Abstract
Objective
To examine the effect of downhill running on immunoglobulin responses.
Method
Eleven untrained men performed 2 × 60 minute bouts of downhill running (−13.5% gradient), at a speed eliciting 75% of their V̇o2peak on a level grade. Two runs were spaced 14 days apart. Serum samples were collected before, after, and every hour for 12 hours and every 24 hours for six days. Serum total creatine kinase and immunoglobulin isotypes and subclasses were measured, and results were analysed using a repeated measures analysis of variance (12 hour period, 2 × 14; 24 hour intervals, 2 × 6, p⩽0.05).
Results
There was a significant interaction effect for creatine kinase (activity lower after run 2 than after run 1, 6–24 h) and exercise effect, with the serum concentrations of IgG1, IgG2, IgG4, and IgE lower, and IgM higher, after run 2.
Conclusion
Lower concentrations of IgG1, IgG2, and IgE after run 2 may reflect a dampened autoimmune inflammatory response to autoantigens and enhanced autoantigen clearance mediated by the upregulation of IgM.
Keywords: antibody, autoantigens, immunoglobulin response, inflammation, eccentric exercise
Eccentric exercise has been used as a model to examine tissue damage and the subsequent repair response.1,2,3,4 A bout of unaccustomed, eccentrically biased exercise increases systemic markers of tissue damage, which usually peak 24–48 hours after the exercise.5 When a similar bout is repeated within a few days to six weeks, these markers are significantly lower, suggesting some form of adaptation, a phenomenon referred to as the “repeated bout effect”.6 As would be expected in response to exercise induced tissue damage, immune and inflammatory alterations have also been examined, but the focus has been on the innate immune system.3,7,8
Adaptive immunity is associated with primary and/or secondary immunoglobulin responses to pathogens such as bacteria and viruses. For this reason, the aim of previous studies has been to determine the relation between exercise induced alterations in immunoglobulins and susceptibility to upper respiratory infections.9 However, immunoglobulins have additional functions that have received little attention in the exercise immunology literature. These include (a) the mediation of inflammatory responses to injured skeletal muscle,10,11 (b) regulating autoimmune responses,12 and (c) clearing autoantigens, such as tissue damage fragments.13 In support of this, Nieman and Nehlsen‐Cannarella14 have suggested that IgM assists in the removal or elimination of exercise induced tissue breakdown products. In addition, Smith15 recently hypothesised that exercise induced tissue damage may shift the systemic cytokine milieu from a Th1 to a Th2 profile, thereby altering humoral immunity, including immunoglobulin responses.
Therefore, because of the involvement of immunoglobulins in various aspects of the body's response to tissue damage,10,11,12,13 it has been hypothesised that immunoglobulins would be altered by tissue damaging exercise such as downhill running. To date there is limited research reporting immunoglobulin responses to single or repeated bouts of exercise that may induce tissue damage. Therefore, the purpose of this study was to determine the effect of two equivalent bouts of downhill running on immunoglobulin isotype and subclass responses.
Materials and methods
Subjects
Eleven, healthy, active but untrained, white men were recruited to participate. Initial selection criteria included: age 18–30 years; no history of leg injury or any other medical condition that would be exacerbated by two bouts of downhill running; no regular use of any anti‐inflammatory drug. Physical characteristics were (mean (SD)): age 19.7 (0.4) years; height 1.79 (4.3) m; mass 78.5 (3.0) kg; percentage body fat 4.6 (3.2); V̇o2max 47.8 (3.6) ml O2/kg/min. Subjects read and signed an informed consent, which had been approved by the institution's ethics committee.
Assessment of V̇o2peak and determination of running speeds
A standard Bruce protocol was used to determine V̇o2peak using a Quinton 90 treadmill (Quinton Instrument Co, Seattle, Washington, USA). Continuous respiratory measurements were recorded using a MedGraphics CardiO2 combined V̇o2/electrocardiograph exercise system (Medical Graphics Corporation, Chicago, Illinois, USA). Heart rate was recorded at the end of each minute using a Polar heart rate monitor, and ratings of perceived exertion were recorded at the end of each stage (every three minutes), as well as when subjects reached volitional exhaustion. The test was accepted as V̇o2peak if two of the following criteria were attained: respiratory exchange ratio ⩾ 1.1 and/or rating of perceived exertion ⩾ 19 on the 15 point scale, and/or maximum heart rate within 20 beats of the age predicted value. Seventy five per cent of V̇o2peak was calculated, and metabolic equations were used to determine the speed, on a level grade, that would elicit this V̇o2.16 This treadmill speed was the designated speed for each subject for both downhill runs.
Downhill runs
Subjects were instructed to ingest a normal mixed diet, to be well hydrated, and to refrain from any strenuous exercise for at least 72 hours before the downhill runs. They performed two bouts of downhill running spaced 14 days apart. The runs on both days were between 5 and 11 am and at approximately the same time for each subject. The subjects performed the runs in a fasted state (at least eight hours after absorption). At the start of both runs, subjects warmed up for five minutes by running on a level grade at the predetermined speed. The treadmill was then lowered to a −13.5% level and subjects ran for 60 minutes. The subjects remained in the exercise teaching laboratory for 12 hours after both runs. They were provided with food and fluid and encouraged to eat, and especially to drink water, ad libitum.
Biological fluid sampling and processing
On arriving in the exercise teaching laboratory, subjects were required to sit quietly for 10 minutes before both runs. A qualified phlebotomist then inserted a venous catheter (22 gauge, 2.2 cm), which was kept patent using saline solution. Blood was drawn at the following times: before exercise, immediately after exercise, and one, two, three, four, five, six, seven, eight, nine, 10, 11, and 12 hours after (14 samples × 15 ml per sample = 210 ml blood over about 14 hours). In addition, subjects were required to return to the exercise teaching laboratory at 24, 48, 72, 96, 120, and 144 hours after, for additional blood draws (6 days × 15 ml = 90 ml). At these times, a standard venepuncture was performed using an antecubital vein. Plasma was collected in EDTA tubes and used for assessment of total and differential white cell count (data not presented). Serum was collected in serum separator tubes, allowed to stand at room temperature for 30 minutes, and then spun down for 10 minutes at 2000 g. Aliquots were frozen at −80°C in 0.5 ml Eppendorf tubes.
Analysis of biological fluids
Although the usefulness of creatine kinase activity as a reliable and valid indicator of muscle damage has been questioned,17 it is currently used as an indicator of muscle damage in exercise and clinical studies.17,18 Therefore total serum creatine kinase activity was measured to indicate muscle damage in the present study.
For the measurement of immunoglobulin concentrations, each set of blood specimens from an individual subject—that is, 20 samples—was assayed in a single run with a single lot number of reagents and consumables used by a single operator. All blood samples were analysed in triplicate. The analytical intra‐assay coefficients of variation obtained from the measurements in this study were as follows: IgD, 2.95%; IgG (isotypes), 3.33%; IgM, 1.40%; IgE, 3.50%.
Total creatine kinase activity
Total creatine kinase activity in serum was measured before the run, immediately after, and at three, six, nine, 12, and every 24 hours for six days after both runs. Concentrations were determined using a Reflotron analyser (Boehringer Mannheim, Mannheim, Germany).
Serum immunoglobulin
The total amount of serum IgM, IgD, IgA, IgG, and IgG subclasses was quantified by incubation with appropriate antisera (anti‐human IgM, IgA, IgG and IgG1, IgG2, IgG3 and IgG4; Behring, Germany). The amount of complex (immunoglobulin–anti‐immunoglobulin) formed was measured by light scatter, using laser nephelometry (Behring), and the amount of antibody present was quantified by comparison with standards of known concentration.
The total amount of serum IgE was determined with the use of the Alastat Microplate Total IgE kit (Diagnostic Products, Los Angeles, California, USA) according to the manufacturer's instructions and by comparisons with a known range of standard IgE concentrations.
Statistical analysis
Each dependent variable was analysed before and after each run using a repeated measures analysis of variance, with significance set at p⩽0.05. Data were analysed separately for each run day (2 × 14 time points) as well as for the 24 hour periods (2 × 6 time points). A post hoc Bonferroni test was used to test for significant interaction or main effects.
Results
Creatine kinase
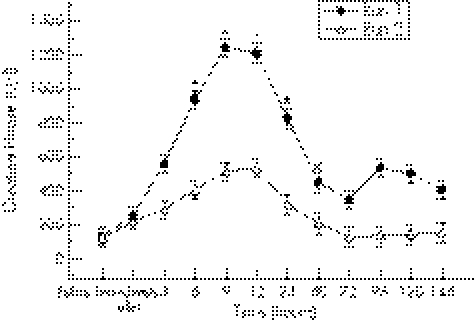
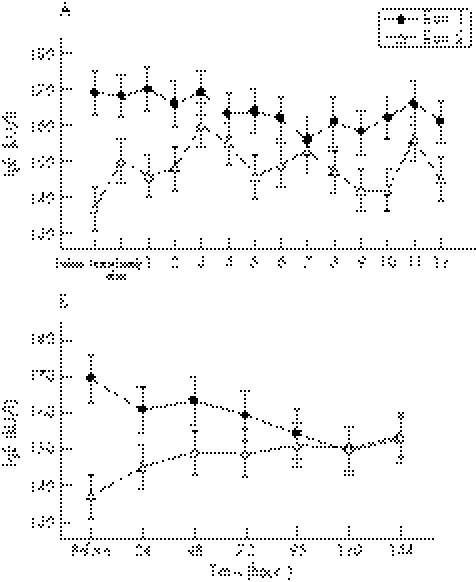
There was a significant interaction effect for creatine kinase. Creatine kinase activity at six, nine, 12, and 24 hours (fig 1) was significantly lower after run 2 than after run 1: 402.55 (41.57) v 925.05 (148.58) U/l (p = 0.0002), 514.60 (54.97) v 1241.92 (134.61) U/l (p = 0.0001), 534.97 (58.93) v 1133.73 (118.08) U/l (p = 0.0001), and 319.40 (36.00) v 826.85 (105.34) U/l (p = 0.0001) respectively.
Figure 1 Total creatine kinase activity before and after both bouts of downhill running. *Significant interaction effect. Values are mean (SE).
Immunoglobulin results
IgD
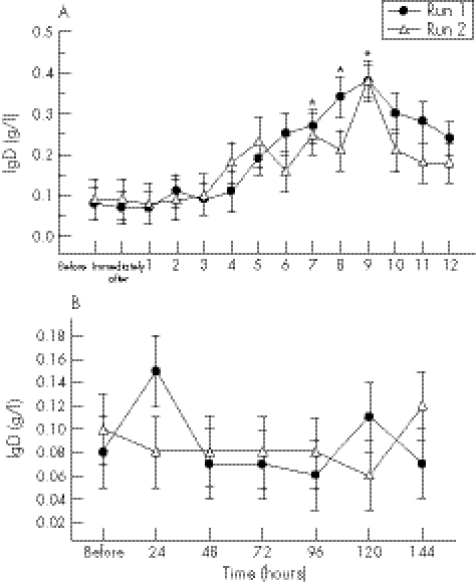
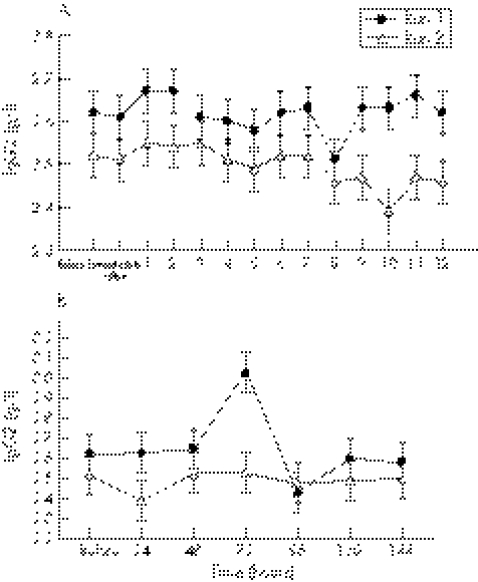
During the first 12 hours after exercise, IgD concentrations began to rise from four hours and were significantly higher at seven to nine hours (threefold at seven hours (p = 0.04) and eight hours (p = 0.01); fourfold at nine hours (p<0.0001)) on both exercise days compared with initial baseline values (fig 2A).
Figure 2 (A) IgD concentration before and at hourly intervals after both bouts of downhill running. *Significant time effect for both runs compared with baseline values. Values are mean (SE). (B) IgD concentration before and at 24 hour intervals after both bouts of downhill running. There were no significant interaction, run, or time effects during this period.
IgM
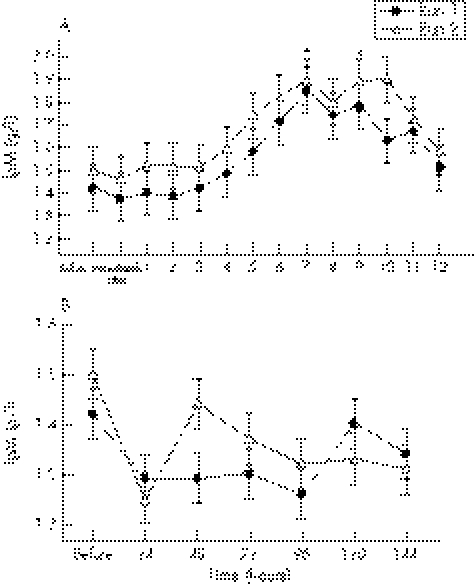
During the first 12 hours after exercise, there was a significant (p = 0.04) run effect, with IgM significantly higher (+5%) after run 2 (1.64 (0.02) g/l) than after run 1 (1.57 (0.02) g/l) (fig 3A). IgM concentrations began to rise from four hours and were significantly higher at seven hours (+21%, p = 0.005) and nine hours (+29%, p = 0.02) into the recovery period on both exercise days compared with initial baseline values (fig 3A).
Figure 3 (A) IgM concentration before and at hourly intervals after both bouts of downhill running. There was a significant run effect, with the means (SE) for run 2 being significantly higher than for run 1. *Significant time effect for both runs compared with baseline values. (B) IgM concentration before and at 24 hour intervals after both bouts of downhill running. There were no significant interaction, run, or time effects during this period.
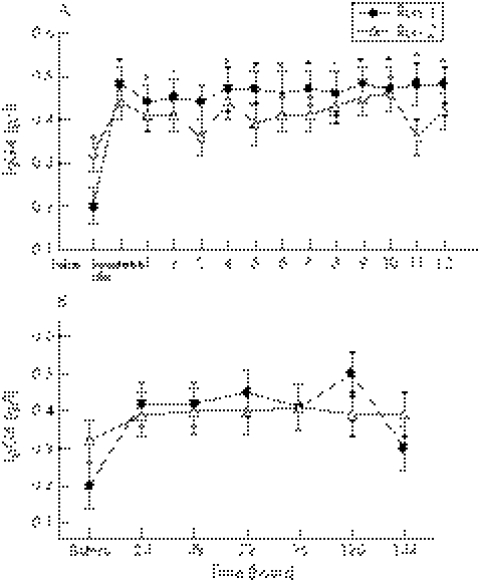
Immunoglobulins G1, G2, and G4
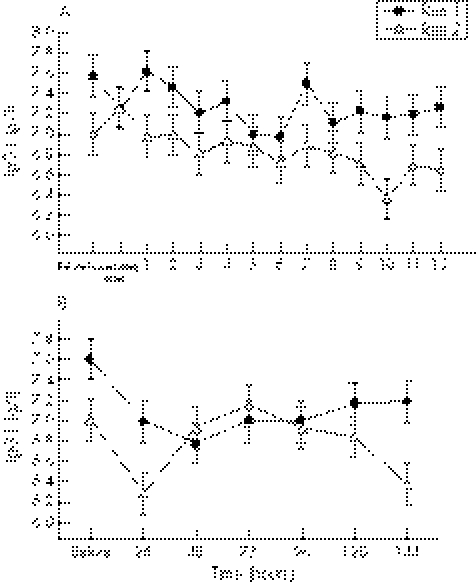
During the first 12 hours after exercise, there was a significant (p<0.0001) run effect for IgG1, with lower concentrations (−5%) after run 2 (6.84 (0.05) g/l) than after run 1 (7.22 (0.05) g/l) (fig 4A). In addition, at six days after exercise, there was a significant (p = 0.02) run effect, with IgG1 concentrations remaining lower (−5%) after run 2 (6.79 (0.08) g/l) than after run 1 (7.09 (0.08) g/l) (fig 4B). During the first 12 hours after exercise, there was also a significant (p<0.0001) run effect for IgG2, with lower concentrations (−5%) after run 2 (2.5 (0.01) g/l) than after run 1 (2.62 (0.01) g/l) (fig 5A). Similarly to IgG1, at six days after exercise, there was also a significant (p = 0.02) run effect, with IgG2 concentrations remaining lower (−6%) after run 2 (2.49 (0.04) g/l) than after run 1 (2.65 (0.04) g/l) (fig 5B). During the initial 12 hours after exercise, there was also a significant (p = 0.02) run effect for IgG4, with concentrations significantly lower (−7%) after run 2 (0.40 (0.01) g/l) than after run 1 (0.45 (0.01) g/l) (fig 6A). In addition, during the first 12 hours after exercise, IgG4 concentrations were significantly higher (+40–70%) immediately after (p = 0.005), and at one (p = 0.01), two (p = 0.008), four (p = 0.001), five (p = 0.009), six (p = 0.008), seven (p = 0.004), eight (p = 0.003), nine (p = 0.0002), 10 (p = 0.0005), 11 (p = 0.02), and 12 (p = 0.001) hours into the recovery period on both exercise days compared with initial baseline values (fig 6A).
Figure 4 (A) IgG1 concentration before and at hourly intervals after both bouts of downhill running. There was a significant run effect, with the means (SE) for run 2 being significantly lower than for run 1. (B) IgG1 concentration before and at 24 hour intervals after both bouts of downhill running. There was a significant run effect, with the means (SE) for run 2 being significantly lower than for run 1.
Figure 5 (A) IgG2 concentration before and at hourly intervals after both bouts of downhill running. There was a significant run effect, with the means (SE) for run 2 being significantly lower than for run 1. (B) IgG2 concentration before and at 24 hour intervals after both bouts of downhill running. There was a significant run effect, with the means (SE) for run 2 being significantly lower than for run 1.
Figure 6 (A) IgG4 concentration before and at hourly intervals after both bouts of downhill running. There was a significant run effect, with the means (SE) for run 2 being significantly lower than for run 1. *Significant time effect for both runs compared with baseline values. (B) IgG4 concentration before and at 24 hour intervals after both bouts of downhill running. There was a significant run effect, with the means (SE) for run 2 being significantly lower than for run 1.
IgE
During the initial 12 hours after exercise, there was a significant (p<0.0001) run effect for IgE, with concentrations significantly lower (−10%) after run 2 (148 (1.53) kU/l) than after run 1 (163 (1.67) kU/l) (fig 7A). In addition, at six days after exercise, there was a significant (p<0.001) run effect, with IgE concentrations remaining lower (−7%) after run 2 (147 (2.33) kU/l) than after run 1 (158 (2.48) kU/l) (fig 7B).
Figure 7 (A) IgE concentration before and at hourly intervals after both bouts of downhill running. There was a significant run effect, with the means (SE) for run 2 being significantly lower than for run 1. (B) IgE concentration before and at 24 hour intervals after both bouts of downhill running. There was a significant run effect, with the means (SE) for run 2 being significantly lower than for run 1.
Total serum IgG, IgG3, and IgA concentrations were not significantly altered by the downhill runs, and therefore results are not reported.
Discussion
Whereas numerous studies have compared secretory and serum immunoglobulin responses to acute and chronic bouts of exercise and training,14,19,20 to the best of our knowledge, this is the first to report serum immunoglobulin responses to exercise induced muscle damage. Although the specific biological and clinical significance of the alterations in immunoglobulin concentrations to muscle damage require further investigation, the findings do suggest additional functions of immunoglobulins, other than pathogen defence.
The result for IgM during the initial 12 hours, with a higher concentration of serum IgM after run 2, as well as an increase in IgM (seven hours and nine hours) after both runs, are unique findings. IgM is typically produced as a primary antibody response to pathogens. However, additional functions have recently been reported for IgM in clinical studies, suggesting that this antibody is involved in immune responses to tissue damage,10,11 as well as in the clearance of autoantigens, which may include tissue damage fragments.13 Natural IgM antibodies are a subclass of IgM antibody, and are the first antibodies to bind to non‐infectious autoantigens, with the formation of immune complexes.13 Natural IgM antibodies perform two crucial functions to prevent the development of autoimmune disease. Firstly, they present autoantigens to immature B cells resident in the bone marrow, which induces B cell tolerance to autoantigens.13 Secondly, these immune complexes bind to C1q and C4, components of the classical complement pathway, resulting in the rapid removal of the immune complexes to the spleen.13 The interaction occurs independently of C3 activation, and therefore the inflammatory, tissue damaging mechanisms of the complement cascade that follow the activation of C3 are avoided.13 In support of this mechanism, the increase in IgM at seven and nine hours occurred simultaneously with an increase in C4 observed in the same study,21 suggesting an interaction between IgM and complement. It is proposed therefore that the increase in IgM in the present study at seven and nine hours is a response to the increase in circulating autoantigens due to tissue damage induced by the downhill running. The ultimate function of this increase in IgM would be to rapidly clear autoantigens to prevent the development of a pathological autoimmune response.
It has recently been shown that IgM B cells function as memory B cells and may enhance secondary immune responses by synthesising high affinity IgM.22 It is speculated that the raised IgM during the first 12 hours after exercise, after run 2 compared with run 1, reflects a secondary antibody response to autoantigens. IgM memory to tissue damage autoantigens, developed after run 1, would result in the identification of autoantigens earlier, as well as an enhanced IgM antibody synthesis after run 2. This response would be protective, with a more pronounced removal of autoantigens from the circulation. In the light of this explanation, it is possible that the lower creatine kinase activities may be due to enhanced clearance of this sarcoplasmic enzyme by an IgM (and complement) memory response. Further investigation is required to determine whether there is an IgM response to sarcoplasmic enzymes such as creatine kinase.
The exact function of IgD is yet to be determined.23 However, IgD and IgD receptors on mature naive (no prior exposure to antigen) B cells have recently been shown to promote primary IgM antibody responses.23 However, IgD is decreased before a decrease in IgM expression and/or IgM isotype switching to IgG.23 Therefore it has been suggested that IgD expression on B cells prevents the activation of IgG memory B cells.23
IgG is typically involved in secondary antibody responses to pathogens. However, IgG is also implicated in promoting pathological autoimmune responses to autoantigens.13 The depressed IgG1 and IgG2 concentrations after run 2 suggest that immunoglobulin isotype switching from IgM to IgG1/IgG2 or synthesis of IgG1/IgG2 antibody was dampened. It is proposed that the increase in IgD, from 7–11 h, after both runs, represents an increased migration of mature naive B cells from the bone marrow into the circulation. IgD is typically co‐expressed with IgM on these B cells, indicating that there may be a signal to increase the naive IgD/IgM B cell concentration in the circulation.24 In addition, it is proposed that this increase in IgD prevents IgM antibody switching to IgG and/or memory B cell IgG response autoantigens. This would represent a protective response to prevent the development of a pathological IgG mediated inflammatory/autoimmune response to autoantigens.
It was recently proposed that exercise induced tissue damage might induce an immune shift to a TH2 phenotype.15 A TH2 immune shift is accompanied by an increase in interleukins 4 and 13, which induces immunoglobulin isotype switching from IgM to IgG4 and/or IgE.25 IgE mediates acute allergic inflammatory responses,25 and has also been shown to increase in response to tissue damage as part of the acute phase response.26 The initial synthesis of IgG4 and IgE is dependent on interleukin 4, but they are regulated independently.27
Importantly, individuals may display a TH2 immune shift without experiencing an increase in IgE or becoming allergic. During this “modified” TH2 response, there is an increase in IgG4 and a decrease in IgE.27 An increase in systemic IgG4 typically indicates an immune “tolerance” response to allergens in allergic patients.27 This has been shown in allergic patients receiving specific treatment to reduce their symptoms.27 It has been shown that when this “modified” TH2 response occurs, patients experience a reduction in atopic related symptoms.27 This IgG4 response typically coincides with a suppression of IgE concentrations. The mechanism responsible for this is not well understood, but the anti‐inflammatory cytokine interleukin 10 has been implicated.27
What is already known on this topic
Downhill running has been used as a model to examine immune and inflammatory responses to muscle damage, with the focus on the innate immune system
Clinical literature has suggested that immunoglobulins may also be involved in the immune response to muscle damage, although this has not been examined in the exercise literature
What this study adds
Immunoglobulin concentrations are altered by exercise induced muscle damage
The results indicate additional functions of immunoglobulin isotypes and subclasses other than their involvement in the defence against pathogens, including the mediation of inflammatory responses, the prevention of autoimmune responses, and the clearance of autoantigens, such as damaged muscle fragments or sarcoplasmic components
The increase in IgG4 during the initial 12 hours after both runs, together with the suppression of IgE during the first 12 hours and from 24 to 144 hours, after run 2, suggests that a “modified” TH2 response may occur in response to exercise induced damage. This would reflect a protective response to exercise induced tissue damage resulting in the downregulation of an IgE mediated acute inflammatory response. In addition, as IgE forms part of the acute phase response to tissue damage,26 the reduction in IgE after run 2 may reflect a reduced level of exercise induced tissue damage in response to this second bout. The reduced creatine kinase activity after run 2 indicates that there was reduced tissue damage. Immunoglobulin isotype switching to IgG4 and not IgG1 or IgG2 may also explain the decreased concentrations of IgG1 and IgG2. IgG4 does not activate complement or inflammatory responses, but rather induces immune tolerance to antigens.27
The IgG4 and IgE results may have significant implications for the use of exercise training for the treatment of atopic disease. The result suggests that eccentrically biased exercise may induce a “modified” TH2 response, reducing systemic IgE concentrations. Recently, exercise has been shown to reduce allergen specific IgE concentrations and airway inflammatory responses in a mouse model of atopic asthma.28 Therefore the use of eccentrically biased exercise in the treatment of atopic patients requires further research.
In addition, this “modified” TH2 response may also provide an explanation for why certain people do not show an increase in systemic IgE, despite an increase in the synthesis of TH2 cytokines during exercise.29 A “modified” TH2 response with an increase in IgG4 may occur in most people, whereas an inappropriate TH2 response, with an increase in systemic IgE and atopic related symptoms, only occurs in those who are genetically predisposed to switching to IgE and developing atopic disease. Further research is required to examine this possibility.
Conclusions
In summary, two bouts of downhill running induced alterations in immunoglobulin concentrations that suggested additional functions for immunoglobulins, separate from their role in pathogen defence. The alterations in IgD and IgM indicate involvement in the clearance of autoantigens as well as prevention of the development of highly specific autoantibodies. This response may prevent the development of pathological inflammatory/autoimmune responses to exercise induced tissue damage. The increase in IgG4 and suppression of IgE may represent a “modified” TH2 response to tissue damage that prevents the development of an IgE mediated, acute inflammatory response, while enhancing immune tolerance to autoantigens.
Footnotes
Competing interests: none declared
References
- 1.Brown S J, Child R B, Day S H.et al Exercise‐induced skeletal muscle damage and adaptation following repeated bouts of eccentric muscle contractions. J Sports Sci 199715215–222. [DOI] [PubMed] [Google Scholar]
- 2.Friden J, Seger J, Sjostrom M.et al Adaptive response in human skeletal muscle subjected to prolonged eccentric training. Int J Sports Med 19834177–183. [DOI] [PubMed] [Google Scholar]
- 3.Smith L L, Bond J A, Holbert D.et al Differential white cell count after two bouts of downhill running. Int J Sports Med 199819432–437. [DOI] [PubMed] [Google Scholar]
- 4.Thompson H S. The repeated bout effect and heat shock proteins: intramuscular HSP72 and HSP70 expression following two bouts of eccentric exercise in humans. Acta Physiol Scand 200217447–56. [DOI] [PubMed] [Google Scholar]
- 5.Clarkson P M, Tremblay I. Exercise‐induced muscle damage, repair, and adaptation in humans. J Appl Physiol 1988651–6. [DOI] [PubMed] [Google Scholar]
- 6.Byrnes W C, Clarkson P M, White J S.et al Delayed onset muscle soreness following repeated bouts of downhill running. J Appl Physiol 198559710–715. [DOI] [PubMed] [Google Scholar]
- 7.MacIntyre D L, Reid W D, McKenzie D C. Delayed muscle soreness. The inflammatory response to muscle injury and its clinical implications. Sports Med 19952024–40. [DOI] [PubMed] [Google Scholar]
- 8.Pizza F X, Mitchell J B, Davis B H.et al Exercise‐induced muscle damage: effect on circulating leukocyte and lymphocyte subsets. Med Sci Sports Exerc 199527363–370. [PubMed] [Google Scholar]
- 9.Mackinnon L T.Advances in exercise immunology. Torrens Park, Australia: Human Kinetics, 1999
- 10.Chan R K, Ding G, Verna N.et al IgM binding to injured tissue precedes complement activation during skeletal muscle ischemia‐reperfusion. J Surg Res 200412229–35. [DOI] [PubMed] [Google Scholar]
- 11.Chan R K, Verna N, Afnan J.et al Attenuation of skeletal muscle reperfusion injury with intravenous 12 amino acid peptides that bind to pathogenic IgM. Surgery 2006139236–243. [DOI] [PubMed] [Google Scholar]
- 12.Haahr P M, Pedersen B K, Fomsgaard A.et al Effect of physical exercise on in vitro production of interleukin 1, interleukin 6, tumour necrosis factor‐α, interleukin 2 and interferon‐γ. Int J Sports Med 199112223–227. [DOI] [PubMed] [Google Scholar]
- 13.Boes M. Role of natural and immune IgM antibodies in immune responses. Mol Immunol 2000371141–1149. [DOI] [PubMed] [Google Scholar]
- 14.Nieman D C, Nehlsen‐Cannarella S L. The effects of acute and chronic exercise and immunoglobulins. Sports Med 199111183–201. [DOI] [PubMed] [Google Scholar]
- 15.Smith L L. Overtraining, excessive exercise, and altered immunity: is this a T helper‐1 versus T helper‐2 lymphocyte response? Sports Med 200333347–364. [DOI] [PubMed] [Google Scholar]
- 16.Medicine ACoS ACSM's guidelines for exercise testing and prescription. 6th ed. Philadelphia: Lippincott, Williams and Wilkins, 2000
- 17.Martinez‐Amat A, Boulaiz H, Prados J.et al Release of α‐actin into serum after skeletal muscle damage. Br J Sports Med 200539830–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clarkson P M, Hubal M J. Exercise‐induced muscle damage in humans. Am J Phys Med Rehabil 20028152–69. [DOI] [PubMed] [Google Scholar]
- 19.Gleeson M, Pyne D B. Exercise effects on mucosal immunity. Immunol Cell Biol 200078536–544. [DOI] [PubMed] [Google Scholar]
- 20.Mackinnon L T. Immunoglobulin, antibody, and exercise. Exerc Immunol Rev 199621–35. [Google Scholar]
- 21.Semple S J, Smith L L, McKune A J.et al Systemic inflammatory responses to a repeated bout of eccentrically biased exercise. Eur J Appl Physiol. 2006;in press
- 22.Shi Y, Agematsu K, Ochs H D.et al Functional analysis of human memory B‐cell populations: IgD+CD27+ B cells are crucial in secondary immune response by producing high affinity IgM. Clin Immunol 2003108128–137. [DOI] [PubMed] [Google Scholar]
- 23.Yuan D, Dang T, Bibi R. Inappropriate expression of IgD from a transgene inhibits the function of antigen‐specific memory B cells. Cell Immunol 200121161–70. [DOI] [PubMed] [Google Scholar]
- 24.Sagaert X, De Wolf‐Peeters C. Classification of B‐cells according to their differentiation status, their micro‐anatomical localisation and their developmental lineage. Immunol Lett 200390179–186. [DOI] [PubMed] [Google Scholar]
- 25.Romagnani S. T‐cell responses in allergy and asthma. Curr Opin Allergy Clin Immunol 2001173–78. [DOI] [PubMed] [Google Scholar]
- 26.Szczeklik A, Jawien J. Possible role of IgE in acute‐phase response. Allergy 1997521149–1150. [DOI] [PubMed] [Google Scholar]
- 27.Roitt I, Brostoff J, Male D.Immunology. 6th ed. Edinburgh: Mosby, 2001
- 28.Pastva A, Estell K, Schoeb T R.et al Aerobic exercise attenuates airway inflammatory responses in a mouse model of atopic asthma. J Immunol 20041724520–4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki K, Nakaji S, Yamada M.et al Systemic inflammatory response to exhaustive exercise. Cytokine kinetics. Exerc Immunol Rev 200286–48. [PubMed] [Google Scholar]