Abstract
Objective
To assess if ultrasound guided autologous blood injection is an effective treatment for medial epicondylitis.
Methods
Twenty patients (13 men, 7 women) with refractory medial epicondylitis with symptom duration of 12 months underwent sonographic evaluation. Tendinosis was confirmed according to three sonographic criteria: echo texture, interstitial tears and neovascularity. The tendon was then dry needled and autologous blood was injected. Patients were reviewed at 4 weeks and at 10 months. VAS scores and modified Nirschl scores were assessed pre‐procedure and post‐procedure.
Results
There was significant reduction in VAS pain score between pre‐procedure and 10 months post‐procedure when it had a median (IQR) of 1.00 (1–1.75), range 0–7. The median (IQR) Nirschl score, which at pre‐procedure was 6.00 (5–7), range 4–7, had decreased at 4 weeks to 4.00 (2.25–5), range 2–7, and at 10 months to 1.00 (1–1.75), range 0–7, revealing a significant decrease (z = 3.763, p<0.001). The hypo‐echoic change in the flexor tendon significantly decreased between pre‐procedure, when there was a mean (SD) of 6.45 (1.47), and at 10 months, when it was 3.85 (2.37) (p<0.001). Doppler ultrasound showed that neovascularity decreased between pre‐procedure, when there was a mean (SD) of 6.10 (1.62), range 4–9, and at 10 months, when it was 3.60 (2.56), range 0–9 (p<0.001).
Discussion
The combined action of dry needling and autologous blood injection under ultrasound guidance appears to be an effective treatment for refractory medial epicondylitis as demonstrated by a significant decrease in VAS pain and a fall in the modified Nirschl scores.
Keywords: autologous blood, medial epicondylitis, treatment, ultrasound
Medial epicondylitis, more commonly known as golfer's elbow, is characterised by pathological changes to the musculo‐tendinous origin of the common flexors where it arises from the medial epicondyle. Lateral epicondylitis, also known as tennis elbow, has received most of the attention, probably because it is diagnosed 7–10 times more often than medial epicondylitis.1 Although medial epicondylitis affects patients from 12 to 80 years of age, it is most common in the 40–50 year age group. The dominant arm is affected in 75% of patients2 and the condition has been attributed to repetitive forearm pronation and wrist flexion. Changes are most often seen in the flexor carpi radialis and pronator teres, although large diffuse tears can occur in the palmaris longus,1 flexor digitorum superficialis and flexor carpi ulnaris. The condition has been reported frequently in baseball pitchers due to intense valgus force but has also been seen in those engaged in golf, tennis, bowling, racquetball and javelin throwing.3
Medial epicondylitis is thought to represent an incomplete healing response to repetitive microtrauma and interstitial tearing of the common flexor tendon. Non‐surgical management is the established method of treatment for medial epicondylitis.2 However, autologous blood injection is described as a novel method of treatment for tennis elbow.4,5 The hypothesis for the mechanism is that the transforming growth factor‐β and basic fibroblast growth factor carried in the blood act as humoral mediators to induce the healing cascade.6 We postulate that such injections could also benefit patients with medial epicondylitis. As this has not been previously reported in the medical literature, a study was designed to evaluate whether autologous blood injection is an effective non‐surgical treatment for medial epicondylitis.
Methods
Review board approval was obtained for the study and 20 patients with refractory medial epicondylitis were recruited. There were 13 men and 7 women, with a mean age of 48.5 years (range 31–68 years). The mean duration of symptoms was 12 months (range 2–36 months).
Inclusion criteria included symptoms lasting for 6 months following presentation, failure of conservative treatment including rest, physiotherapy and steroid injection, and confirmation of the diagnosis on MRI. Full thickness tears of the common flexor tendon and tears of the common flexor origin were excluded from the study. Also, patients who had had a steroid injection or other intervention in the preceding 3 months were excluded from the study.
Patients were asked to rate their pain on a 0–10 visual analogue scale (VAS), with 0 representing no pain and 10 the worst pain. In addition, patients were categorised according to the modified Nirschl 0–7 staging scheme (table 1).7
Table 1 Modified Nirschl score.
| Phase 1 – Mild pain with exercise, resolves within 24 h |
| Phase 2 – Pain with exercise, exceeds 48 h |
| Phase 3 – Pain with exercise, does not alter activity |
| Phase 4 – Pain with exercise, alters activity |
| Phase 5 – Pain with heavy activities of daily living |
| Phase 6 – Pain with light activities of daily living, intermittent pain at rest |
| Phase 7 – Constant pain at rest, disrupts sleep |
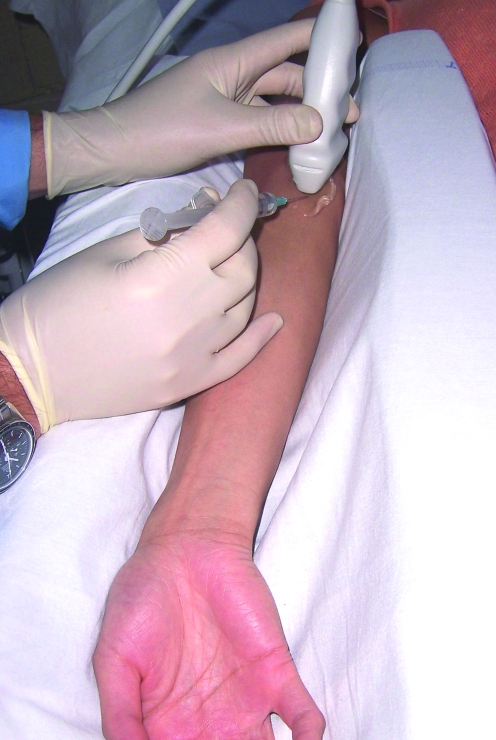
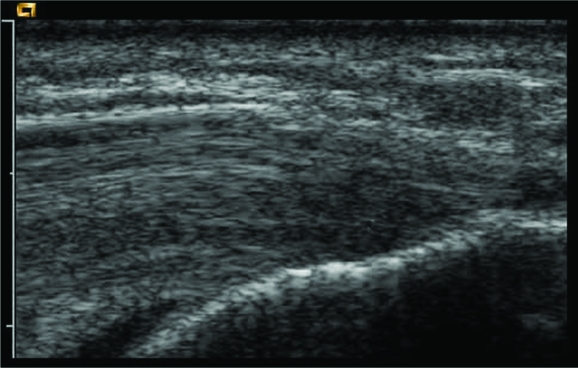
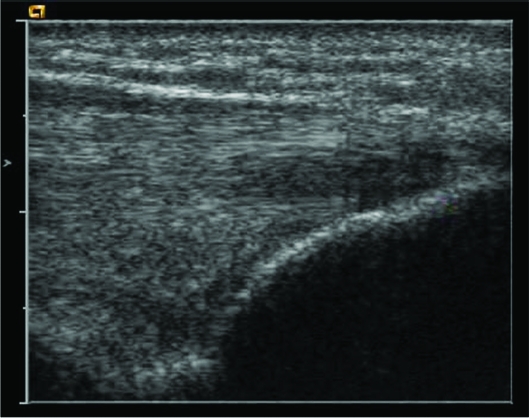
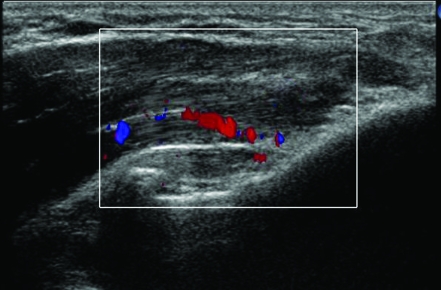
All patients were examined with a 15L8W transducer on a Siemens (Acuson) Sequoia ultrasound machine (fig 1). The common flexor origin was evaluated with ultrasound (a normal appearance is shown in fig 2), and the diagnosis of tendinosis was confirmed according to three sonographic criteria: echo texture, interstitial tears and neovascularity.8 Tendon size is also a recognised criterion for diagnosis of tendinosis but was not included due to difficulty in reproducing the tendon measurements. Echo texture was evaluated by identifying areas of hypo‐echoic change within the tendon (fig 3). A semi‐quantitative score of 1–10 was awarded, with 0 representing a normal echogenic tendon and 10 representing diffuse hypo‐echoic change throughout the tendon. Discrete tears were seen as tendons with focal anechoic areas with no fibres intact or as distinct hypo‐echoic areas of fibril discontinuity (fig 4A,B). The numbers and sizes of these tears were recorded. Neovascularity (fig 5) was recorded by placing the colour Doppler sensitivity at threshold and turning back the gain. The maximum number of pixels occupying the tendon origin was estimated as a percentage and given a score between 1 and 10.
Figure 1 The patient lies supine with the arm fully extended and wrist in supination with the probe on the medial epicondyle. Patient consent was obtained for publication of this figure.
Figure 2 Normal flexor origin in a volunteer.
Figure 3 Hypo‐echoic area indicating focal tendinosis.
Figure 5 Doppler ultrasound of medial epicondylitis demonstrating neovascularity prior to autologous blood injection.
Following ultrasound examination, 2 ml of autologous blood was withdrawn from the contralateral antecubital fossa of the patient and 2 ml of bupivacaine was infiltrated along the surface of the tendon using a 23G needle. After a few minutes to allow the anaesthetic to work, the needle tip was then positioned into the site of the maximum tendon injury and the tendon was dry needled to create fenestrations and so cause fibril disruption and internal bleeding. The autologous blood was then slowly injected into the site of tendinosis and fibril discontinuity. The time from blood aspiration to injection was approximately 3–4 min.
Patients were told to continue their normal daily activities but avoid those that would aggravate their symptoms. They were rescheduled for a follow‐up appointment 4 weeks later at which time a second sonographic evaluation was carried out, followed by a second autologous blood injection. Final sonographic and clinical follow‐up was performed at 10 months. All ultrasound assessments and injections were carried out by a single musculoskeletal radiologist with more than 10 years' experience (DC).
Statistical analysis
Pre‐procedural VAS and modified Nirschl scores and those at 4 weeks and 10 months post‐procedure were compared. Differences in hypo‐echoic change, neovascularity, and interstitial tears at pre‐procedure and at 10 months were also noted.
Normality of the difference between pre‐procedure and post‐procedure measures was assessed using the Kolmogorov‐Smirnov test. Data that did not follow a normal distribution were analysed using the Wilcoxon signed ranks test; all other data were analysed using the paired sample t test.
All statistical analysis was performed using SPSS for Windows, version 14.0 (SPSS, Chicago, Illinois, USA) and p values of <0.05 were considered statistically significant.
Figure 4 (A) Tendon thickening and an interstitial tear as an anechoic area in longitudinal scan. (B) Tendon thickening and an interstitial tear as an anechoic area in short axis in the same patient.
Results
Between January 2004 and May 2005, 26 patients were referred for autologous blood injection. Six patients were excluded following MRI and/or ultrasound as one had both medial and lateral epicondylitis, one had rotator cuff injury, one had adhesive capsulitis, one had a partial tear of the biceps tendon at the insertion, one had acute synovitis and one had medial collateral ligament injury. Twenty patients (13 men, 7 women) completed the course of treatment and were followed up for a period of up to 10 months after the initial injection. The mean duration of symptoms was 12 months (range 12–36 months).
All 20 patients had two autologous blood injections. Two patients returned for a third autologous blood injection. Of three patients who felt the procedure had failed, two subsequently went on to tendon repair at surgery and one is still awaiting surgery.
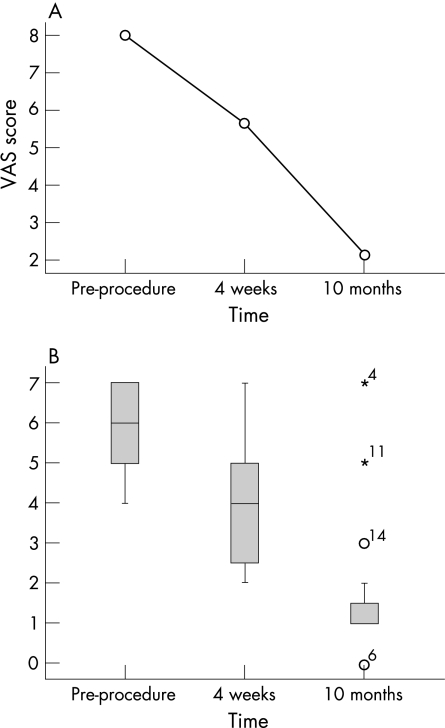
In the remaining 17 patients, the mean VAS pain score, which at pre‐procedure was 8.00 (range 5–10), at 4 weeks had decreased to 5.65 (range 2–9). Statistical analysis found the difference to be significant (z = 4.88, p<0.001). In addition, statistical analysis of the VAS pain score at pre‐procedure and at 10 months, when it was a mean of 2.15 (range 0–9), revealed a significant decrease (z = 5.16, p<0.001).
The modified Nirschl score, which at pre‐procedure had a median (IQR) of 6.00 (5–7), range 3–11, at 4 weeks had significantly decreased (z = 4.64, p<0.001) to a median (IQR) of 4 (2.25–5), range 2–7. The difference was found to be statistically significant. In addition, analysis of the Nirschl score at pre‐procedure and at 10 months, when it had a median (IQR) of 1.00 (1–1.75), range 0–7, revealed a significant decrease (z = 3.763, p<0.001).
The graph in fig 6A shows the mean VAS change over time; the box and whisker plot in fig 6B shows the modified Nirschl scores pre‐ and post‐procedure at 4 weeks and at 10 months. The midline inside the boxes represents the median score, the box itself represents the lower and upper quartiles (inter‐quartile range), and the whiskers represent the remainder of the range, with the outliers indicated by a circle or asterisk.
Figure 6 (A) Mean VAS score change over time. (B) Nirschl scores pre‐ and post‐procedure at 4 weeks and at 10 months.
Hypo‐echoic changes decreased between pre‐procedure, when there was a mean (SD) of 6.45 (1.47), range 4–9, and post‐procedure at 10 months, when there was a mean (SD) of 3.85 (2.37), range 1–9; this decrease was found to be statistically significant (t = 4.815, df = 19, p<0.001).
Neovascularity decreased between pre‐procedure, when there was a mean (SD) of 6.10 (1.62), range 4–9, and post‐procedure at 10 months when there was a mean (SD) of 3.60 (2.56), range 0–9; this decrease was found to be statistically significant (t = 5.415, df = 19, p<0.001).
The median (IQR) number of interstitial tears for 11/20 patients was 6.00 (5–8), range 3–11, at baseline and 3.00 (0–4), range 0–11, at post‐procedure. Statistical analysis found the difference to be significant (z = 2.726, p = 0.006).
There was no infection, neurovascular damage or rupture of the tendon in any patient after autologous blood injection.
Although there was a general improvement in all the sonographic parameters, none of the treated tendons had a normal appearance.
Discussion
Medial epicondylitis primarily affects the pronator teres and flexor carpi radialis, which attach to the anterior aspect of the medial epicondyle. These muscles are subject to repeated trauma and micro‐tearing either because of repetitive overuse or less often because of a direct blow or sudden extreme contraction.2 Repetitive forearm pronation and wrist flexion is thought to be the most likely culprit. The term epicondylitis was used to describe this condition as early theories postulated an inflammatory process involving the bursa, periosteum, synovium and annular ligament.9,10 However, the preferred term now is tendinoses, as there are many pathological studies which have shown that there is micro‐tearing with disruption of the collagen bundles, vascular and fibroblast proliferation, focal hyaline degeneration and an incomplete reparative process with no evidence of any inflammatory process.11 Histological analysis by Nirschl and Pettrone has shown that there is disruption of normal architecture with later stages demonstrating micro‐tearing, tendon degeneration and aborted, incomplete neurovascular response.11 They have called these changes “angiofibroblastic hyperplasia”. Macroscopically, the pathologic tissue appears grey and friable. A chronic cycle of tendon micro‐tearing, degeneration and repair ensues with weakening of the common flexor origin and potential for rupture.
Nirschl has proposed four descriptive stages of epicondylar tendinosis.12 Stages 1 and 2 represent pathologic tissue alteration with angiofibroblastic degeneration. Structural failure is the hallmark of stage 3. Stage 4 shows fibrosis or calcification.
Diagnosis is usually based on clinical findings. Ultrasound or MR imaging is useful for diagnosing other conditions such as ulnar neuritis and ulnar collateral ligament instability as these may co‐exist. Very few studies describe the imaging findings in medial epicondylitis, probably because of the relatively low prevalence of this condition compared to lateral epicondylitis. A study by Kijowski and de Smet evaluating the MR findings in medial epicondylitis concluded that intermediate to high T2 signal intensity within the common flexor tendon and the presence of paratendinous soft tissue oedema were the most specific findings.13 Three out of the 15 patients in their study had tears of the ulnar collateral ligament and one had an abnormal ulnar nerve. No studies discuss the sonographic features of medial epicondylitis, but two reports describe the sonographic features of lateral epicondylitis4,8 and a third examines dynamic ultrasound in the evaluation of the ulnar collateral ligament.14
Non‐surgical treatment has been the mainstay of treatment for epicondylitis2 and includes RICE (rest, ice, compression and elevation), topical medications such as dimethylsulfoxide, oral non‐steroidal anti‐inflammatory analgesics, counter‐force braces and physiotherapy. Although conservative treatment is described as being the most successful, one study has shown that 26% of such patients have recurrence of symptoms and 40% have prolonged minor discomfort.15
Surgical treatment is recommended in patients who fail to respond to conservative treatment. Again there are few reports on the surgical treatment of medial epicondylitis. However, one study reported an 87% success rate in patients (n = 26) after an average follow‐up of 7 years,16 while another reported good results in 14 of 17 patients.17
The use of local steroid injection for tendinosis is well documented. The mechanism of short‐term relief following steroid injection or needling is not understood. However, it is postulated that fenestration of an area of tendinosis with needling may promote beneficial bleeding into new channels created through mucoid degeneration. The mechanical disruption may initiate a healing response in the tendon.4,18 One study showed a significant decrease in pain after 6 weeks, but there was recurrence of the pre‐injection pain after 3 months and 1 year.19 Another study showed pain relief in up to 89% of patients, but again there was recurrence of symptoms in 54%.20
Autologous blood injection is a novel treatment for tendinosis. No studies have investigated the efficacy of this treatment for medial epicondylitis, although two studies have evaluated its use in lateral epicondylitis. One report by Edwards and Calandruccio showed that 22/28 patients responded to autologous blood injections, with average Nirschl scores decreasing from 6.5 to 2.0 with a mean follow‐up of 9.5 months.4 Another study by Connell et al showed good response, with decreased modified Nirschl scores from a median pre‐procedure score of 6 (6–7) to 0 (0–1) at 6 months and significant reductions in VAS scores in 33/35 patients with refractory lateral epicondylitis.5
In our study, local anaesthesia was achieved by injecting all patients with 0.25% bupivacaine along the superficial surface of the tendon. Rigorous dry needling was followed by injection under guidance of autologous blood into the areas of tendinosis. Dry needling fenestrates the tendon causing further fibril disruption and local bleeding before the blood injection. All patients had a second injection after 4 weeks which we postulate is the optimal time interval. Two patients returned for a third injection.
In our group of 20 patients, there were three clear treatment failures with recurrence of the pain to the pre‐injection level. Two of these patients have had a successful tendon repair and one is still awaiting surgery. There were no identifiable features at baseline ultrasound to suggest a poor outcome in this group. We excluded patients with intra‐articular pathology or substantive tears of the tendon. As in most procedures, we believe that patient selection is critical to the success of treatment. All patients with partial or full‐thickness tears of the common flexor tendons or involvement of the ulnar collateral ligament are referred directly to surgery.
There are a number of limitations to our study. The most obvious one is that all sonographic observations were made by a single radiologist and hence we have no measure of either intra‐ or inter‐observer variability. There is no control group and the subject bias inherent in our study was unavoidable. At study outset, we opted to use colour Doppler, although it could be argued that power Doppler may be more sensitive and less susceptible to flow direction. Although we believe that dry needling and autologous blood injected into a area of tendinosis induces a healing response with the formation of scar tissue, we have no histopathological correlation and the exact mechanism is not completely understood.
What is already known on this topic
Medial epicondylitis or golfer's elbow represents an incomplete healing response to repetitive micro‐trauma and interstitial tearing of the common flexor tendon.
Non‐surgical treatment is the established method of treatment for medial epicondylitis.
Autologous blood injection is described as a novel method of treatment for tennis elbow.
The hypothesis for the mechanism is that the transforming growth factor‐β and basic fibroblast growth factor carried in the blood act as humoral mediators to induce the healing cascade.
What this study adds
Our study demonstrates that the combined action of dry needling and autologous blood injection under ultrasound guidance is an effective way to treat patients with refractory medial epicondylitis.
We have also demonstrated a significant decrease in pain scores and the modified Nirschl scores.
Although autologous blood injection has been described as a novel method of treatment for tennis elbow, this method of treatment has not been previously reported in the medical literature for refractory medial epicondylitis.
In summary, we feel that the combined action of dry needling and autologous blood injection under ultrasound guidance is an effective treatment for patients with refractory medial epicondylitis as demonstrated by a significant decrease in VAS pain and a fall in the modified Nirschl scores. However, further research to explain the exact mechanism of healing by this technique and analysis of the various studies regarding the use of this technique in other tendinopathies is essential.
Footnotes
Funding: None.
Competing interests: None declared.
Patient consent was obtained for publication of figure 1.
References
- 1.Leach R E, Miller J K. Lateral and medial epicondylitis of the elbow. Clin Sports Med 1987659–72. [PubMed] [Google Scholar]
- 2.Ciccotti M C, Schwaetz M A, Ciccotti M G. Diagnosis and treatment of medial epicondylitis of the elbow. Clin Sports Med 200423693–705. [DOI] [PubMed] [Google Scholar]
- 3.Vangsness C, Jobe F W. Surgical technique of medial epicondylitis: results in 35 elbows. J Bone Joint Surg Br 199173409–411. [DOI] [PubMed] [Google Scholar]
- 4.Edwards S G, Calandruccio J H. Autologous blood injections for refractory lateral epicondylitis. J Hand Surg 200328A272–278. [DOI] [PubMed] [Google Scholar]
- 5.Connell D A, Ali K E, Ahmad M.et al Ultrasound guided autologous blood injection for tennis elbow. Skeletal Radiol 200635(6)371–377. [DOI] [PubMed] [Google Scholar]
- 6.Iwasaki M, Nakahara H, Nakata K.et al Regulation of proliferation and osteochondrogenic differentiation of periosteum‐derived cells by transforming growth factor‐β and basic fibroblast growth factor. J Bone Joint Surg 199577A543–554. [DOI] [PubMed] [Google Scholar]
- 7.Nirschl R P. Elbow tendinosis/tennis elbow. Clin Sports Med 199211851–870. [PubMed] [Google Scholar]
- 8.Connell D, Burke F, Coombes P.et al Sonographic examination of lateral epicondylitis. AJR Am J Roentgenol 2001176777–782. [DOI] [PubMed] [Google Scholar]
- 9.Carp L. Tennis elbow epicondylitis caused radiohumeral bursitis. Arch Surg 193224905 [Google Scholar]
- 10.Garden R S. Tennis elbow. J Bone Joint Surg Br 196143100–106. [Google Scholar]
- 11.Nirschl R P, Pettrone F A. Tennis elbow: the surgical treatment of lateral epicondylitis. J Bone Joint Surg 197961‐A832–839. [PubMed] [Google Scholar]
- 12.Nirschl R P. Prevention and treatment of elbow and shoulder injuries in tennis players. Clin Sports Med 19887289–294. [PubMed] [Google Scholar]
- 13.Kijowski R, de Smet A A. Magnetic resonance imaging findings in patients with medial epicondylitis. Skeletal Radiol 200534196–202. [DOI] [PubMed] [Google Scholar]
- 14.Nazarian L N, McShane J M, Ciccotti M G.et al Dynamic US of the anterior band of the ulnar collateral ligament of the elbow in asymptomatic major league baseball pitchers. Radiology 2003227(1)149–154. [DOI] [PubMed] [Google Scholar]
- 15.Binder A I, Hazelman B L. Lateral humeral epicondylitis‐‐a study of natural history and the effect of conservative therapy. Br J Rheumatol 198322(2)73–76. [DOI] [PubMed] [Google Scholar]
- 16.Gabel G T, Morrey B T. Operative treatment of medical epicondylitis. Influence of concomitant ulnar neuropathy at the elbow. J Bone Joint Surg Am 199577(7)1065–1069. [DOI] [PubMed] [Google Scholar]
- 17.Wittenberg R H, Schaal S, Muhr G. Surgical treatment of persistent elbow epicondylitis. Clin Orthop Relat Res 199227873–80. [PubMed] [Google Scholar]
- 18.Amiel D, Akeson W, Harwood F L.et al Stress deprivation effect on metabolic turnover of medial collateral ligament collagen. A comparison between 9‐ and 12‐week immobilization. Clin Orthop 1983172265–270. [PubMed] [Google Scholar]
- 19.Stahl S, Kaufman T. The efficacy of an injection of steroids for medial epicondylitis: a prospective study of sixty elbows. J Bone Joint Surg Am 1997791648–1652. [DOI] [PubMed] [Google Scholar]
- 20.Price R, Sinclair H, Heinrich I.et al Local injection treatment of tennis elbow‐‐hydrocortisone, triamcinolone and lidocaine compared. Br J Rheumatol 19913039–51. [DOI] [PubMed] [Google Scholar]