Abstract
The case is reported of a male track and field athlete with breathing difficulties at rest and during exercise, which were exacerbated in the supine position and during water immersion. Right hemidiaphragmatic paralysis was diagnosed. The cause of this relatively benign disorder is not known and there are no serious clinical implications. There is no treatment, but a continuation of exercise together with interventions to strengthen the subsidiary inspiratory muscles is recommended.
Keywords: diaphragm paralysis, electromagnetic stimulation, phrenic nerve
In December 2003, a 39 year old white male track and field athlete presented to the Olympic Medical Institute for routine physiological assessment. After a standard pre‐exercise questionnaire had been completed and informed consent had been obtained, it was established that the athlete had no history of note. The questionnaire revealed that he was experiencing breathing difficulties at rest and during exercise. Questioning established that he found inspiration difficult at rest with an exacerbation of symptoms in a supine position (nocturnally) and during water immersion (bathing or swimming). Furthermore, he described excessive dyspnoea during exercise and reduced exercise performance, particularly in events requiring a substantial aerobic contribution—for example, running. Symptoms had appeared four months previously after competition in a Highland Games event; however, the athlete had no recollection of trauma at that time. Treatment with anti‐inflammatories and antibiotics had been ineffective.
Standard assessment of lung function revealed a forced vital capacity (FVC) of 3.15 litres (66% of predicted) in a seated position, which fell by 48% to 1.6 litres in a supine position. Physical examination found an absence of breath sounds and abnormal percussion at the right lung base. Further, a slight atrophy of the right trapezius was observed with no other peripheral muscle weakness. A radiograph (fig 1) showed evidence of an elevated right hemidiaphragm in the absence of lung or pleural lesions. A computed tomography scan confirmed the elevation in the right hemisphere of the diaphragm, but was otherwise normal.
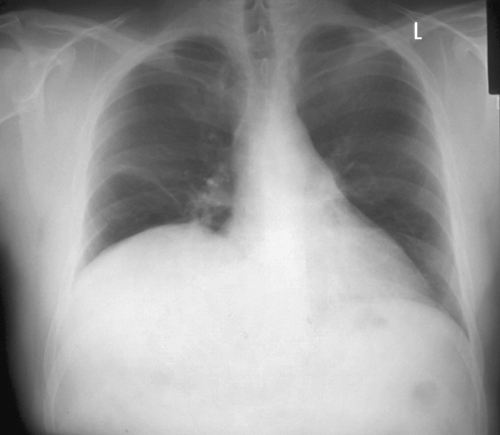
Figure 1 Radiograph showing elevated right hemidiaphragm.
A resting electrocardiogram (ECG) showed left axis deviation with low voltage in the limb leads. ECG anomalies are often observed in athletes, but left axis deviation and low voltage limb leads are rare.1 Echocardiography showed normal cardiac morphology and function, indicating that the observed left axis deviation and low voltage limb leads were associated with an altered orientation of the heart caused by an increased abdominal excursion into the thoracic cavity on the right side.
The athlete was referred to a specialist unit for assessment of phrenic nerve function. Unilateral magnetic stimulation of the phrenic nerve was performed, and transdiaphragmatic pressure was assessed using oesophageal and gastric balloon catheters.2 The results revealed hemidiaphragm paralysis with absent twitch response on the right.
A magnetic resonance image of the phrenic nerve and cervical roots was ordered to establish a possible cause for the observed paralysis. T1 and T2 weighted sagittal and axial scans with gadolinium contrast showed a tiny area of altered signal in the chord at C3. This altered signal probably represented a tiny syrinx and is of doubtful clinical significance (fig 2). Otherwise, there was no swelling in the chord, no compression on the chord, and no evidence of disc lesion.
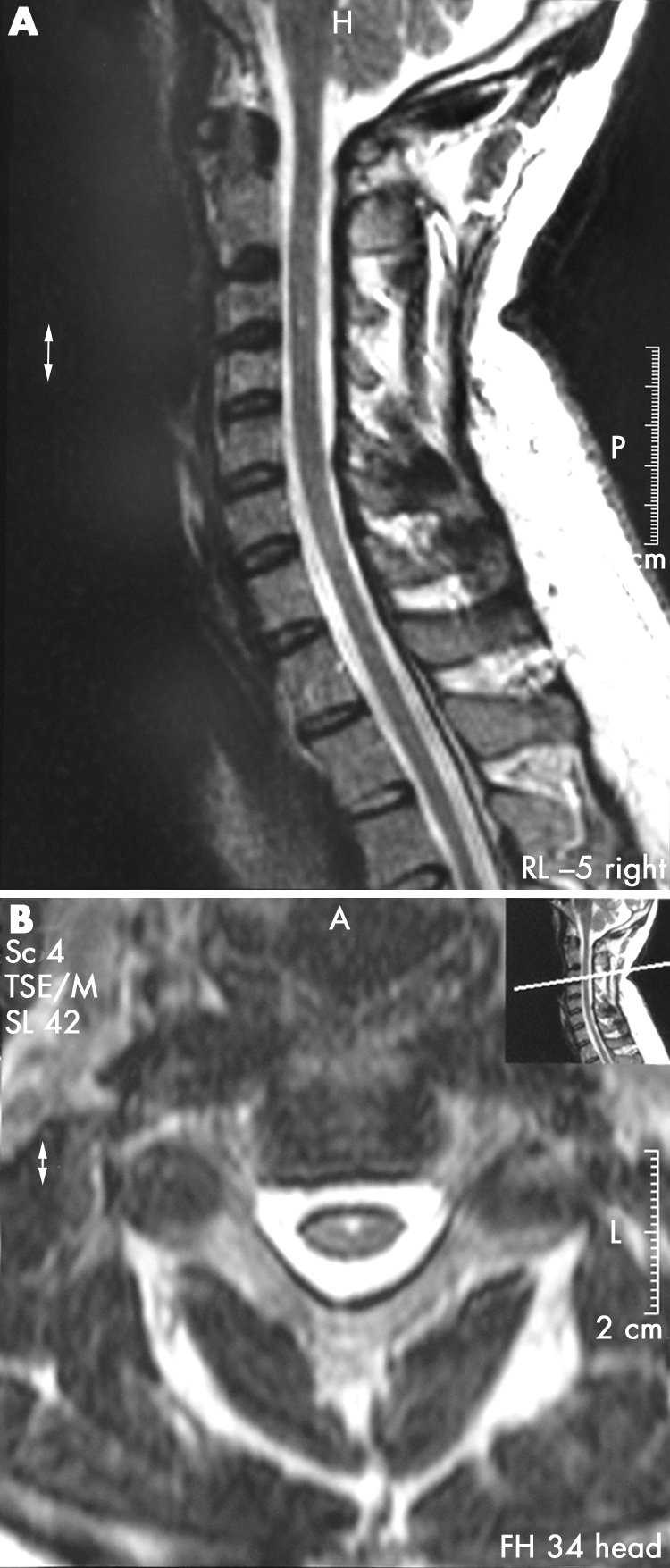
Figure 2 Magnetic resonance image showing altered signal in the chord at the C3 level (arrow), probably indicative of a tiny syrinx of no clinical significance.
The athlete was diagnosed with right hemidiaphragmatic paralysis of unknown aetiology, but probably associated with a neuralgic amyotrophy. No treatment currently exists for this condition, but the athlete was prescribed a course of inspiratory muscle training to strengthen his subsidiary inspiratory muscles. Unilateral diaphragmatic paralysis is considered a relatively benign disorder with no serious clinical implications. Exercise is not contraindicated in this condition and should in fact be encouraged. Therefore the athlete was advised to continue training and competing to his symptom limiting maximum.
Discussion
Paralysis of the diaphragm occurs as a result of an interruption of the phrenic nerve from infectious, iatrogenic, or malignant causes. The most common cause of diaphragm paralysis, however, is not known. Previously, hemidiaphragm elevation on chest radiograph, in the absence of ipsilateral lung disease, was assumed to indicate severe hemidiaphragm dysfunction or paralysis. Recent evidence, however, suggests that a radiograph, although having moderate sensitivity (a low number of false negatives), has poor specificity (a large number of false positives) for the diagnosis of diaphragm dysfunction.3 Isolated hemidiaphragm elevation on a chest radiograph is probably of little value in the diagnosis of unilateral hemidiaphragm paralysis. Although the condition is unlikely if diaphragm elevation is absent on the chest radiograph, transdiaphragmatic pressure is the “gold standard” assessment of hemidiaphragm function and therefore the diagnosis of diaphragmatic dysfunction/paralysis.3
The athlete in this case study described classical symptoms of severe diaphragmatic dysfunction/paralysis. Dyspnoea at rest that is exacerbated during exercise is universal in this condition. At rest, the reduction in FVC observed in diaphragmatic paralysis is compensated for by a concomitant increase in breathing frequency to normalise minute ventilation (Ve) .4 Aggravation of dyspnoea as a result of immersion is common in diaphragmatic paralysis because of an increase in intra‐abdominal pressure leading to a reduced FVC and increased work of breathing. Reduced exercise capacity associated with impaired Ve is a common finding in unilateral and bilateral diaphragm paralysis.5 In patients with long standing unilateral diaphragmatic paralysis, however, submaximal exercise capacity appears to be well preserved. This preservation of submaximal exercise capacity is associated with increased activity of the inspiratory intercostal and abdominal muscles.5 Despite the recruitment of subsidiary muscles, however, the reduced FVC observed in diaphragmatic paralysis results in a reduced peak ventilation (Vepeak) leading to an abnormal Vo2peak/Ve peak ratio, and a resultant reduction in maximal exercise performance.5
The long term recovery of severe diaphragmatic dysfunction/paralysis is not fully understood. Data on the long term recovery of diaphragm strength in patients with neuralgic amyotrophy suggest that symptomatic improvement is common in the short term. Functional improvement is expected in most patients, but this may take a considerable time, with data suggesting that a minimum of three years is required for substantial recovery.6 Functional recovery is associated with re‐innervation of the phrenic nerve and is therefore slow. Enhancing the strength of inspiratory intercostal muscles and abdominal muscles may attain improvement in symptoms and pulmonary function at rest and during exercise. Therefore respiratory training is likely to be of benefit (and without significant side effects) in the short and long term treatment of diaphragmatic paralysis. Owing to the likely recovery of neuralgic amyotrophy in the present case study, however, conclusions on the efficacy of inspiratory muscle training are difficult to draw.
Footnotes
Competing interests: none declared
References
- 1.Sharma S, Whyte G, Elliott P M.et al Electrocardiographic changes in 1000 highly trained elite athletes. Br J Sports Med 199930319–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mills G, Kyroussis D, Hamnegard C.et al Unilateral magnetic stimulation of the phrenic nerve. Thorax 1995501162–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chetta A, Rehman A, Moxham J.et al Chest radiography cannot predict diaphragm function. Respir Med 20059939–44. [DOI] [PubMed] [Google Scholar]
- 4.Schoenhofer B, Koehler D, Polkey M. Influence of immersion in water on muscle function and breathing pattern in patients with severe diaphragm weakness. Chest 20041252069–2074. [DOI] [PubMed] [Google Scholar]
- 5.Hart N, Nickol A, Cramer D.et al Effect of severe isolated unilateral and bilateral diaphragm weakness on exercise performance. Am J Respir Crit Care Med 20021651265–1270. [DOI] [PubMed] [Google Scholar]
- 6.Hughes P, Polkey M, Moxham J.et al Long‐term recovery of diaphragm strength in neuralgic amyotrophy. Eur Respir J 199913379–384. [DOI] [PubMed] [Google Scholar]


