical ICU. N Engl J Med 2006354449–461. [DOI] [PubMed] [Google Scholar]
- 19.Rubin H R, Pronovost P, Diette G B. From a process of care to a measure: the development and testing of a quality indicator. Int J Qual Health Care 200113489–496. [DOI] [PubMed] [Google Scholar]
- 20.Institute for Healthcare Improvement Ventilator bundle (IHI Tool). Boston, MA: BMJ Publishing Group, 2004
- 21.Wacholder S. Binomial regression in GLIM: estimating risk ratios and risk differences. Am J Epidemiol 1986123174–184. [DOI] [PubMed] [Google Scholar]
- 22.Zeger S L, Liang K Y. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 198642121–130. [PubMed] [Google Scholar]
- 23.Donabedian A. The quality of medical care. Science 1978200856–864. [DOI] [PubMed] [Google Scholar]
- 24.Kelley M A, Angus D, Chalfin D B.et al The critical care crisis in the United States: a report from the profession. Chest 20041251514–1517. [DOI] [PubMed] [Google Scholar]
- 25.Kahn J M, Matthews F A, Angus D C.et al Barriers to implementing the Leapfrog Group recommendations for intensivist physician staffing: a survey of ICU directors. J Crit Care 20072297–103. [DOI] [PubMed] [Google Scholar]
- 26.Brook A D, Ahrens T S, Schaiff R.et al Effect of a nursing‐implemented sedation protocol on the duration of mechanical ventilation. Crit Care Med 1999272609–2615. [DOI] [PubMed] [Google Scholar]
- 27.Ely E W, Meade M O, Haponik E F.et al Mechanical ventilator weaning protocols driven by nonphysician health‐care professionals: evidence‐based clinical practice guidelines. Chest 2001120S454–S463. [DOI] [PubMed] [Google Scholar]
- 28.Holcomb B W, Wheeler A P, Ely E W. New ways to reduce unnecessary variation and improve outcomes in the intensive care unit. Curr Opin Crit Care 20017304–311. [DOI] [PubMed] [Google Scholar]
- 29.Aiken L H, Clarke S P, Sloane D M.et al Hospital nurse staffing and patient mortality, nurse burnout, and job dissatisfaction. JAMA 20022881987–1993. [DOI] [PubMed] [Google Scholar]
- 30.Young M P, Gooder V J, Oltermann M H.et al The impact of a multidisciplinary approach on caring for ventilator‐dependent patients. Int J Qual Health Care 19981015–26. [DOI] [PubMed] [Google Scholar]
- 31.Shortell S M, Zimmerman J E, Rousseau D M.et al The performance of intensive care units: does good management make a difference? Med Care 199432508–525. [DOI] [PubMed] [Google Scholar]
- 32.Montazeri M, Cook D J. Impact of a clinical pharmacist in a multidisciplinary intensive care unit. Crit Care Med 1994221044–1048. [DOI] [PubMed] [Google Scholar]
- 33.Pronovost P J, Weast B, Holzmueller C G.et al Evaluation of the culture of safety: survey of clinicians and managers in an academic medical center. Qual Saf Health Care 200312405–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubin H R, Pronovost P, Diette G B. The advantages and disadvantages of process‐based measures of health care quality. Int J Qual Health Care 200113469–474. [DOI] [PubMed] [Google Scholar]
- 35.Pronovost P J, Miller M R, Dorman T.et al Developing and implementing measures of quality of care in the intensive care unit. Curr Opin Crit Care 20017297–303. [DOI] [PubMed] [Google Scholar]
- 36.McGlynn E A, Asch S M. Developing a clinical performance measure. Am J Prev Med 19981414–21. [DOI] [PubMed] [Google Scholar]
- 37.Keroack M A, Cerese J, Cuny J.et al The relationship between evidence‐based practices and survival in patients requiring prolonged mechanical ventilation in academic medical centers. Am J Med Qual 20062191–100. [DOI] [PubMed] [Google Scholar]