Short abstract
Aerobic exercise can favourably modify arterial compliance in middle‐aged and older adults
Keywords: arterial stiffness, ageing, cardiovascular disease, physical activity
Cardiovascular diseases are prevalent in industrialised societies, and age is the dominant risk factor for morbidity and mortality.1 Consequently, a primary public health concern is to understand the mechanisms by which ageing is associated with cardiovascular diseases and to establish appropriate prevention and intervention strategies. One putative mechanism that has emerged as an important risk factor for cardiovascular disease is the age‐associated decline in large artery compliance.2,3 This single degenerative alteration to the vasculature has profound effects on cardiovascular health, contributing to increased systolic blood pressure and pulse pressure, isolated systolic hypertension, reduced cardiovagal baroreflex sensitivity, increased aortic input impedance, left ventricular hypertrophy and diastolic dysfunction, atherosclerosis, and congestive heart failure.1,2,3,4 As such, the age‐associated decline in arterial compliance is an important therapeutic target for habitual physical activity in the prevention of cardiovascular diseases. The purpose of this article is to highlight recent studies from our laboratory and other peer‐reviewed literature that provide compelling experimental evidence that aerobic exercise can favourably modify arterial compliance in middle‐aged and older adults.5,6,7,8 We will also present evidence suggesting that resistance training is potentially detrimental to arterial compliance unless combined with aerobic exercise.
Arterial compliance and ageing
Arterial compliance (and its inverse, arterial stiffness) describes the ability of an artery to distend in response to a change in intravascular (transmural) pressure. By distending and recoiling, the large “elastic” arteries in the cardiothoracic region—for example, aorta and carotid arteries—function to buffer the oscillation in blood pressure caused by ventricular pumping, and help to convert intermittent blood flow from the ventricle into continuous blood flow in the vasculature.9 This compliant property of the artery is typically determined by simultaneous measurements of changes in arterial blood pressure and volume during the cardiac cycle6 or by indirect measurement of indices of arterial stiffness. Indirect indices include the velocity of the pulse wave along an arterial segment or measurement of systolic pressure augmentation caused by early wave reflection from peripheral sites such as the aortic bifurcation.9
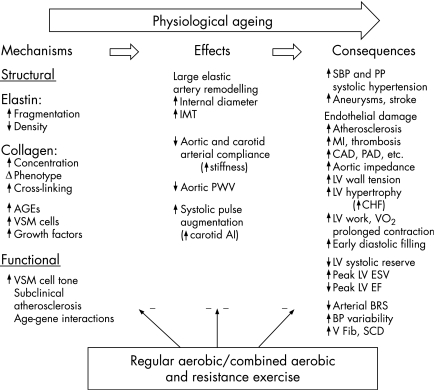
In healthy sedentary men and women, arterial compliance is progressively reduced by 40–50% between the ages of ∼25 and 75 years.6 The mechanisms by which arteries stiffen with advancing age are complex and involve both structural and functional alterations4 (fig 1). The structural integrity of the arterial wall is principally provided by the scaffolding proteins, elastin and collagen.10 Elastin becomes frayed and fragmented and total elastin content of the arterial wall is diminished with age.1,10 The collagen matrix becomes unravelled, cross‐linking occurs, total collagen content increases, and collagen fibre distribution becomes disorganised and dysfunctional.10 An unfavourable balance between matrix metalloproteinases and their inhibitors is induced by a number of activator molecules, often in the context of a pro‐inflammatory/oxidative stress environment.10 Both elastin and collagen are susceptible to non‐enzymatic cross‐linking, with advanced glycation end products that further stiffen the arterial wall.10 Vascular smooth muscle cells (VSMCs) hypertrophy, proliferate and invade the intima of the arterial wall, with consequent thickening of the wall and intima‐media layer.10 The intimal layer of stiffened arteries also contains mononuclear cells and macrophages, cell adhesion molecules, cytokines and transforming growth factors,10 all of which probably contribute to the age‐associated degeneration and stiffening of the arterial wall.
Figure 1 Contrasting effects of physiological ageing and exercise with an aerobic component on artery compliance, including possible mechanisms and clinical end points. AGEs, Advanced glycation end products; AI, augmentation index; BRS, baroreflex sensitivity; CAD, coronary artery disease; CHF, congestive heart failure; EF, ejection fraction; ESV, end‐systolic volume; IMT, intima‐media thickness; LV, left ventricle; MI, myocardial infarction; PAD, peripheral arterial disease; PP, arterial pulse pressure; PWV, pulse wave velocity; SBP, systolic blood pressure; SCD, sudden cardiac death; V Fib, ventricular fibrillation; VSM, vascular smooth muscle. Adapted from Seals4 to include the possible role of combined aerobic and resistance training.
Functional changes to vascular endothelial cells and VSMCs influence large artery compliance and have been reviewed extensively.1,2,3,4,10,11 Vascular endothelial function is impaired with age, nitric oxide bioavailability is reduced, and vasoconstrictor reactivity is increased.12 VSMC tone is probably increased with advancing age through increased sympathetic nervous system activity and α adrenergic receptor stimulation, increased circulating vasoconstrictor molecules (for example, angiotensin II, norepinephrine) and altered bioavailability of locally synthesised vasodilator molecules (for example, nitric oxide, prostacyclin) and vasoconstrictor molecules (for example, endothelin‐1).1,3,12 Changes in myogenic tone, subclinical atherosclerosis and a genetic predisposition for altered arterial function with age may also be involved.4 Many of these factors are probably mediated by age‐associated changes in inflammation and oxidative stress,3,10 although the relative contribution of these factors to the decline in arterial compliance and the importance of an inflammatory/oxidative stress phenotype are uncertain.
Aerobic exercise favourably modifies arterial compliance with ageing
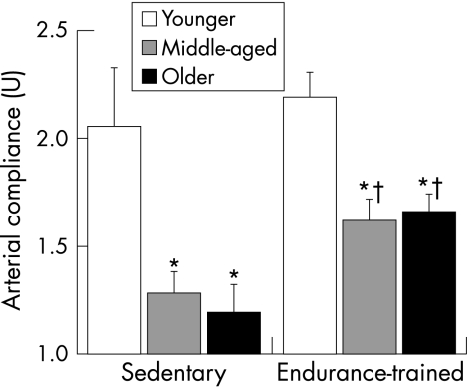
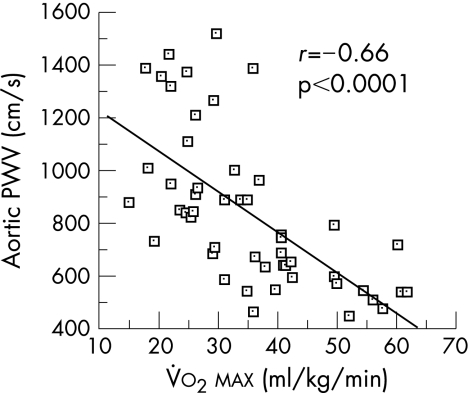
Regular aerobic exercise appears to attenuate the age‐associated decline in arterial stiffness and at least partially restores arterial compliance in previously sedentary older adults. Cross‐sectional data from the Baltimore Longitudinal Study on Ageing were the first to show that older endurance‐trained adults had lower aortic pulse wave velocity than their sedentary peers, indicating greater arterial compliance.8 By including cohorts of younger adults, later cross‐sectional studies were able to establish the effect of habitual physical activity on the age‐related decline in arterial compliance. The first of these studies showed that, in women who undertook chronic vigorous aerobic exercise, the age‐associated decline in large artery compliance was slowed or even prevented.7 A follow‐up study showed that, in a cross‐section of men, the decline in arterial compliance with age in endurance‐trained men was only half that observed in sedentary men (fig 2).6 These studies found that maximal aerobic capacity was the strongest physiological correlate of arterial compliance in men and women (fig 3) and, in combination, suggest that regular aerobic exercise attenuates age‐associated reductions in large elastic artery compliance.
Figure 2 The decline in carotid artery compliance with age is smaller in endurance‐trained than sedentary men. *p<0.05 compared with younger men of similar activity status. †p<0.05 compared with sedentary men of similar age. Adapted from Tanaka et al.6
Figure 3 Aortic pulse wave velocity (PWV) is inversely related to maximal aerobic capacity (V̇o2max) in pooled aerobically trained premenopausal and postmenopausal women. Adapted from Tanaka et al.7
Intervention studies have determined the effect of an aerobic exercise programme on arterial compliance in previously sedentary middle‐aged and older adults. Men who undertook 3 months of moderate intensity aerobic exercise (primarily walking) increased carotid artery compliance by ∼25%.6 Arterial compliance was still lower than in younger subjects but was not significantly different from middle‐aged and older endurance‐trained men.6 Importantly, the change in arterial compliance was independent of changes in body composition, blood pressure and other cardiovascular disease risk factors.
Findings from intervention studies in women are also promising. A moderate aerobic exercise intervention improved arterial compliance in previously sedentary postmenopausal women to levels that were not different from endurance‐trained postmenopausal women.5 Interestingly, arterial compliance was restored to levels similar to those of younger premenopausal women after aerobic training in previously sedentary women who were taking hormone replacement therapy.5
Greater arterial compliance in habitually active men and women appears to confer benefit on cardiovascular function in at least two respects. Firstly, there is a strong relationship between age‐associated increases in 24‐hour systolic and pulse pressure and arterial stiffness.13 As such, in contrast with their sedentary peers, aerobic exercise‐trained women show no age‐associated increases in arterial stiffness or arterial blood pressure.13 Secondly, the progressive loss of cardiovagal baroreflex sensitivity with ageing in healthy men is strongly related to carotid artery stiffening, but the decline in both is markedly attenuated in endurance‐trained men.14 Indeed, differences in arterial compliance explain much or all of the differences in cardiovagal baroreflex sensitivity associated with age and endurance training.14 Taken together, these findings suggest that habitual aerobic exercise may be an effective first line of defence against the age‐related decline in arterial compliance and associated changes in cardiovascular function and disease risk.
Mechanisms
There is little direct evidence about the mechanisms by which habitual physical activity influences arterial compliance, but presumably the age‐associated changes to large artery structure and/or function are attenuated or reversed.3,4 Greater total elastin and reduced collagen calcification are observed in the aorta of aerobically trained compared with sedentary rats,15 and collagen cross‐linking is <50% in the left ventricle of aerobically trained middle‐aged and older rats compared with age‐matched sedentary control animals.16 If collagen and elastin in the large arteries of humans are similarly protected by aerobic exercise, physical activity across the lifespan may benefit arterial compliance by attenuating detrimental changes to the structural properties of the arterial wall. It is not known if short‐term exercise interventions alter the structural composition of the arterial wall, but it is thought that this is unlikely and any such alterations would require a longer period.10
Changes in large artery compliance following an aerobic exercise intervention are relatively rapid and may reflect changes in VSMC tone, mediated at least partially by vascular endothelial cell function. Vascular endothelial cells synthesise nitric oxide which diffuses to VSMCs causing relaxation.12 This vascular endothelium‐dependent vasodilation declines with sedentary ageing, but is preserved in endurance‐trained men, and a short‐term programme of aerobic exercise completely restores the age‐related loss of endothelium dependent vasodilation in previously sedentary men.17 An increase in nitric oxide bioavailability with habitual exercise may tonically suppress VSMC tone in the arterial wall with a corresponding increase in arterial compliance.11 Arterial stiffness (aortic pulse wave velocity) is increased in rats if nitric oxide synthesis is pharmacologically abolished,18 and mRNA and protein expression of endothelium nitric oxide synthase are increased in aerobically trained rats.19 Taken together, these studies indicate that aerobic exercise may increase nitric oxide bioavailability with a consequent reduction in VSMC tone and increase in arterial compliance. However, at present, there is no direct evidence linking habitual exercise‐related improvements in vascular endothelial function to increases in large elastic artery compliance.
Several other mechanisms are probably involved and require further investigation, including alterations in other vasodilator factors (for example, prostaglandins, endothelial‐derived hyperpolarising factor), vasoconstrictor molecules (for example, endothelin‐1 and angiotensin II), inflammatory mediators, reactive oxygen species and/or anti‐oxidant defences. One of the challenges of future investigations is to provide further insight into the specific cellular and molecular mechanisms by which habitual exercise favourably modulates the age‐associated decline in arterial compliance in humans, and the consequences of this modulation for overall cardiovascular health.1,2,3,4,10,11
Resistance training and arterial compliance with ageing
Evidence has emerged to show that arterial compliance is lower in both younger and middle‐aged men who habitually perform intense resistance training compared with age‐matched sedentary controls.20,21 This potentially detrimental effect of resistance training on arterial compliance was subsequently supported by intervention studies in both men and women.22,22a In a randomised control trial, arterial compliance was reduced after 2 months of resistance training without further decline after 4 months.22 This effect was fully reversed after 4 months of detraining, showing relatively rapid plasticity in arterial compliance with resistance training. One possible explanation is that the reduction in arterial compliance is an immediate, temporary response to a bout of resistance training without longer‐term change in arterial structure and function. A recent study found that arterial compliance was reduced immediately after a single bout of resistance exercise, but returned to pre‐exercise values by 60 min after exercise.23 In combination, these studies suggest that arterial stiffening induced by resistance training is an adaptive response that is reversed by a period of detraining. Whether detraining improves arterial compliance in adults who have engaged in intensive resistance training over many years is unknown.
The adverse effect of resistance training on arterial compliance is of concern because moderate strength training has been recommended as a counter‐measure to the loss of muscle mass (sarcopenia) and frailty with ageing. However, recent evidence indicates that combining aerobic exercise training with a programme of resistance training is an effective strategy to prevent the negative consequences of resistance training on arterial stiffness. Cross‐sectional data showed that middle‐aged and older rowers who engaged in both resistance and aerobic exercise training had greater arterial compliance than their sedentary peers.24 Further studies will need to determine if the addition of aerobic training improves arterial compliance in middle‐aged and older adults who have engaged in long‐term resistance training. The mechanisms by which resistance training reduces arterial compliance also need to be elucidated to determine the optimal aerobic training strategy to complement resistance training.
Summary
Arterial compliance progressively declines with advancing age and increases the risk of cardiovascular diseases. Aerobic exercise training attenuates this decline and improves arterial compliance in previously sedentary middle‐aged and older adults. Intensive resistance training alone may increase arterial stiffness, but this is not observed with combined aerobic and resistance training. As such, the evidence to date suggests that habitual physical activity that includes an aerobic exercise component is a useful therapeutic strategy in the management of the age‐associated decline in arterial compliance.
Footnotes
References
- 1.Najjar S S, Scuteri A, Lakatta E G. Arterial aging: is it an immutable cardiovascular risk factor? Hypertension 200546454–462. [DOI] [PubMed] [Google Scholar]
- 2.Lakatta E G, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises. Part I. Aging arteries: a “set up” for vascular disease. Circulation 2003107139–146. [DOI] [PubMed] [Google Scholar]
- 3.Seals D R, Moreau K L, Gates P E.et al Modulatory influences on ageing of the vasculature in healthy humans. Exp Gerontol November200641501–507. [DOI] [PubMed] [Google Scholar]
- 4.Seals D R. Habitual exercise and the age‐associated decline in large artery compliance. Exerc Sport Sci Rev 20033168–72. [DOI] [PubMed] [Google Scholar]
- 5.Moreau K L, Donato A J, Seals D R.et al Regular exercise, hormone replacement therapy and the age‐related decline in carotid arterial compliance in healthy women. Cardiovasc Res 200357861–868. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka H, Dinenno F A, Monahan K D.et al Aging, habitual exercise, and dynamic arterial compliance. Circulation 20001021270–1275. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka H, DeSouza C A, Seals D R. Absence of age‐related increase in central arterial stiffness in physically active women. Arterioscler Thromb Vasc Biol 199818127–132. [DOI] [PubMed] [Google Scholar]
- 8.Vaitkevicius P V, Fleg J L, Engel J H.et al Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation 1993881456–1462. [DOI] [PubMed] [Google Scholar]
- 9.Nichols W, O'Rourke M.McDonald's blood flow in arteries: theoretical, experimental and clinical principles. 4th edn. London: Edward Arnold, 1998
- 10.Zieman S J, Melenovsky V, Kass D A. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol 200525932–943. [DOI] [PubMed] [Google Scholar]
- 11.Wilkinson I B, Franklin S S, Cockcroft J R. Nitric oxide and the regulation of large artery stiffness: from physiology to pharmacology. Hypertension 200444112–116. [DOI] [PubMed] [Google Scholar]
- 12.Brandes R P, Fleming I, Busse R. Endothelial aging. Cardiovasc Res 200566286–294. [DOI] [PubMed] [Google Scholar]
- 13.Seals D R, Stevenson E T, Jones P P.et al Lack of age‐associated elevations in 24‐h systolic and pulse pressures in women who exercise regularly. Am J Physiol 1999277H947–H955. [DOI] [PubMed] [Google Scholar]
- 14.Monahan K D, Tanaka H, Dinenno F A.et al Central arterial compliance is associated with age‐ and habitual exercise‐related differences in cardiovagal baroreflex sensitivity. Circulation 20011041627–1632. [DOI] [PubMed] [Google Scholar]
- 15.Matsuda M, Nosaka T, Sato M.et al Effects of physical exercise on the elasticity and elastic components of the rat aorta. Eur J Appl Physiol Occup Physiol 199366122–126. [DOI] [PubMed] [Google Scholar]
- 16.Thomas D P, Zimmerman S D, Hansen T R.et al Collagen gene expression in rat left ventricle: interactive effect of age and exercise training. J Appl Physiol 2000891462–1468. [DOI] [PubMed] [Google Scholar]
- 17.DeSouza C A, Shapiro L F, Clevenger C M.et al Regular aerobic exercise prevents and restores age‐related declines in endothelium‐dependent vasodilation in healthy men. Circulation 20001021351–1357. [DOI] [PubMed] [Google Scholar]
- 18.Fitch R, Vergona R, Sullivan M.et al Nitric oxide synthase inhibition increases aortic stiffness measured by pulse wave velocity in rats. Cardiovasc Res 200151351–358. [DOI] [PubMed] [Google Scholar]
- 19.Maeda S, Iemitsu M, Miyauchi T.et al Aortic stiffness and aerobic exercise: mechanistic insight from microarray analysis. Med Sci Sports Exer 2005371710–1716. [DOI] [PubMed] [Google Scholar]
- 20.Bertovic D A, Waddell T K, Gatzka C D.et al Muscular strength training is associated with low arterial compliance and high pulse pressure. Hypertension 1999331385–1391. [DOI] [PubMed] [Google Scholar]
- 21.Miyachi M, Donato A J, Yamamoto K.et al Greater age‐related reductions in central arterial compliance in resistance‐trained men. Hypertension 200341130–135. [DOI] [PubMed] [Google Scholar]
- 22.Miyachi M, Kawano H, Sugawara J.et al Unfavorable effects of resistance training on central arterial compliance: a randomized intervention study. Circulation 20041102858–2863. [DOI] [PubMed] [Google Scholar]
- 22a.Cortez‐Cooper M Y, De Van A E, Anton M M.et al Effects of high intensity resistance training on arterial stiffness and wave reflection in women. Am J Hypertens 200518930–934. [DOI] [PubMed] [Google Scholar]
- 23.DeVan A E, Anton M M, Cook J N.et al Acute effects of resistance exercise on arterial compliance. J Appl Physiol 2005982287–2291. [DOI] [PubMed] [Google Scholar]
- 24.Cook J N, DeVan A E, Schleifer J L.et al Arterial compliance of rowers: implications for combined aerobic and strength training on arterial elasticity. Am J Physiol Heart Circ Physiol 2006290H1596–H1600. [DOI] [PubMed] [Google Scholar]