Abstract
Objective
To evaluate the short‐term effects of exercise in patients with major depression.
Design
Prospective, randomised, controlled study.
Setting
A university hospital.
Patients
A consecutive series of 38 inpatients with a major depression episode undergoing standard clinical antidepressant drug treatment.
Interventions
Patients were randomly assigned to an exercise (walking, n = 20) or placebo (low‐intensity stretching and relaxation exercises, n = 18) group. Training was carried out for 10 days.
Main outcome measurements
Severity of depression assessed with the Bech‐Rafaelsen Melancholy Scale (BRMS) and the Center for Epidemiologic Studies Depression scale (CES‐D).
Results
After 10 days, reduction of depression scores in the exercise group was significantly larger than in the placebo group (BRMS: 36% v 18%; CES‐D: 41% v 21%; p for both = 0.01); the proportion of patients with a clinical response (reduction in the BRMS scores by more than six points) was also larger for the exercise group (65% v 22%, p<0.01).
Conclusions
Endurance exercise may help to achieve substantial improvement in the mood of selected patients with major depression in a short time.
Exercise has been shown to improve mood and to reduce anxiety in healthy people.1,2 These findings have led to a growing interest in the effects of physical activity in patients with affective disorders. However, although exercise is often used as an additional treatment for depression, scientific evidence about the effects of this intervention is lacking. Although two meta‐analyses suggested that exercise may be as effective as psychotherapy3,4 and more effective than other behavioural interventions4 for treating depression, a meta‐analysis could not determine the effects of exercise on depression because of a lack of good‐quality research on clinical populations.5
A growing body of evidence shows that regular physical activity results in functional and morphological adaptations in the brain. Exercise increases the expression of growth factors (insulin‐like growth factor‐I, nerve growth factor and brain‐derived neurotrophic factor), which trigger the production of proteins of signal transduction cascades associated with memory processes.6,7 Indeed, research in animal models has shown that endurance training increases cortical capillary supplies, the number of synaptic connections and the development of new neurones.8 These processes may result in a higher efficiency, plasticity and adaptability of the brain.
Several randomised controlled trials have shown that physical activity improves the mood of patients with mild to moderate depression after several weeks.9,10,11 However, experiments in animals indicate that even a single exercise bout generates considerable changes in the brain concentration of neurotransmitters involved in the pathophysiology of depression.12 We have previously reported that exercise may substantially improve the mood of patients undergoing bone marrow transplantation13 or with therapy‐resistant depression14 in a short time. The results of a trial suggest that even a single exercise bout may improve the mood of patients with clinical depression.14 Further, two randomised controlled trials showed an association between exercise amount15 and duration11,15 and reduction of symptoms in patients with depression. Finally, a recent study provided evidence for exercise as a possible adjuvant treatment for patients with poor response to antidepressant drugs.16
These findings could be of clinical relevance, as about 30% of patients do not respond to conventional pharmacotherapy, and antidepressants require 1–4 weeks before they show any therapeutic effect. The introduction of exercise programmes in the early treatment of depression could help reduce the duration of therapeutic latency. However, there is a lack of information about several critical features of exercise in the treatment of mood disorders. The diversity of potential clinical populations, the multiple therapeutic settings (single or adjuvant treatment, augmentation to improve remission rates or long‐term treatment) and the variety of exercise programmes are factors that may substantially affect treatment response. Therefore, there is a need for information about the possibilities and mechanisms of action of diverse exercise programmes in patients with depression in different settings. In the present study, we evaluated the effect of a short‐time exercise programme as adjuvant treatment on patients with major depression undergoing standard clinical antidepressant drug treatment.
Methods
A consecutive series of patients admitted to a university hospital for treatment of a major depressive episode according to the Diagnostic and statistical manual of mental disorders, 4th edn criteria17 were considered for participation in the study. Inclusion criteria were depressive episodes with a score of >12 on the Bech‐Rafaelsen Melancholy Scale (BRMS),18 corresponding to moderate to severe depression, age 20–70 years, ability to walk and to understand written German. Exclusion criteria were associated organic disease, schizophrenic symptoms, epilepsy and referral for electroconvulsive therapy.
We expected the reduction in the BRMS depression scores to be about 20% greater in the intervention than in the control group on the basis of pilot research.19 A calculation of the sample size showed that a minimum of 20 patients was required in each group to detect this difference with a probability of a 5% and 20% α and β error. In all, 39 of 45 patients who fulfilled the inclusion criteria agreed to participate in the study and were recruited (table 1).
Table 1 Characteristics of the study population.
| Characteristics | Training group | Placebo group |
|---|---|---|
| Number | 20 | 18 |
| Age (in years), mean (SD) | 49 (13) | 50 (13) |
| Sex | 9 men, 11 women | 8 men, 10 women |
| Diagnosis | ||
| Bipolar affective disorder, moderate to severe | 6 | 1 |
| Depressive episode, moderate to severe | 7 | 6 |
| Recurrent depressive disorder, moderate to severe | 6 | 10 |
| Dysthymia | 1 | – |
| Persistent affective disorder | – | 1 |
| Days between hospital admission and recruitment, mean (SD) (range) | 8.1 (6) (3–27) | 6.6 (5.3) (2–26) |
| Beginning of antidepressant treatment | ||
| >6 weeks before recruitment | 5 | 2 |
| Between 15 and 21 days before recruitment | 1 | 1 |
| Between 8 and 14 days before recruitment | 1 | 2 |
| <7 days before recruitment | 10 | 11 |
| After recruitment | 3 | 2 |
| Treatment*: | ||
| Tricyclic and tetracyclic antidepressant | 9 | 6 |
| Specific serotonin re‐uptake inhibitors | 5 | 5 |
| Monoaminooxidase inhibitors | 1 | 3 |
| Lithium carbonate | 5 | 3 |
| Trazodone | 1 | 1 |
| Serotonin and noradrenaline re‐uptake inhibitors | 1 | 2 |
| Noradrenalin re‐uptake inhibitors | 3 | 1 |
| No pharmacological treatment | 1 | 2 |
| Sleep deprivation therapy | 7 | 3 |
*Five patients in the training group and three patients in the placebo group received more than one drug.
Patients were stratified according to the antidepressive medication they received at the time of recruitment (see table 1 for a description of strata) and randomly assigned to an endurance exercise or a placebo activity group on the basis of a computer‐generated number list. For allocating patients into the groups, two study collaborators contacted the randomisation centre by telephone. The randomisation sequence was not revealed until the assignment of interventions.
To quantify the training intensity, maximal physical performance in the endurance exercise group was assessed by a modified Bruce treadmill test under continuous electrocardiogram monitoring on admission and at the end of the study. The test was started at 3 km/h and carried out until patients reached 80% of the expected maximal heart rate, defined as 220−age. Lactate concentration in capillary blood was assessed every 3 min. Maximal oxygen uptake in ml/kg/min was calculated by using the maximal physical performance according to the guidelines of the American College of Sports Medicine.20
Endurance exercise consisted of daily walking on a treadmill for 10 days. The exercise regimen was designed according to an interval‐training pattern. Patients walked five times for 3 min with a mean (SD) intensity corresponding to a lactate concentration of 3 (0.5) mmol/l in capillary blood and a heart rate of 80% of the maximum. Between these workloads, patients walked at half speed for 3 min. Intensity of effort was evaluated daily with the Borg Rating of Perceived Exertion Scale,21 a visual scale ranging from 6, “very easy”, to 20, “very strenuous”. The selected training intensity on this scale corresponded to a value of 13–14 (somewhat strenuous). Heart rate was continuously monitored during training and was used to evaluate the training intensity. As heart rate during exercise decreased due to training adaptation and improved economy,22 the treadmill elevation was increased to maintain the training intensity. During training, patients were continuously supervised by instructed study personnel. Interaction was limited to general comments about the walking technique and training‐related bodily sensations such as amount of exertion or muscle complaints.
Patients in the placebo exercise group carried out a daily 30‐min programme. It consisted of light stretching exercises for the calves, thighs, back, shoulders and pectoral muscles, as well as relaxation exercises. Each muscle group was stretched for 20 s, with a resting interval of 40 s between stretching series. Thus, the total activity time was less than 10 min daily. Participants were instructed not to put themselves under stress. Special attention was given to avoiding any major physical exertion. Both programmes, endurance exercises and relaxation exercises, were carried out individually in the same room and supervised by the same two study collaborators. The total amount of time for the treadmill walking and stretching was equivalent for both groups (30 min daily for 10 days). There was no contact between patients during the training sessions.
Severity of depression was rated at the beginning and at the end of the programme by a psychologist using the BRMS.18 This instrument was developed from the Hamilton Depression scale to measure more strictly the quantitative aspect of depression. The scale consists of 11 items with scores ranging from 0 to 44; scores between 6 and 14 show mild, between 15 and 25 moderate and between 26 and 44 severe depression. The BRMS has been shown to have a higher sensitivity and internal construct validity, and to be more sensitive than the Hamilton Depression Scale in detecting mood changes during antidepressive treatment.23,24 All patients were rated by the same investigator, who was unaware of the participants' group assignment. To evaluate the subjective mood changes, patients rated the severity of depression symptoms before and at the end of the programme using the Center for Epidemiologic Studies Depression scale (CES‐D).25 All tests were carried out before exercise.
The study was approved by the institutional ethics committee. All patients gave their written informed consent. Owing to the characteristics of the study, it was not possible to blind patients to the intervention. Furthermore, we did not consider it practical to inform patients about the expected outcomes, as it would have introduced an obvious source of bias. Therefore, patients were told that the primary aim of the study was to compare the effects of two different types of exercise, resistance training and stretching, on mood.
Statistical analysis
The study was carried out following the “intention to treat” principle. Three patients (two in the placebo group, one in the endurance exercise group) dropped out of the study for personal reasons before the end of the programme. A complete assessment of mood changes in these patients was carried out 11 days after training started, and these data were included in the statistical analysis. One patient in the placebo group decided to leave the hospital after 4 days, and there were no data on her mood status at discharge. One patient in the endurance exercise group completed the training programme but refused to give a subjective evaluation of his mood status. The data of these patients were included in the statistical analysis using the worst rank assumption.26
According to the usual criteria, a response to therapeutic intervention was defined as a reduction of six or more points in the BRMS. An analysis of covariance with two factors (training or control group, and drug coded according to pharmacological group of the antidepressant) controlling for baseline depression scores was carried out to evaluate the effect of treatment and drug on the end points. Differences between groups and changes in a group were evaluated by using the Fisher, the Wilcoxon or the Mann–Whitney test.
We also compared the length of hospitalisation of both groups. For this purpose, hospitalisation was defined as the number of days between the beginning of training and discharge from hospital. To avoid an intentional or unintentional manipulation of results, medical teams were not informed about this secondary end point of the study. A value of p<0.05 was considered significant. As most end points were closely related, no Bonferroni corrections were carried out. Statistical analyses were performed with the software SAS V.8.2.
Results
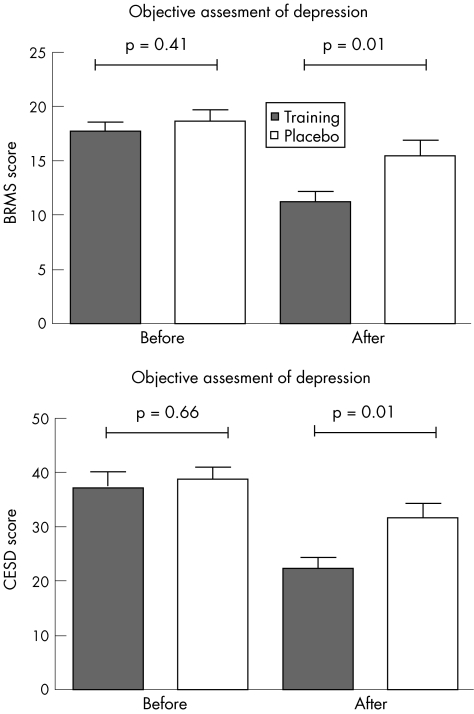
The rating of depression severity (BRMS score) on admission showed no difference between the groups (p = 0.41; fig 1 and table 2). After 10 days, the exercise group had a substantially greater reduction in depression scores (36%) than the control group (18%; p = 0.01). This difference resulted in significantly lower depression scores for the training group at the end of the study, on adjusting for baseline scores. Exercise (b = −4.57, se(b) = 1.32, F = 15.61, df = 1.26, p = 0.001) and depression scores on admission (b = 0.86, se(b) = 0.12, F = 34.96, df = 1.26, p<0.001) had a significant effect on the end points; the effect of the concomitant drug on the end points was not significant (F = 2.24, df = 4.26, p = 0.09).
Figure 1 Depression scores before and after the interventions. BRMS, Bech–Rafaelsen Melancholy Scale; CES‐D, Center for Epidemiologic Studies Depression scale.
Table 2 Depression scores at recruitment and at the end of the study.
| Training | Placebo | p Value | |
|---|---|---|---|
| BRMS | |||
| Recruitment | 17.6 ( 3.7) (12–25) | 18.7 (4.2) (12–26) | 0.41 |
| End | 11.2 (4) (2–19) | 15.5 (6.1)(4–28) | 0.01 |
| Difference between scores | −6.45 (0.8) (−1 to −15) | −3.2 (3.7) (+5 to −12) | 0.01 |
| CES‐D | |||
| Recruitment | 37.6 (12.9) (13–52) | 39.2 (8.5) (21–56) | 0.66 |
| End | 22.4 (10.0) (1–37) | 31.8 (11.2) (10–46) | 0.01 |
| Difference between scores | −15.2 (2.2) (0 to −33) | −7.4 (20.0) (+10 to −22) | 0.01 |
BRMS, Bech–Rafaelsen Melancholy Scale; CES‐D, Center for Epidemiologic Studies Depression scale.
Values are shown as mean (SD), followed by ranges in parentheses.
A clinical response (reduction of BRMS scores by six or more points) was observed in 13 patients in the endurance training group (65%) and four patients in the placebo group (22%; p = 0.009).
The subjective evaluation of depression (CES‐D scores) of the patients of the two groups did not differ at the beginning of the study (p = 0.66, table 2). After the intervention, the reduction in the CES‐D scores was substantially greater in the endurance training group (41%) than in the placebo group (21%, p = 0.01). Thus, the training group had significantly lower CES‐D scores at the end of the study than the control group (b = −10.15, se(b) = 3.39, F = 10.51, df = 1.25, p = 0.003), on adjusting for baseline scores (b = 0.59, se(b) = 0.15, F = 18.05, df = 1.25, p = 0.001) and medication (F = 0.24, df = 4.25, p = 0.91).
Patients reported no negative effects of exercise (muscle pain, tightness or fatigue) in the course of the programme. All participants in both groups tolerated the intervention without complications. The clinical records of all patients between the end of the training programme and discharge from hospital were evaluated after completing the study. In the 2 weeks after the completion of training, one patient in the placebo group required gastric lavage after intentionally ingesting a toxic dose of carbamazepine, and one patient in the exercise group inflicted a superficial cut in her arm. There were no other incidents.
The maximum oxygen uptake of the endurance training group remained unchanged during the study (27.9 (10.3) ml/kg/min before, v 28.1 (10.1) after; p = 0.99).
The length of hospitalisation (calculated as days between the beginning of the exercise programme and discharge from hospital) was shorter for the training group; however, the difference between the two groups was not significant (endurance exercise group: 47 (2) days; placebo group: 58 (38) days; p = 0.32).
Discussion
Antidepressants, which may relieve symptoms in about 60% of patients with depression, have a latency of several weeks until taking effect. During this time, patients and physicians are confronted with a lack of therapeutic options. Thus, there is a need for new treatments which can reduce symptoms of depression in a short time. The results of our study indicate that endurance training can quickly lead to a substantial improvement of mood in patients with severe depression. In fact, 13 (65%) patients in the endurance training group, but only 4 (22%) patients in the placebo group, had a reduction of BRMS scores by >6 points, corresponding to a clinical response. Further, at the end of the study, the depression scores in the endurance training group were considerably lower than in the placebo group. Therefore, the results of our study suggest that exercise can be a useful complementary treatment for major depression in the first 3 weeks of hospitalisation until antidepressive drugs take effect.
Our findings are in contrast with the results of a randomised controlled trial which compared the response to exercise, drugs or a combination of both in older patients with a major depressive disorder.11 In this study, antidepressants facilitated a more rapid initial therapeutic response than exercise. However, the patients in the mentioned trial exercised only three times a week and at a lower intensity than the participants in our study. The different outcomes of the two trials suggest that it is necessary to reach a certain threshold of energy expenditure or intensity of effort to achieve a clinical reduction of depression scores in a short time.
Several mechanisms can explain the reduction in depression scores observed in the study population. Exercise may provide a feeling of body control, help to release anger and hostility, and distract patients from depressive thoughts. Motivation, expectations and human contact may also influence the mood of participants and partially explain the improvement observed in the two groups. However, psychological or motivational factors alone cannot explain the observed outcomes, as the reduction in depression scores was substantially greater in the training group despite a comparable duration and frequency of intervention in both groups. This indicates an additional effect of endurance training on mood.
Endurance exercise generates changes in the concentration of several biologically active molecules such as adrenocorticotropic hormone, cortisol, catecholamines, opioid peptides and cytokines, which have been described to have effects on mood or are involved in the physiopathology of affective disorders.27 Moreover, endurance exercise has been shown to modify the concentration of neuroactive substances in the central nervous system of rats.12,28 Further, some indirect evidence suggests that intense physical effort may result in increased serotonin synthesis and re‐uptake in the brain.29 New evidence suggests that regular physical activity may increase neurogenesis in the hippocampus,30 a mechanism that has been related to the mechanism of action of antidepressants. One or more of these mechanisms could be responsible for the mood improvement observed in the endurance exercise group.
A sample of <20 patients per group was sufficient to show a statistically significant difference in the reduction in depression scores in both groups. This indicates a substantial and consistent improvement in mood in the endurance training group. However, the reduced number of patients is also a limitation of our study. Possibly, some imbalance between the groups remained undetected because of the small sample size. Further, it is not possible to differentiate between the effects of exercise on patient subgroups. A larger carefully controlled study is obviously needed to evaluate the effects of endurance exercise in different diagnostic groups.
A further limitation of the present study is the short duration of the training programme. The goal of the trial was to evaluate the short‐term effects of endurance exercise in patients with major depression. As we did not perform any further assessments of mood in the weeks after concluding the study, no conclusions could be drawn about the duration of the antidepressive effect of endurance exercise. A recent review suggests that the benefits of exercise do not persist beyond the end of treatment.5 Furthermore, it is not known whether a sudden interruption of an exercise programme may result in a rebound effect with a relevant increase in depression symptoms. However, the substantially shorter (although statistically not significant) hospitalisation of the endurance training group can be seen as an argument at least against the negative long‐term effects of the endurance training programme on mood.
A source of potential bias was the use of further treatments with training. In fact, most patients in our study received antidepressive drugs. However, several factors speak against an effect of pharmacotherapy on the observed outcomes. Firstly, the stratified randomised procedure generated groups that were comparable regarding the type of antidepressive drug they received (table 1). Secondly, at the time of recruitment, most patients had been taking antidepressants for too long (>6 weeks, n = 7, 18%) or too short (<7 days or even after the start of the study, n = 26, 69%) a time to improve mood during the 10 days of the study. Finally, the statistical analysis showed no significant effect of pharmacotherapy on the reduction in depression scores during the study.
A similar potentially confounding factor was the use of sleep deprivation in some patients. Seven patients in the training group and three patients in the placebo group were subjected to this intervention, which is often used in the initial stage of depression treatment. Sleep deprivation is known to substantially reduce symptoms in about 30% of patients with depressive disorder.31 However, the improvement in mood usually lasts <48 h after the end of sleep deprivation. Therefore, an effect of this intervention on the observed outcomes is unlikely, as none of the patients were subjected to sleep deprivation in the 2 days before the second examination. Moreover, in a retrospective statistical analysis, the reduction in depression scores was still significantly greater in the training group than in the control group, even after excluding patients under sleep deprivation in the last 7 days of the training programme (p = 0.009).
What is already known on this topic
There is growing evidence showing that physical activity can be a helpful adjuvant treatment in patients with mood disorders.
What this study adds
Endurance training may substantially reduce the severity of symptoms in patients with major depression in a short time.
We conclude that an endurance training programme substantially improves symptoms in selected patients with moderate to severe depression. Endurance training can thus be a helpful complementary treatment for patients with severe affective disorders in the first 3 weeks of pharmacotherapy.
Abbreviations
BRMS - Bech‐Rafaelsen Melancholy Scale
CES‐D - Center for Epidemiologic Studies Depression scale
Footnotes
Competing interests: None.
References
- 1.Scully D, Kremer J, Meade M M.et al Physical exercise and psychological well being: a critical review. Br J Sports Med 199832111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yeung R R. The acute effects of exercise on mood state. J Psychosom Res 199640123–141. [DOI] [PubMed] [Google Scholar]
- 3.North T C, McCullagh P, Tran Z V. Effect of exercise on depression. Exerc Sport Sci Rev 199018379–415. [PubMed] [Google Scholar]
- 4.Craft L L, Landers D M. The effect of exercise on clinical depression and depression resulting from mental illness: a meta‐analysis. J Sport Exerc Psychol 199820339–357. [Google Scholar]
- 5.Lawlor D A, Hopker S W. The effectiveness of exercise as an intervention in the management of depression: systematic review and meta‐regression analysis of randomised controlled trials. BMJ 2001322763–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ding Q, Vaynman S, Akhavan M.et al Insulin‐like growth factor i interfaces with brain‐derived neurotrophic factor‐mediated synaptic plasticity to modulate aspects of exercise‐induced cognitive function. Neuroscience 2006140823–833. [DOI] [PubMed] [Google Scholar]
- 7.Gold S M, Schulz K H, Hartmann S.et al Basal serum levels and reactivity of nerve growth factor and brain‐derived neurotrophic factor to standardized acute exercise in multiple sclerosis and controls. J Neuroimmunol 200313899–105. [DOI] [PubMed] [Google Scholar]
- 8.Colcombe S J, Kramer A F, Erickson K I.et al Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci USA 20041013316–3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh N A, Clements K M, Fiatarone M A. A randomized controlled trial of progressive resistance training in depressed elders. J Gerontol A Biol Sci Med Sci 199752M27–M35. [DOI] [PubMed] [Google Scholar]
- 10.Mather A S, Rodriguez C, Guthrie M F.et al Effects of exercise on depressive symptoms in older adults with poorly responsive depressive disorder: randomised controlled trial. Br J Psychiatry 2002180411–415. [DOI] [PubMed] [Google Scholar]
- 11.Blumenthal J A, Babyak M A, Moore K A.et al Effects of exercise training on older patients with major depression. Arch Intern Med 19991592349–2356. [DOI] [PubMed] [Google Scholar]
- 12.Chaouloff F. Effects of acute physical exercise on central serotonergic systems. Med Sci Sports Exerc 19972958–62. [DOI] [PubMed] [Google Scholar]
- 13.Dimeo F, Bertz H, Finke J.et al An aerobic exercise program for patients with haematological malignancies after bone marrow transplantation. Bone Marrow Transplant 1996181157–1160. [PubMed] [Google Scholar]
- 14.Bartholomew J B, Morrison D, Ciccolo J T. Effects of acute exercise on mood and well‐being in patients with major depressive disorder. Med Sci Sports Exerc 2005372032–2037. [DOI] [PubMed] [Google Scholar]
- 15.Dunn A L, Trivedi M H, Kampert J B.et al Exercise treatment for depression: efficacy and dose response. Am J Prev Med 2005281–8. [DOI] [PubMed] [Google Scholar]
- 16.Trivedi M H, Greer T L, Grannemann B D.et al Exercise as an augmentation strategy for treatment of major depression. J Psychiatr Pract 200612205–213. [DOI] [PubMed] [Google Scholar]
- 17.American Psychiatric Association Diagnostic & statistical manual of mental disorders, 4th edn (DSM‐IV‐TR). Washington: American Psychiatric Press, 2000
- 18.Bech P, Rafaelsen O J. The Melancholia‐Scale: development, consistency, validity and utility. In: Satorius N, Ban TA, eds. Assessment of depression. Berlin: Springer, 1986259–269.
- 19.Dimeo F, Bauer M, Varahram I.et al Benefits from aerobic exercise in patients with major depression: a pilot study. Br J Sports Med 200135114–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American College of Sports Medicine Guidelines for exercise testing and prescription. 3rd edn Philadelphia: Lea & Feibiger, 1995
- 21.Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med 1970392–98. [PubMed] [Google Scholar]
- 22.Beneke R, Meyer K. Walking performance and economy in chronic heart failure patiens pre and post exercise training. Eur J Appl Physiol 199775246–251. [DOI] [PubMed] [Google Scholar]
- 23.Maier W, Philipp M, Heuser I.et al Improving depression severity assessment‐‐I. Reliability, internal validity and sensitivity to change of three observer depression scales. J Psychiatr Res 1988223–12. [DOI] [PubMed] [Google Scholar]
- 24.Maier W, Heuser I, Philipp M.et al Improving depression severity assessment‐‐II. Content, concurrent and external validity of three observer depression scales. J Psychiatr Res 19882213–19. [DOI] [PubMed] [Google Scholar]
- 25.Radloff L S. The CES‐D scale: a self‐report depression scale for research in the general population. Appl Psychol Meas 19771385–401. [Google Scholar]
- 26.Lachin J M. Worst‐rank score analysis with informatively missing observations in clinical trials. Control Clin Trials 199920408–422. [DOI] [PubMed] [Google Scholar]
- 27.Wilmore J H, Costill D L.Physiology of sports and exercise. 1st edn. Champaign, IL: Human Kinetics, 1994
- 28.Pagliari R, Peyrin L. Norepinephrine release in the rat frontal cortex under treadmill exercise: a study with microdialysis. J Appl Physiol 1995782121–2130. [DOI] [PubMed] [Google Scholar]
- 29.Struder H K, Weicker H. Physiology and pathophysiology of the serotonergic system and its implications on mental and physical performance. Part ii. Int J Sports Med 200122482–497. [DOI] [PubMed] [Google Scholar]
- 30.Ernst C, Olson A K, Pinel J P.et al Antidepressant effects of exercise: evidence for an adult‐neurogenesis hypothesis? J Psychiatry Neurosci 20063184–92. [PMC free article] [PubMed] [Google Scholar]
- 31.Wirz‐Justice A, Van den Hoofdakker R H. Sleep deprivation in depression: what do we know, where do we go? Biol Psychiatry 199946445–453. [DOI] [PubMed] [Google Scholar]