Abstract
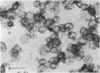
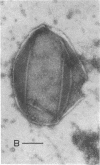
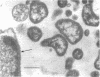
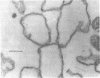
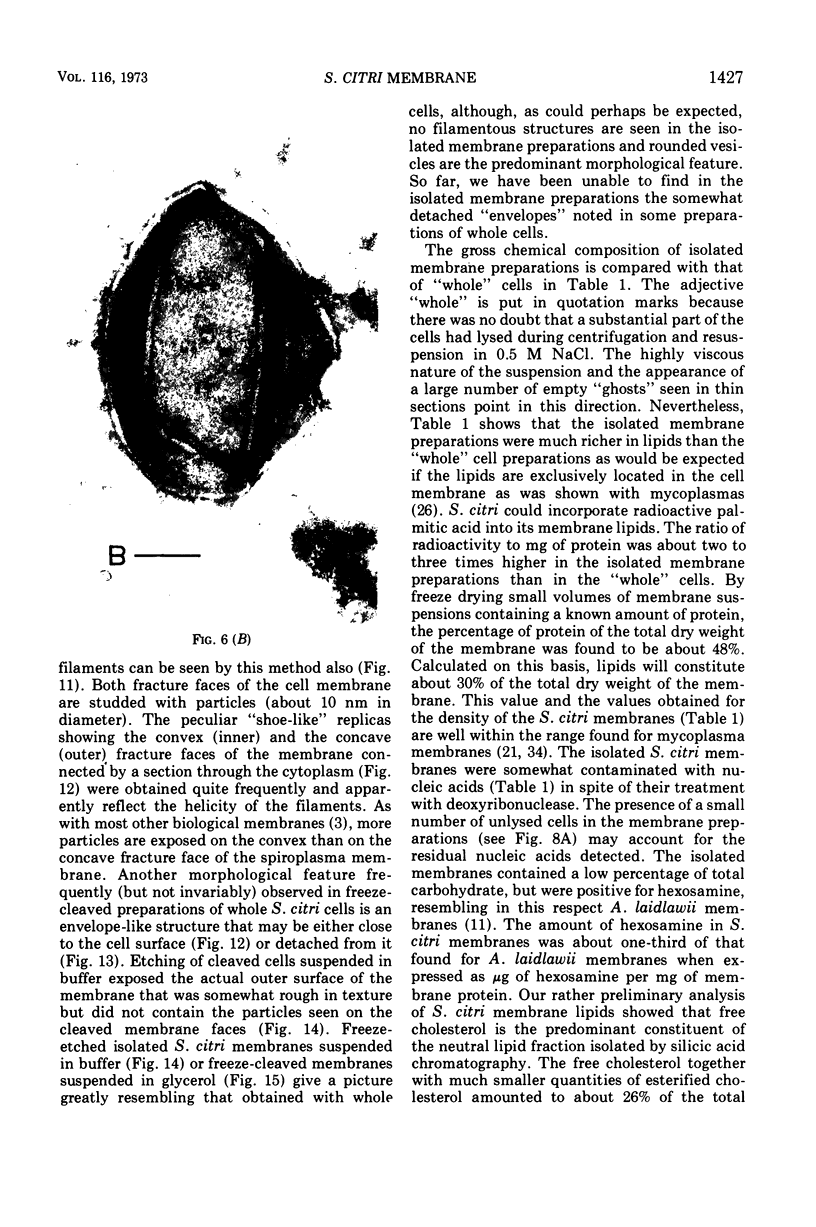
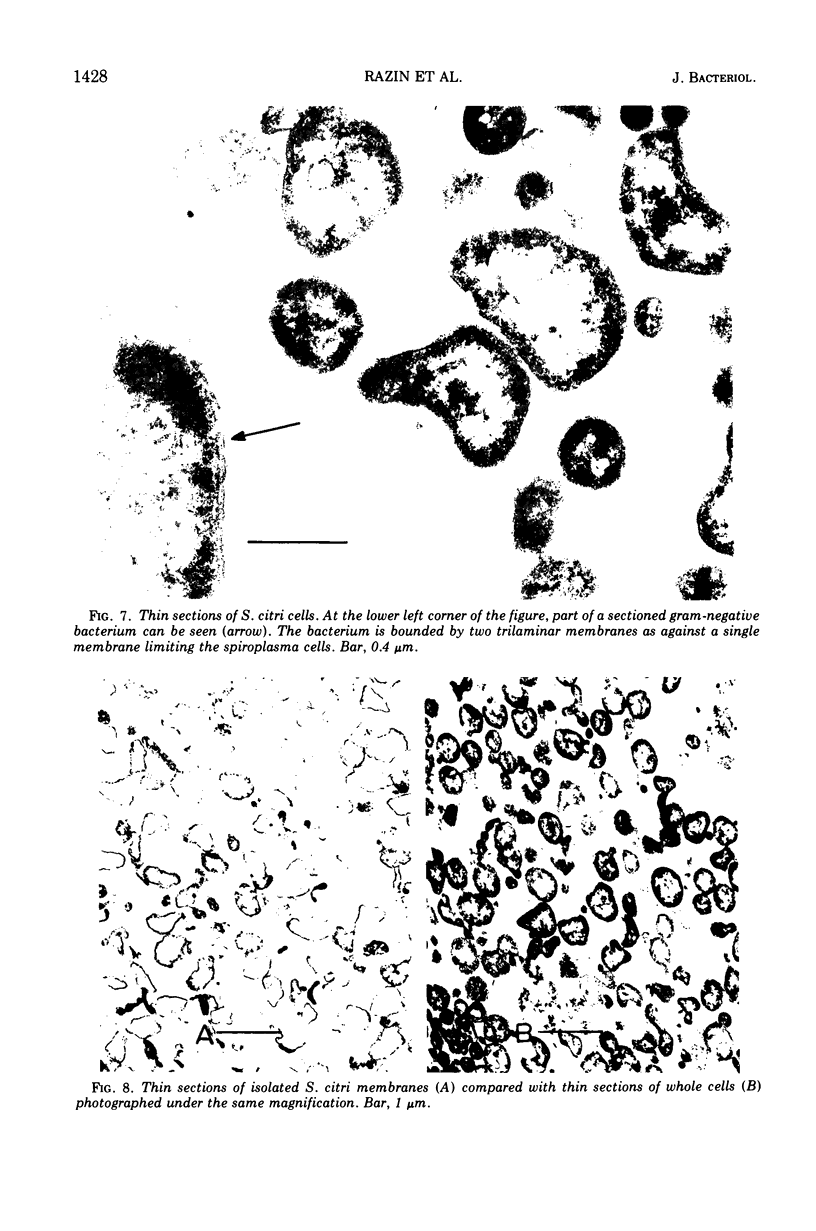
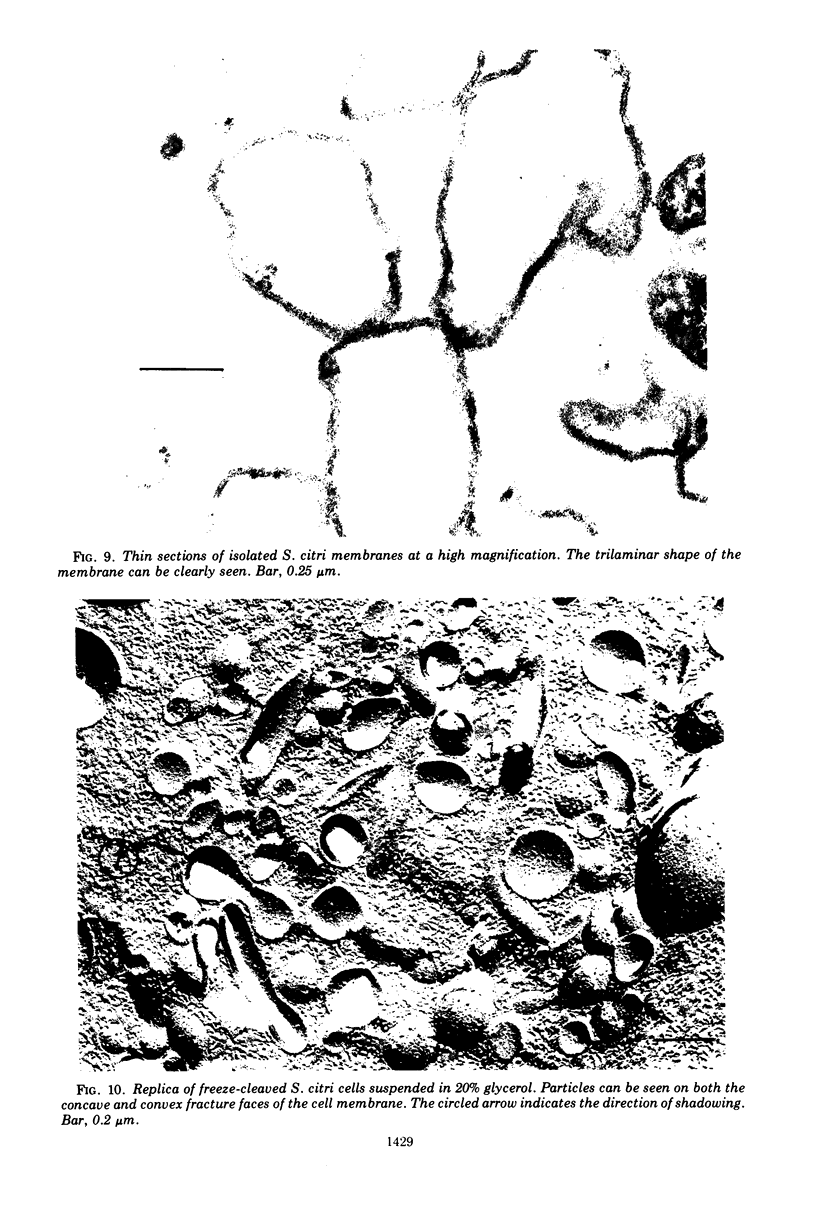
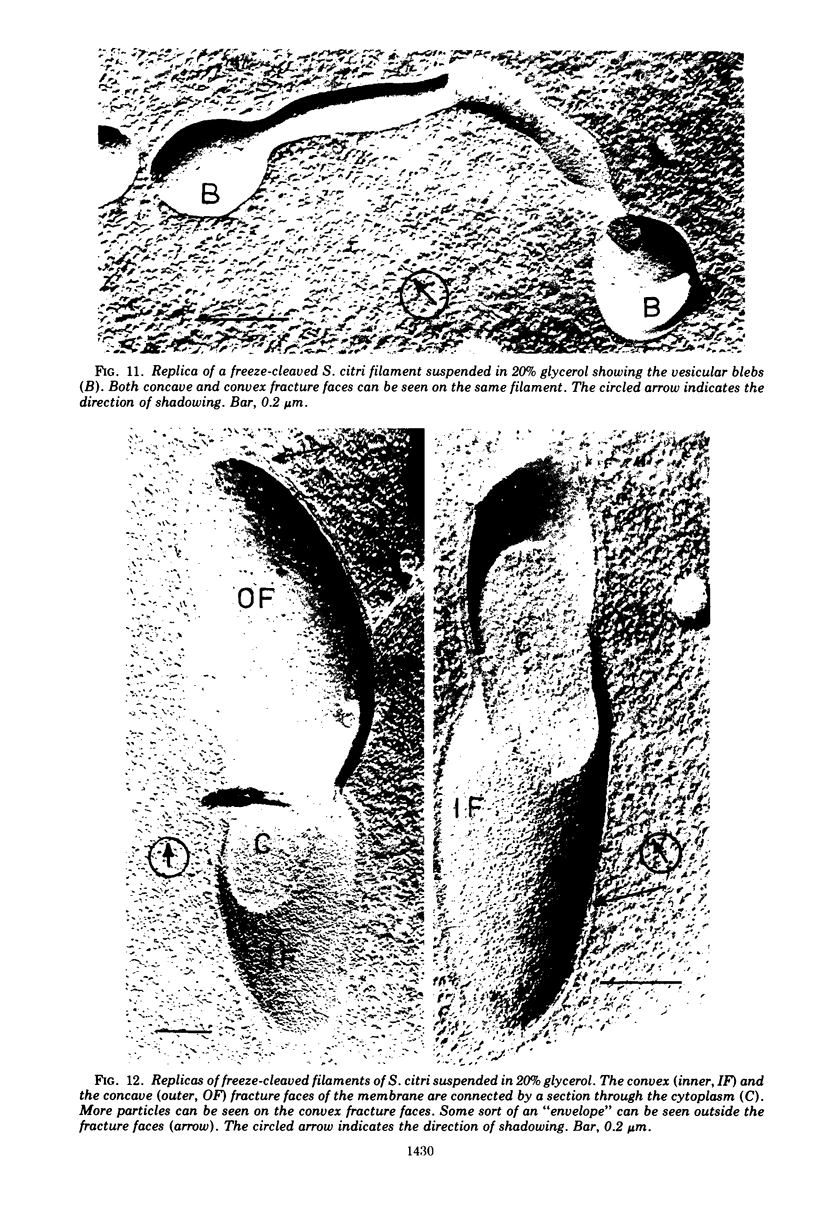
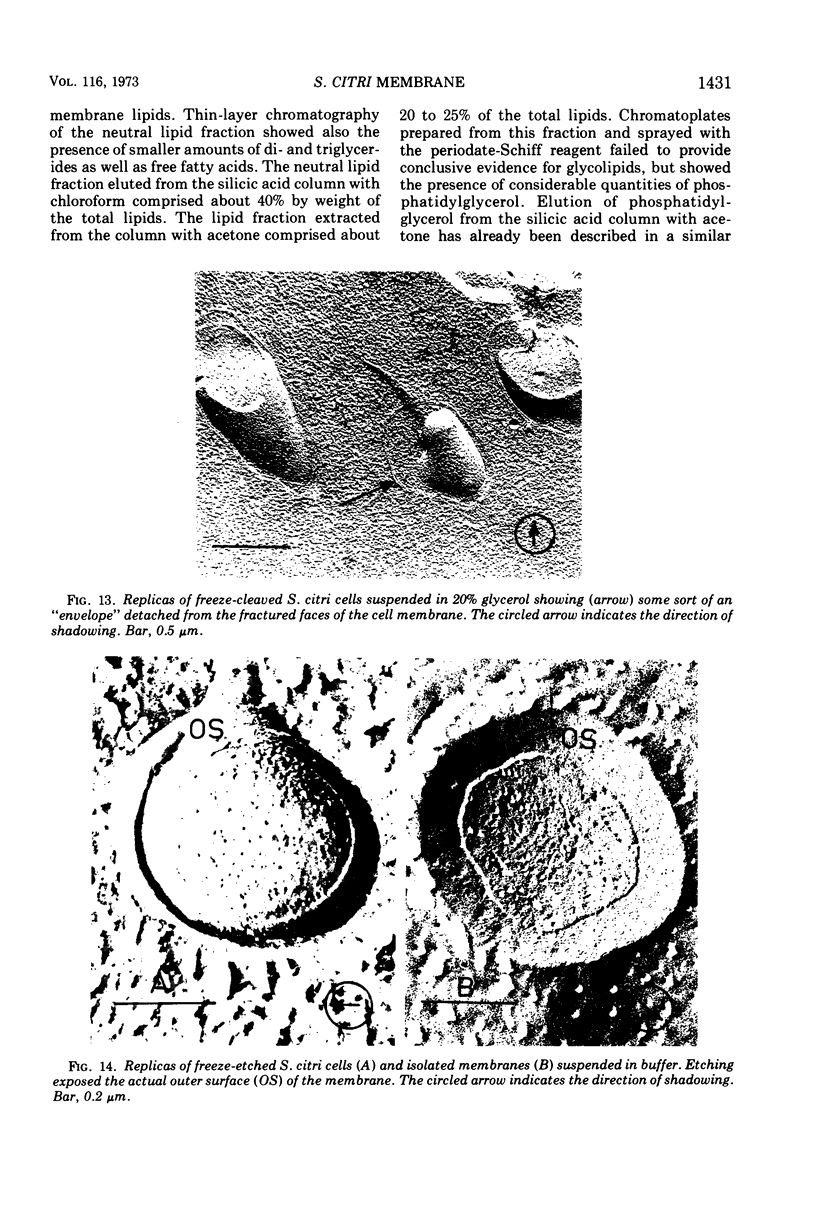
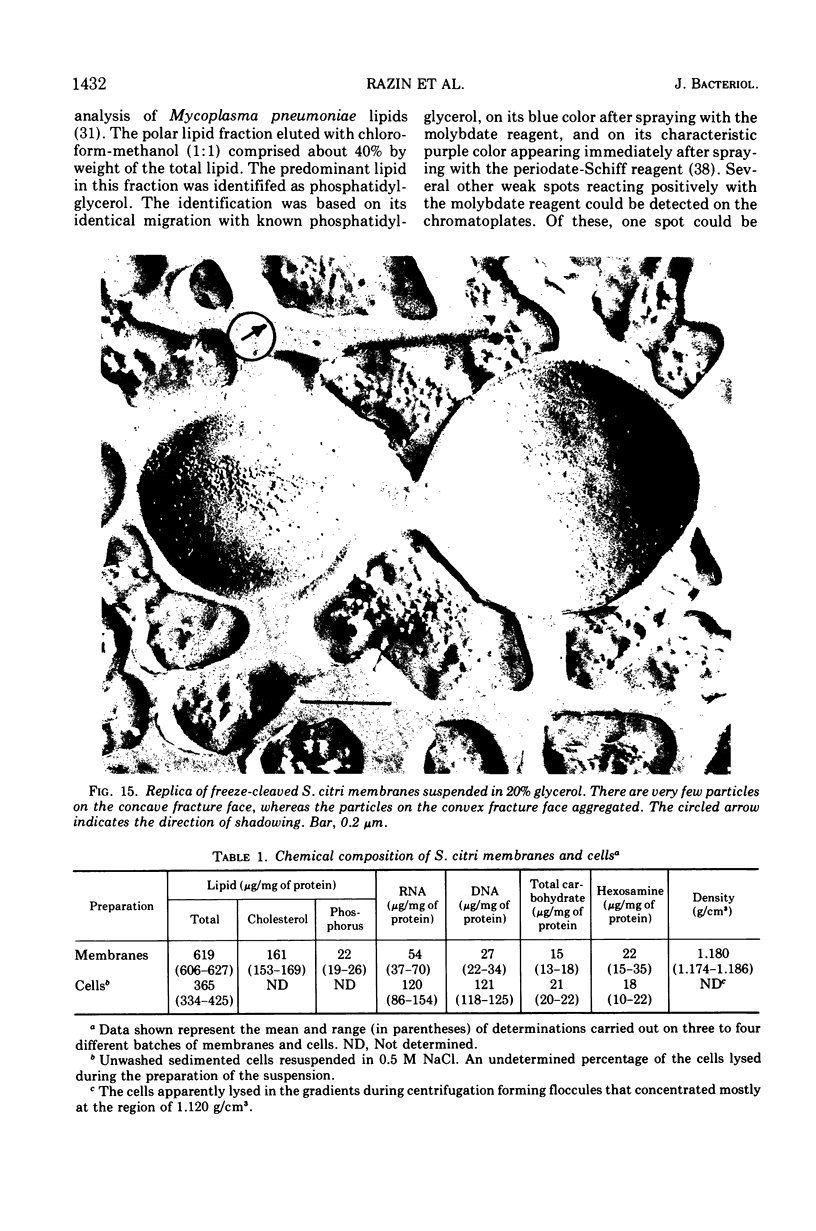
Thin sections of Spiroplasma citri, a mycoplasma-like organism isolated from citrus infected with “Stubborn” disease, showed the organisms to be limited by a single trilaminar plasma membrane. An additional outer layer could, however, be frequently seen in freeze-etched preparations of unwashed cells. The organisms were found to be extremely sensitive to lysis by osmotic shock. The cell membrane of S. citri isolated in this way resembled that of mycoplasmas in ultrastructure and gross chemical composition. The isolated membranes showed the characteristic trilaminar shape in section and the typical particle-studded fracture faces in freeze-etched preparations. Protein and lipid formed over 80% of the total dry weight of the membrane, which had a density of ~1.180 g/cm3. Cholesterol constituted over 20% of the total membrane lipid. Phosphatidyl-glycerol, synthesized by the organisms, was the major phospholipid. Significant amounts of hexosamine (15 to 35 μg/mg of membrane protein) could be found in the membrane preparations. Our results support the thesis that S. citri does not possess a cell wall, either of the gram-positive or the gram-negative type, though it may be coated by some other type of an envelope or by a slime layer, at least temporarily.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARGAMAN M., RAZIN S. CHOLESTEROL AND CHOLESTEROL ESTERS IN MYCOPLASMA. J Gen Microbiol. 1965 Jan;38:153–160. doi: 10.1099/00221287-38-1-153. [DOI] [PubMed] [Google Scholar]
- Cole R. M., Popkin T. J., Prescott B., Chanock R. M., Razin S. Electron microscopy of solubilized Acholeplasma laidlawii membrane proteins reaggregated with Mycoplasma pneumoniae glycolipids. Biochim Biophys Acta. 1971 Mar 9;233(1):76–83. doi: 10.1016/0005-2736(71)90359-2. [DOI] [PubMed] [Google Scholar]
- Cole R. M., Tully J. G., Popkin T. J., Bové J. M. Morphology, ultrastructure, and bacteriophage infection of the helical mycoplasma-like organism (Spiroplasma citri gen. nov., sp. nov.) cultured from "stubborn" disease of citrus. J Bacteriol. 1973 Jul;115(1):367–384. doi: 10.1128/jb.115.1.367-386.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DITTMER J. C., LESTER R. L. A SIMPLE, SPECIFIC SPRAY FOR THE DETECTION OF PHOSPHOLIPIDS ON THIN-LAYER CHROMATOGRAMS. J Lipid Res. 1964 Jan;5:126–127. [PubMed] [Google Scholar]
- Davis R. E., Worley J. F., Whitcomb R. F., Ishijima T., Steere R. L. Helical filaments produced by a Mycoplasma-like organism associated with corn stunt disease. Science. 1972 May 5;176(4034):521–523. doi: 10.1126/science.176.4034.521. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Morowitz H. J. Characterization of the plasma membrane of Mycoplasma laidlawii. IV. Structure and composition of membrane and aggregated components. Biochim Biophys Acta. 1968 Apr 29;150(3):385–396. doi: 10.1016/0005-2736(68)90137-5. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- FREUNDT E. A. Morphology and classification of the PPLO. Ann N Y Acad Sci. 1960 Jan 15;79:312–325. doi: 10.1111/j.1749-6632.1960.tb42693.x. [DOI] [PubMed] [Google Scholar]
- Freeman C. P., West D. Complete separation of lipid classes on a single thin-layer plate. J Lipid Res. 1966 Mar;7(2):324–327. [PubMed] [Google Scholar]
- Gatt R., Berman E. R. A rapid procedure for the estimation of amino sugars on a micro scale. Anal Biochem. 1966 Apr;15(1):167–171. doi: 10.1016/0003-2697(66)90262-4. [DOI] [PubMed] [Google Scholar]
- Gilleland H. E., Jr, Stinnett J. D., Roth I. L., Eagon R. G. Freeze-etch study of Pseudomonas aeruginosa: localization within the cell wall of an ethylenediaminetetraacetate-extractable. J Bacteriol. 1973 Jan;113(1):417–432. doi: 10.1128/jb.113.1.417-432.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliam J. M., Morowitz H. J. Characterization of the plasma membrane of Mycoplasma laidlawii. IX. Isolation and characterization of the membrane polyhexosamine. Biochim Biophys Acta. 1972 Aug 9;274(2):353–363. doi: 10.1016/0005-2736(72)90183-6. [DOI] [PubMed] [Google Scholar]
- Kahane I., Razin S. pH-dependent changes in density of plasma membranes of growing Mycoplasma laidlawii cells. FEBS Lett. 1970 Oct 16;10(4):261–264. doi: 10.1016/0014-5793(70)80643-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maramorosch K., Granados R. R., Hirumi H. Mycoplasma diseases of plants and insects. Adv Virus Res. 1970;16:135–193. doi: 10.1016/s0065-3527(08)60023-8. [DOI] [PubMed] [Google Scholar]
- Morowitz H. J., Terry T. M. Characterization of the plasma membrane of Mycoplasma laidlawii. V. Effects of selective removal of protein and lipid. Biochim Biophys Acta. 1969 Jul 15;183(2):276–294. doi: 10.1016/0005-2736(69)90084-4. [DOI] [PubMed] [Google Scholar]
- PARSONS J., WYCOFF H. D. Chromatographic microassay for cholesterol and cholesterol esters. Science. 1957 Feb 22;125(3243):347–348. doi: 10.1126/science.125.3243.347. [DOI] [PubMed] [Google Scholar]
- RAZIN S., ARGAMAN M., AVIGAN J. CHEMICAL COMPOSITION OF MYCOPLASMA CELLS AND MEMBRANES. J Gen Microbiol. 1963 Dec;33:477–487. doi: 10.1099/00221287-33-3-477. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S., Cosenza B. J. Growth phases of Mycoplasma in liquid media observed with phase-contrast microscope. J Bacteriol. 1966 Feb;91(2):858–869. doi: 10.1128/jb.91.2.858-869.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S., Morowitz H. J., Terry T. M. Membrane subunits of Mycoplasma laidlawii and their assembly to membranelike structures. Proc Natl Acad Sci U S A. 1965 Jul;54(1):219–225. doi: 10.1073/pnas.54.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S., Ne'eman Z., Ohad I. Selective reaggregation of solubilized Mycoplasma-membrane proteins and the kinetics of membrane reformation. Biochim Biophys Acta. 1969;193(2):277–293. doi: 10.1016/0005-2736(69)90189-8. [DOI] [PubMed] [Google Scholar]
- Razin S. Physiology of mycoplasmas. Adv Microb Physiol. 1973;10:1–80. doi: 10.1016/s0065-2911(08)60086-7. [DOI] [PubMed] [Google Scholar]
- Razin S., Prescott B., Caldes G., James W. D., Chanock R. M. Role of Glycolipids and Phosphatidylglycerol in the Serological Activity of Mycoplasma pneumoniae. Infect Immun. 1970 Apr;1(4):408–416. doi: 10.1128/iai.1.4.408-416.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S., Tourtellotte M. E., McElhaney R. N., Pollack J. D. Influence of lipid components of Mycoplasma laidlawii membranes on osmotic fragility of cells. J Bacteriol. 1966 Feb;91(2):609–616. doi: 10.1128/jb.91.2.609-616.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S., Razin S. Isolation of mycoplasma membranes by digitonin. J Bacteriol. 1972 May;110(2):699–705. doi: 10.1128/jb.110.2.699-705.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S., Stein O., Razin S. Reassembly of Mycoplasma membranes disaggregated by detergents. Arch Biochem Biophys. 1968 Apr;125(1):46–56. doi: 10.1016/0003-9861(68)90637-1. [DOI] [PubMed] [Google Scholar]
- Shaw N., Smith P. F., Koostra W. L. The lipid composition of Mycoplasma laidlawii strain B. Biochem J. 1968 Apr;107(3):329–333. doi: 10.1042/bj1070329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw N. The detection of lipids on thin-layer chromatograms with the periodate-Schiff reagents. Biochim Biophys Acta. 1968 Oct 22;164(2):435–436. doi: 10.1016/0005-2760(68)90171-9. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Terry T. M., Zupnik J. S. Weak association of glucosamine-containing polymer with the Acholeplasma laidlawii membrane. Biochim Biophys Acta. 1973 Jan 2;291(1):144–148. doi: 10.1016/0005-2736(73)90069-2. [DOI] [PubMed] [Google Scholar]
- Tillack T. W., Carter R., Razin S. Native and reformed Mycoplasma laidlawii membranes compared by freeze-etching. Biochim Biophys Acta. 1970;219(1):123–130. doi: 10.1016/0005-2736(70)90067-2. [DOI] [PubMed] [Google Scholar]
- Tourtellotte M. E., Branton D., Keith A. Membrane structure: spin labeling and freeze etching of Mycoplasma laidawii. Proc Natl Acad Sci U S A. 1970 Jul;66(3):909–916. doi: 10.1073/pnas.66.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]