Abstract
Background
Little information is available on the effect of strength training on vascular function, particularly in older people.
Objective
To determine the effect of resistance training on arterial stiffness and endothelial function in older adults.
Method
Eleven healthy men (mean (SEM) age 64 (1) years) performed 12 weeks of resistance training involving knee flexion and extension (three sets a day, two days a week).
Results
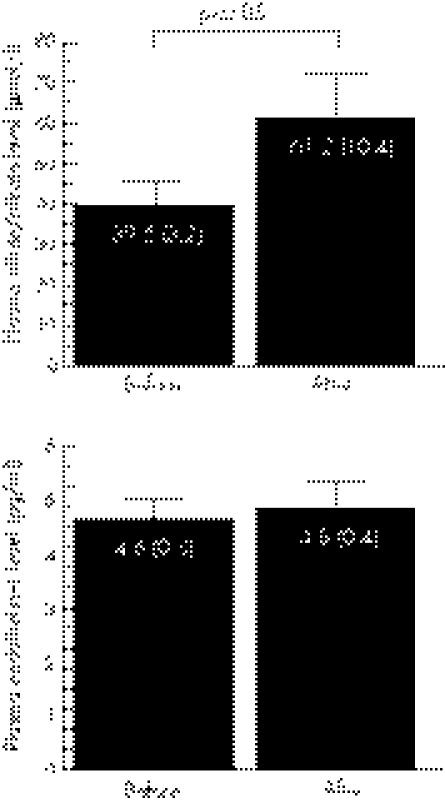
Resistance training increased maximal muscle power by 16% (p<0.0001). Arterial stiffness as assessed by aortic pulse wave velocity did not change with resistance training. Plasma concentration of nitric oxide (NO), measured as its stable end product (nitrite/nitrate), had increased (p<0.05) after resistance training (61.2 (10.4) v 39.6 (3.2) μmol/l). There was no change in plasma concentration of endothelin‐1.
Conclusion
The results suggest that short term resistance training may increase NO production without stiffening central arteries in healthy older men.
Keywords: strength training, arterial stiffness, endothelial function, nitric oxide
Vascular disease is the leading cause of mortality and morbidity in most modern countries. The vasculature was once considered to be a simple passive conduit. However, it is now recognised that it is a complex organ capable of producing a number of local vasoactive factors and actively buffering cardiac pulsation. With advancing age, the functional abilities of the vasculature decline, contributing to the increased incidence of vascular disease.1 Indeed, reduced bioavailability of nitric oxide (NO) and hardening of the central arteries are important factors in the initiation and progression of vascular disease.2,3
In the past, clinical practice for dealing with vascular disease placed major importance on diagnosis and treatment. However, the emphasis has now shifted to the primary and secondary prevention of these diseases.4 It has been well established that regular aerobic (endurance) exercise is an important strategy for prevention of vascular disease.5,6,7 In recent years, resistance training has been increasingly incorporated into the overall exercise programme for older adults because of its impact on muscle strength, functional capacity, and osteoporosis.8 Yet relatively little information is available on the effect of resistance training on vascular function, including endothelial function and arterial stiffness. Moreover, available data in young adults are not encouraging, suggesting that resistance training does not enhance endothelial function, as assessed by flow mediated dilatation,9 and increases, rather than decreases, arterial stiffness.10,11 Currently, the effects of resistance training on vascular functions in older adults are not known.
Accordingly, the major aim of this study was to determine effects of resistance training on arterial stiffness and endothelial function in older adults. We measured aortic pulse wave velocity (PWV), an established measure of arterial stiffness, and plasma concentrations of NO (measured as its stable end product, nitrite/nitrate (NOx)) and endothelin‐1, markers of endothelial function, before and after a period of resistance training. We hypothesised that resistance training would improve endothelial function and would not further increase arterial stiffness in older adults.
Methods
Subjects
Eleven healthy sedentary men (60–67 years old) volunteered to participate. All were normotensive (<140/90 mm Hg) and non‐obese, with no signs, symptoms, or history of any overt chronic diseases. None had smoked or were currently taking any drugs. The study was approved by the ethics committee of the University of Tsukuba. All subjects gave their written informed consent before inclusion in the study.
Resistance exercise training
After an adequate warm up, the subjects completed resistance exercise for two days a week for 12 weeks. They performed three sets of isokinetic (concentric and eccentric) knee extension and flexion exercises (10 repetitions/set at one repetition maximums) at 60°/s using a Biodex System 3 dynamometer (Biodex Medical System, Shirley, New York, USA). Training was performed on both legs. Trained assistants verbally encouraged the subjects and ensured proper technique. Subjects were instructed to refrain from any other regular exercise workouts during the entire study period.
Experimental procedure
All 11 subjects completed the resistance exercise intervention. Before they were tested, subjects fasted for 12 hours. Because NOx can be affected by dietary intake,12 participants were placed on a nitrite/nitrate‐free diet over the 24 hours before testing. Measurements after the resistance training programme were performed after subjects had rested for at least two days in order to rule out any acute effects from the most recent bout of resistance exercise. All of the measurements were performed at a constant room temperature (25°C).
Arterial stiffness
Carotid and femoral arterial PWVs were obtained in triplicate (formPWV; Colin Medical Technology, Komaki, Japan) after a resting period of at least 20 minutes as previously described.13 The distance travelled by the pulse wave was assessed with a random zero length measurement over the body surface with a non‐elastic tape measure. Pulse wave transit time was determined from the time delay between the proximal and distal “foot” waveforms. PWV was calculated as the distance divided by the transit time. At the time of PWV measurement, brachial blood pressure was also measured using oscillometry in triplicate (formPWV).
Plasma NOx concentration
This was determined as previously described.7,14 Briefly, 80 μl of each sample was incubated in a 270 μl incubation mixture containing 140 μl 125 mM potassium phosphate, 10 μl 87.5 μM FAD (Sigma, St Louis, Missouri, USA), 10 μl 3.5 mM NADPH, 90 μl double distilled water, and 20 μl nitrate reductase (1.75 U/ml; Sigma). The reaction was initiated by the addition of nitrate reductase to convert nitrate into nitrite. The reaction was terminated by the addition of 0.8 ml Griess reagent and 0.45 ml double distilled water. After each mixture had been centrifuged, the supernatants were analysed spectrophotometrically.
Plasma endothelin‐1 concentration
Each blood sample was placed in a chilled tube containing aprotinin and EDTA, and was then centrifuged at 2000 g for 15 minutes at 4°C. Plasma was acidified with 3 ml 4% acetic acid, and immunoreactive endothelin‐1 was extracted with a Sep‐Pak C18 cartridge (Waters, Milford, Massachusetts, USA) as previously described.6,14 The eluates were reconstituted with 0.25 ml assay buffer and subjected to sandwich enzyme immunoassay using immobilised mouse monoclonal antibody.
Maximal muscular strength
Maximal muscular power was assessed before and after resistance training using the Biodex System 3 dynamometer at 60°/s.
Metabolic risk factors for coronary heart disease
Fasting serum concentrations of cholesterol and insulin, and plasma concentration of glucose were determined using standard enzymatic techniques.
Statistical analysis
Student's t tests for paired values were performed to evaluate differences in dependent variables before and after resistance training. p<0.05 was accepted as significant. Values are expressed as mean (SEM).
Results
As shown in table 1, there were no significant differences in body weight, heart rate, blood pressure, and blood concentrations of cholesterol and glucose before and after the training programme, whereas serum insulin concentration had decreased significantly after resistance training. Maximal muscular power had increased significantly after resistance training (120.9 (6.4) and 139.1 (5.4) N.m before and after training).
Table 1 Changes in selected subject characteristics in response to exercise training.
| Before | After | |
|---|---|---|
| Height (cm) | 167 (3) | – |
| Body weight (kg) | 63 (3) | 62 (3) |
| Body mass index (kg/m2) | 22.4 (0.9) | 22.3 (0.8) |
| Heart rate (beats/min) | 59 (2) | 57 (2) |
| Blood pressure (mm Hg) | ||
| Systolic | 123 (3) | 120 (3) |
| Diastolic | 80 (2) | 76 (2) |
| Total cholesterol (mmol/l) | 5.33 (0.25) | 5.31 (0.23) |
| HDL‐cholesterol (mmol/l) | 1.53 (0.12) | 1.58 (0.09) |
| LDL‐cholesterol (mmol/l) | 3.15 (0.24) | 3.24 (0.18) |
| Serum insulin (μU/ml) | 6.6 (0.5) | 4.0 (0.8)* |
| Plasma glucose (mmol/l) | 5.6 (0.2) | 5.6 (0.2) |
| Maximal muscular strength (N.m) | 121 (6) | 139 (5)† |
Values are mean (SEM).
*p<0.01, †p<0.0001 compared with before.
HDL, High density lipoprotein; LDL, low density lipoprotein.
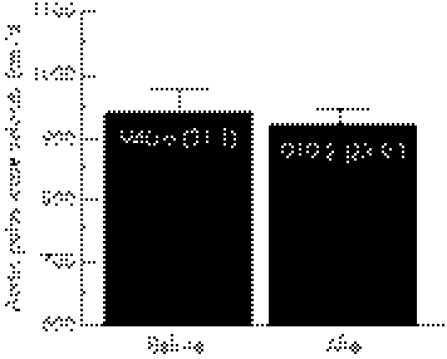
There was no significant change in aortic PWV after resistance training (fig 1). Plasma endothelin‐1 concentration did not change, whereas plasma concentration of NOx increased significantly with resistance training (fig 2).
Figure 1 Aortic pulse wave velocity before and after resistance training in healthy older men (n = 11). Values are mean (SEM).
Figure 2 Plasma nitrite/nitrate and endothelin‐1 concentration before and after resistance training in healthy older men (n = 11). Values are mean (SEM).
Discussion
The primary findings of this study are that the arterial stiffening previously observed with resistance training in young adults did not occur in older men and that short term resistance training improved endothelial function, as assessed by NOx, without affecting endothelin‐1 concentration. These results suggest that resistance training in older adults would produce beneficial effects on the vasculature without any unfavourable effects.
What is already known on this topic
Recent studies have reported that resistance training is associated with greater central arterial stiffness in young healthy men
It is not known whether resistance training influences arterial stiffness in older people
What this study adds
Short term resistance training did not result in arterial stiffening in older men but improved endothelial function
These results suggest that resistance training in older adults would produce beneficial effects on the vasculature without having unfavourable effects
NO produced in the vascular endothelial cells has a potent vasodilatory effect, and has been proposed to have antiatherosclerotic properties.3 On the other hand, endothelin‐1 is a potent vasoconstrictor peptide produced by vascular endothelial cells, and has potent proliferating activity on vascular smooth muscle cells.15 Therefore altered plasma endothelin‐1 and NO concentrations may have important clinical significance. In the present study, plasma NOx concentration had increased significantly after resistance training, whereas no change was observed in plasma endothelin‐1 concentration. We have previously reported that plasma NOx concentrations are increased,7,14 and plasma endothelin‐1 concentrations decreased,6,14 with aerobic exercise training in healthy humans. The present study extends the findings on the beneficial effects of exercise training on endothelial function to resistance training. Given the current trend to prescribe resistance training for older adults, these findings have important implications for the prescription of exercise for older adults.
Central (elastic) arteries become stiffer with age,16,17 and this is associated with the development of several pathological conditions, including hypertension, atherosclerosis, congestive heart failure, stroke, and aortic root regurgitation.2,18 Recent studies have reported that resistance training is associated with greater central arterial stiffness in young healthy men.10,11 However, it is not known whether resistance training influences arterial stiffness in older people. We found that central arterial stiffness, as assessed by aortic PWV, did not change with resistance training. No change in arterial stiffness may be considered a negative finding. However, viewed differently, it is rather encouraging that the arterial stiffening observed in previous studies on young adults did not occur in this study on older adults. Thus older adults may be able to enjoy the benefits of resistance training without accruing arterial stiffening. We cannot exclude the possibilities, however, that no changes in arterial stiffness were due to short term (rather than long term) resistance training, localised leg (rather than whole body) training, and/or the use of a well established yet less robust technique to assess arterial stiffness (PWV changes <10%/decade with aging16). Further studies are needed to address these issues.
In addition to the experimental conditions described above, there are several other important limitations of this study that should be emphasised. Firstly, there was no control group that remained sedentary during the intervention period. Secondly, NOx is a secondary metabolite of NO and may not be the best measure of NO bioavailability.12
In summary, our findings suggest that short term leg resistance training may increase endothelial function without inducing arterial stiffening in older adults.
Acknowledgements
This work was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (18300215, 18650186), the Meiji Yasuda Life Foundation of Health and Welfare, and the NIH (AG20966).
Abbreviations
Nox - nitrite/nitrate
PWV - pulse wave velocity
Footnotes
Competing interests: none declared
References
- 1.Lakatta E G. Age‐associated cardiovascular changes in health: impact on cardiovascular disease in older persons. Heart Fail Rev 2002729–49. [DOI] [PubMed] [Google Scholar]
- 2.Glasser S P, Arnett D K, McVeigh G E.et al Vascular compliance and cardiovascular disease: a risk factor or a marker? Am J Hypertens 1997101175–1189. [DOI] [PubMed] [Google Scholar]
- 3.Moncada S, Palmer R M, Higgs E A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev 199143109–142. [PubMed] [Google Scholar]
- 4.Mosca L, Arnett D K, Dracup K.et al Task Force on Strategic Research Direction: Population/Outcomes/Epidemiology/Social Science Subgroup key science topics report. Circulation 2002106e167–e172. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka H, Dinenno F A, Monahan K D.et al Aging, habitual exercise, and dynamic arterial compliance. Circulation 20001021270–1275. [DOI] [PubMed] [Google Scholar]
- 6.Maeda S, Tanabe T, Miyauchi T.et al Aerobic exercise training reduces plasma endothelin‐1 concentration in older women. J Appl Physiol 200395336–341. [DOI] [PubMed] [Google Scholar]
- 7.Maeda S, Tanabe T, Otsuki T.et al Moderate regular exercise increases basal production of nitric oxide in elderly women. Hypertens Res 200427947–953. [DOI] [PubMed] [Google Scholar]
- 8.Pollock M L, Franklin B A, Balady G J.et al AHA Science Advisory. Resistance exercise in individuals with and without cardiovascular disease: benefits, rationale, safety, and prescription, an advisory from the Committee on Exercise, Rehabilitation, and Prevention, Council on Clinical Cardiology, American Heart Association; position paper endorsed by the American College of Sports Medicine. Circulation 2000101828–833. [DOI] [PubMed] [Google Scholar]
- 9.Rakobowchuk M, McGowan C L, de Groot P C.et al Endothelial function of young healthy males following whole body resistance training. J Appl Physiol 2005982185–2190. [DOI] [PubMed] [Google Scholar]
- 10.Miyachi M, Donato A J, Yamamoto K.et al Greater age‐related reductions in central arterial compliance in resistance‐trained men. Hypertension 200341130–135. [DOI] [PubMed] [Google Scholar]
- 11.Miyachi M, Kawano H, Sugawara J.et al Unfavorable effects of resistance training on central arterial compliance: a randomized intervention study. Circulation 20041102858–2863. [DOI] [PubMed] [Google Scholar]
- 12.Lauer T, Kleinbongard P, Kelm M. Indexes of NO bioavailability in human blood. News Physiol Sci 200217251–255. [DOI] [PubMed] [Google Scholar]
- 13.Sugawara J, Hayashi K, Yokoi T.et al Brachial‐ankle pulse wave velocity: an index of central arterial stiffness? J Hum Hypertens 200519401–406. [DOI] [PubMed] [Google Scholar]
- 14.Maeda S, Miyauchi T, Kakiyama T.et al Effects of exercise training of 8 weeks and detraining on plasma levels of endothelium‐derived factors, endothelin‐1 and nitric oxide, in healthy young humans. Life Sci 2001691005–1016. [DOI] [PubMed] [Google Scholar]
- 15.Rubanyi G M, Polokoff M A. Endothelins: molecular biology, biochemistry, pharmacology, physiology, and pathophysiology. Pharmacol Rev 199446325–415. [PubMed] [Google Scholar]
- 16.Avolio A P, Deng F Q, Li W Q.et al Effects of aging on arterial distensibility in populations with high and low prevalence of hypertension: comparison between urban and rural communities in China. Circulation 198571202–210. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka H, DeSouza C A, Seals D R. Absence of age‐related increase in central arterial stiffness in physically active women. Arterioscler Thromb Vasc Biol 199818127–132. [DOI] [PubMed] [Google Scholar]
- 18.Rowe J W. Clinical consequences of age‐related impairments in vascular compliance. Am J Cardiol 19876068G–71G. [DOI] [PubMed] [Google Scholar]