Abstract
Background
Exercise can alter health in children in both beneficial (eg reduced long‐term risk of atherosclerosis) and adverse (eg exercise‐induced asthma) ways. The mechanisms linking exercise and health are not known, but may rest, partly, on the ability of exercise to increase circulating immune cells. Little is known about the effect of brief exercise, more reflective of naturally occurring patterns of physical activity in children, on immune cell responses.
Objectives
To determine whether (1) a 6‐min bout of exercise can increase circulating inflammatory cells in healthy children and (2) the effect of brief exercise is greater in children with a history of asthma.
Methods
Children with mild–moderate persistent asthma and age‐matched controls (n = 14 in each group, mean age 13.6 years) performed a 6‐min bout of cycle‐ergometer exercise. Spirometry was performed at baseline and after exercise. Blood was drawn before and after exercise, leucocytes were quantified and key lymphocyte cell surface markers were assessed by flow cytometry.
Results
Exercise decreased spirometry only in children with asthma, but increased (p<0.001) most types of leucocytes (eg lymphocytes (controls, mean (SD) 1210 (208) cells/μl; children with asthma, 1119 (147) cells/μl) and eosinophils (controls, 104 (22) cells/μl; children with asthma, 88 (20) cells/μl)) to the same degree in both groups. Similarly, exercise increased T helper cells (controls, 248 (60) cells/μl; children with asthma, 232 (53) cells/μl) and most other lymphocyte subtypes tested. By contrast, although basophils (16 (5) cells/μl) and CD4+ CD45RO+ RA+ lymphocytes (19 (4) cells/μl) increased in controls, no increase in these cell types was found in children with asthma.
Conclusions
Exercise increased many circulating inflammatory cells in both children with asthma and controls. Circulating inflammatory cells did increase in children with asthma, but not to a greater degree than in controls. In fact, basophils and T helper lymphocyte memory transition cells did not increase in children with asthma, whereas they did increase in controls. Even brief exercise in children and adolescents robustly mobilises circulating immune cells.
Although it is well recognised that physical activity can influence health in children, the mechanisms that link exercise and health remain enigmatic. Immune responses to exercise could explain some of the adverse (eg bronchoconstriction, urticaria, anaphylaxis1,2,3) or beneficial (reduction of cardiovascular disease risk4,5) health effects of physical activity, and, indeed, growing evidence in children suggests that exercise is associated with a substantial increase in circulating cytokines (such as interleukin 6) and leucocytes.6,7 However, the vast majority of studies to date designed to explore immune and stress responses to exercise in adults and children have used relatively long exercise protocols,8,9 which do not reflect natural patterns of physical activity in children.
Real‐life patterns of exercise in children are brief.10 Whether or not short episodes of exercise in children and adolescents cause an increase in circulating inflammatory cells has not been well studied. Consequently, the goal of this study was to test the hypothesis that a standard, brief (6‐min) exercise protocol—used commonly to elicit bronchoconstriction in children—would lead to large increases in circulating inflammatory cells, some of which are associated with specific diseases. As habitual levels of physical activity and related factors, such as body composition and nutritional status, can alter peripheral leucocyte function,11,12 testing this hypothesis would be a necessary first step in understanding the potential mechanistic link between exercise and diseases, such as asthma, in which exercise is a potent trigger of airway inflammation.
We chose to measure the levels of the standard circulating leucocytes (ie neutrophils, eosinophils, basophils, lymphocytes and monocytes). In addition, we used flow cytometry to focus on several lymphocyte cell surface markers that have been found, albeit mostly from studies in adults, to have a variety of roles in inflammatory diseases ranging from asthma to atherosclerosis (table 1). Finally, we elected to study this phenomenon in healthy control children and adolescents, as well as in children and adolescents with a known history of mild‐to‐moderate asthma. Exercise‐induced bronchoconstriction (EIB) is a common and well‐described adverse effect of exercise,13,14 and is related to airway dehydration leading to an abnormal airway inflammatory response.2 Consequently, we hypothesised that the effect of brief exercise on circulating immune cells would be greater than in controls without asthma.
Table 1 Cell surface markers evaluated.
| Cell surface marker | Conventional names | Potential role in exercise effects on health |
|---|---|---|
| CD3+/CD4+ | T helper | Pivotal role in the initiation and perpetuation of the inflammatory response in asthma. Plays a part in formation of atherosclerosis |
| CD3+/CD8+ | T cytotoxic/suppressor | CD8+ cells that produce type 2 cytokines have been shown to play a part in asthma inflammation |
| CD19+ | B lymphocytes | Production of IgE, important in the allergic mechanism of inflammation. B cells have a protective role in atherosclerosis |
| CD3−/CD16+ 56+ | Natural killer | The involvement of NK cells in asthma remains unclear. NK cells have been implicated in the development and progression of atherosclerosis, but their role remains unclear |
| CD4+ CD45RA+ | Naive T helper | Naive T helper lymphocytes are essential for responses to new foreign antigens. Adaptive transfer of naive T helper cells accelerates atherosclerosis |
| CD4+ CD45RO+ | Memory T helper | Memory T helper lymphocytes migrate towards inflammatory sites and are preferentially activated relative to naive T helper cells after allergen challenge. Most T lymphocytes in atherosclerotic lesions are memory T helper lymphocytes |
| CD4+ CD45RO+ RA+ | Transitional T helper cell from naive to memory | Probably represent initial steps in the transition from naive to memory cells |
| CD62L+ | L‐selectin | L‐selectin expression on T helper lymphocytes may have a crucial role in bronchospasm. CD62L has been shown to be increased in people with hypertension after stress, and trafficking of lymphocytes to atherosclerotic‐prone aortas is partially CD62L dependent |
| CD29+ | Integrin β‐1 subunit | Lower levels of CD29 are found in atopic patients compared with healthy patients |
IgE, immunoglobulin E; NK cells, natural killer cells.
Methods
Participants
Thirty three children and adolescents aged 8–18 years were recruited for this study. The inclusion criteria included participants who were either healthy or had mild‐to‐moderate persistent asthma, using current National Heart Lung and Blood Institute standards.15 The exclusion criteria in the control group included use of any drugs and the presence of an upper respiratory infection. The exclusion criteria in the comparison group of patients with asthma included the presence of an upper respiratory infection, severe persistent asthma or acute exacerbation within the previous month, use of oral steroids within the month before exercise challenge, use of long‐acting β‐agonists within 24 h and short‐acting β‐agonists within 12 h of exercise challenge. Three participants were excluded as blood drawn after exercise was not available for analysis (two from the control group and one from the group with asthma). Two participants with no history of asthma developed wheezing during the exercise challenge and were excluded, leaving 28 participants available for analysis. The University of California at Irvine Institutional Review Board approved this study, and informed written consent, as well as assent, was obtained from all participants and their parents or guardians. The studies were conducted at the UCI General Clinical Research Center.
Exercise protocol
Participants reported to the General Clinical Research Center Applied Physiology–Human Performance Laboratory for two visits on separate days. On visit 1, the participants performed a standardised 6‐min bout of constant work‐rate exercise on an electronically braked, servo‐controlled, cycle‐ergometer designed to diagnose EIB in children.16 Throughout the test, the work rate was adjusted to maintain heart rate within the target range.
Pulmonary function tests were performed via spirometry at baseline, and then at 5, 10, 15, 20 and 30 min after exercise. In addition, each participant (control and asthma) was auscultated at baseline, and again if either wheezing was audible or if there was a decrease in forced expiratory volume in 1 s (FEV1). A bronchodilator was given if wheezing occurred, patients reported difficulty in breathing, or the drop in FEV1 was >10%.
The participants returned to the laboratory within 14 days and performed a standardised, ramp‐type, progressive cycle‐ergometer exercise test to determine their level of fitness.17 In those participants who had received a bronchodilator after the previous 6‐min exercise challenge, we gave the bronchodilator before the fitness test as a precaution.
Blood sampling and analysis
During visit 1, blood was sampled from an indwelling catheter 30 min after placement and before the onset of exercise (pre‐exercise sampling time point). We waited for 30 min to ensure that measurable physiological parameters of stress (eg heart rate and blood pressure) returned to baseline levels after catheter placement. Participants then completed the 6‐min exercise bout, and additional blood samples were obtained immediately after exercise (post‐exercise sampling time point). All blood samples of the same participant were run in the same batch.
Cell surface antigens by flow cytometry
The following monoclonal antibodies were used in multiparameter flow cytometry: CD3, CD4, CD8, CD19, CD16, CD56, CD45RA, CD45RO, CD29 and CD62L (Becton Dickinson, San Jose, California, USA). Within 2 h of blood collection, 100 μl of blood from each sample was added to 12×75 mm tubes with specific surface antigen fluorescent‐conjugated monoclonal antibodies, mixed well and incubated in the dark at room temperature for 15 min. Then 1× Becton Dickinson‐fluorescence‐activated cell sorter lysing solution (2 ml) was added to lyse red blood cells, mixed gently and incubated for 10 min at room temperature in the dark; Becton Dickinson wash buffer (1× 2 ml) was added, and the mixture centrifuged at 500 g for 5 min. The supernatant was removed without disturbing the cell pellet. Cells were resuspended in 500 μl 5% paraformaldehyde.
Acquisition and analysis
Samples were acquired using a fluorescence‐activated cell sorter calibur flow cytometer (Becton Dickinson). A forward scatter threshold was used to acquire 100 000 events for each prepared sample. Specific cell populations (neutrophils, lymphocytes, monocytes, eosinophils and basophils) were obtained by standard methods from the Clinical Hematology Laboratory, University of California, Irvine, California, USA. Lymphocyte subpopulations were identified by forward and side scatter, and separated by antigenic expressions of CD3+/CD4+, CD3+/CD8+, CD19+, and CD3‐/CD16+56+. CD4+ and CD8+ T cell and natural killer cell subpopulations were further analysed for CD45RA+, CD45RO+, CD29+, and CD62L+ expression (owing to technical problems, only 19 samples were analysed for CD29+).
Statistical analysis
Baseline anthropometric characteristics, pulmonary function tests, leucocytes, cell surface markers and change in pulmonary function from pre‐exercise to the greatest decrease post‐exercise were examined between the control and asthmatic groups using two‐sample t tests. The change in each leucocyte and cell surface marker from pre‐exercise to post‐exercise was evaluated with paired t tests for each group. Those with significant change in either group were then compared using two‐sample t tests for group differences. All tests were two tailed using statistical analysis package SAS V9.1. All group means are presented as mean (standard error), and all comparison results are evaluated at the 0.05 significance level. The p values in supplementary tables are displayed as p<0.001, exact p values if between 0.001 and 0.1, or not significant if p>0.1 (supplementary tables available at http://bjsm.bmjjournals.com/supplemental).
Results
Participants and baseline evaluation
Table 2 summarises anthropometric, fitness, baseline pulmonary function and exercise test results for the 28 participants (8 males per group). There were no differences between the non‐asthmatic and asthma‐comparison groups. In all, 6 (43%) participants with asthma received albuterol treatment after the 6‐min challenge. Consistent with this, compared with controls, the participants with asthma had significantly larger decreases in FEV1 after exercise (−0.086 (0.037) litre in those without asthma v −0.283 (0.043) litre in those with asthma, p = 0.035) and MEF 25–75% (−0.016 (0.118) l/s in those without asthma v −0.434 (0.109) l/s in those with asthma, p = 0.016). There were no significant differences for leucocytes or cell surface markers at pre‐exercise between control and asthmatic groups except CD4+, CD29+, and CD45RO+ cells, which were lower in the patients with asthma (p = 0.046).
Table 2 Anthropometric, fitness, pulmonary function and 6‐min exercise challenge data from the 28 participants*.
| Variable | Controls (n = 14) | Children with asthma (n = 14) |
|---|---|---|
| Age (years) | 13.6 (0.79) | 13.6 (0.51) |
| BMI (kg/m2) | 21.1 (0.99) | 21.6 (0.56) |
| VO2 peak (ml/min/kg) | 33.5 (2.25) | 30.7 (2.10) |
| VC pre‐exercise (l) | 3.6 (0.32) | 3.9 (0.30) |
| FEV1 pre‐exercise (l) | 2.9 (0.24) | 3.3 (0.24) |
| PEFR pre‐exercise (l) | 5.1 (0.51) | 5.9 (0.46) |
| MEF 25–75% pre‐exercise (l/s) | 2.9 (0.26) | 3.5 (0.33) |
| Mean HR (6‐min challenge) | 187.9 (2.28) | 180.6 (3.41) |
| Mean WR (6‐min challenge) | 124.5 (11.22) | 132.1 (12.38) |
Data are mean (SEM).
*No significant differences between the two groups.
BMI, body mass index; FEV1, forced expiratory volume in 1 s; HR, heart rate; MEF, maximal expiratory flow; PEFR, peak expiratory flow rate; VC, vital capacity; VO2, oxygen uptake; WR, work rate.
Leucocyte response to exercise
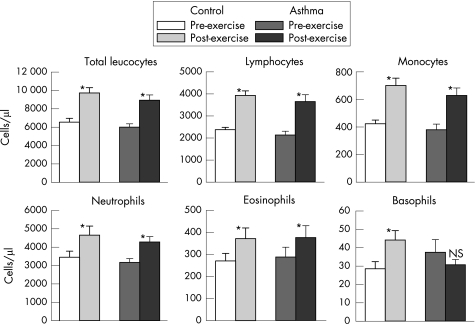
Detailed results are presented in the supplementary tables. Figure 1 shows the effect of the 6‐min exercise on total leucocytes, lymphocytes, monocytes, neutrophils, eosinophils and basophils. Highly significant increases (p<0.001) were observed from pre‐exercise to post‐exercise in both the control and asthmatic groups for total leucocytes, including lymphocytes, monocytes, neutrophils and eosinophils. Basophils showed a different pattern, with a significant increase in the control group (p = 0.006), but an insignificant decrease in the asthmatic group from pre‐exercise to post‐exercise.
Figure 1 Effect of exercise (mean (SEM)) on circulating leucocytes in participants with asthma and controls. The changes from pre‐exercise to post‐exercise were significant (*p<0.01) for all except basophils in the asthmatic group. The basophil response to exercise was significantly different between the control and asthmatic groups (p = 0.019).
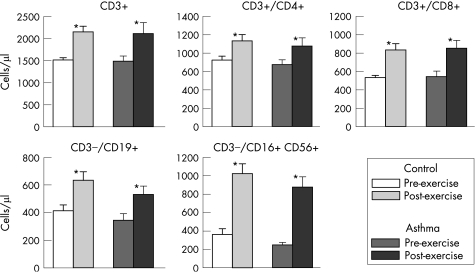
Highly significant increases (p<0.005) in response to the 6‐min exercise were also observed in both study groups for CD3, T helper lymphocytes (CD3+/CD4+), T suppressor lymphocytes (CD3+/CD8+), B cell lymphocytes (CD3−/CD19+) and natural killer cells (CD3−/CD16+ 56+; fig 2).
Figure 2 Effect of exercise (mean (SEM)) on lymphocyte subsets in participants with asthma and controls. The changes from pre‐exercise to post‐exercise were significant (*p<0.005) for all.
Figure 3 shows significant increases (p<0.05) in response to exercise in naive (CD4+ CD45RA+ and CD8+ CD45RA+), memory (CD4+ CD45RO+ and CD8+ CD45RO+), and the CD8+ early transition cells (CD8+ CD45RO+ RA+) in the two study groups. However, for cells expressing CD4+ CD45RO+ RA+, a significant increase after exercise was only observed in the control group (p<0.001).
Figure 3 Effect of exercise (mean (SEM)) on T helper and T suppressor lymphocytes in participants with asthma and controls. The changes from pre‐exercise to post‐exercise were significant (*p<0.05) for all except CD4+ CD45RO+ RA+ in the asthmatic group. The CD4+ CD45RO+ RA+ response to exercise was significantly different between the control and asthmatic groups (p = 0.014).
For both T helper and T suppressor lymphocyte subsets bearing CD29+ adhesion molecules (CD4+ CD29+, CD4+ CD29+ CD45RA+, CD4+ CD29+ CD45RO+, CD8+ CD29+, CD8+ CD29+ CD45RA+, and CD8+ CD29+ CD45RO+), the 12 samples from the asthmatic group showed a highly significant increase (p<0.005) after the 6‐min exercise (fig 4). On the other hand, the seven samples from the control group failed to show a significant change for CD8+ CD29+ CD45RO+ (p = 0.084).
Figure 4 Effect of exercise (mean (SEM)) on T helper and T suppressor lymphocyte subpopulations bearing the CD29+ adhesion molecule in participants with asthma (n = 12) and controls (n = 7). The changes from pre‐exercise to post‐exercise were significant (*p<0.05) for all except CD8+ CD29+ CD45RO+ in the control group. The CD4+ CD29+ CD45RO+ is significantly different at baseline for the two study groups (p = 0.046).
The T helper, T suppressor, and natural killer lymphocytes bearing CD62L+ showed significant increases (p<0.005) in response to exercise for the subsets CD4+ CD62L+, CD4+ CD62L+ CD45RA+, CD8+ CD62L+, CD8+ CD62L+ CD45RA+, and CD3−/CD16+ 56+ CD62L+ for both study groups (fig 5).
Figure 5 Effect of exercise (mean (SEM)) on T helper, T suppressor, and natural killer lymphocytes bearing CD62L+ in participants with asthma and controls. The changes from pre‐exercise to post‐exercise were significant (*p<0.005) for all.
When we compared the differences between controls and children with asthma for leucocyte and lymphocyte subtype responses to the 6‐min exercise, we found that only basophils (p = 0.019, fig 1) and CD4+ CD45RO+ RA+ (p = 0.014, fig 3) showed significant differences. The between group difference observed for CD4+ CD29+ CD45RA+ was marginally significant (p = 0.062, fig 4), and further evaluation with a larger sample size should be considered.
Discussion
The primary aim of this study was to determine whether a brief exercise challenge—one reflective of natural patterns of exercise in children and commonly used to elicit EIB—could alter inflammatory cell numbers and markers in the circulating blood. Consistent with our first hypothesis, we found that the 6‐min challenge substantially increased the total leucocyte count and most of the leucocyte subtypes that we studied in healthy children (fig 1). Moreover, a variety of lymphocyte‐specific cell markers known to be associated with cardiovascular disease, asthma and allergic disorders increased in the peripheral blood in both the healthy children and the children with asthma. Most of the inflammatory cells and markers that we tested increased similarly in both groups (tables A and B; figs 1–5), but, in contrast with our hypothesis, the circulating leucocyte response to exercise in children with asthma did not differ from controls, despite having evidence of bronchoconstriction after exercise. Indeed, the only difference between the controls and children with asthma was the absence of an increase in two leucocyte subgroups in the children with asthma.
A variety of studies support the idea that circulating leucocytes have a role in diseases and conditions influenced by physical activity. CD4+ cells and natural killer cells, both of which increased during exercise in this study, have a role in the genesis of the atherosclerotic plaque.5,18 Moreover, Martin‐Ventura et al19 found that nuclear factor‐κB activity and Fas ligand (FasL) mRNA are increased in circulating monocytes of patients with carotid atherosclerosis, suggesting that the inflammatory cells in the vulnerable region of the plaque could originally be activated in the circulation before they enter the arterial wall. Increasingly, atherosclerosis is viewed as another of the adult diseases, such as osteoporosis,20 that has its origins in childhood.21
The specific effect of habitual physical activity on these cells in children and its long‐term effect on the risk of cardiovascular disease remains unknown. Levels of physical activity, nutritional status and body composition can influence the numbers of circulating leucocytes in children and adults.11,12,22 Moreover, leucocyte function can also be influenced by training status.23,24,25 How fitness status alters circulating leucocyte function and numbers in healthy children or children with asthma remains poorly investigated.
The brief exercise used in this protocol tended to increase circulating lymphocytes positive for cell markers that facilitate adhesion to endothelium (figs 4 and 5), such as L‐selectin26 (identified in this study by the CD62L+ marker) and the integrin β1 subunit27(CD29), which enhances leucocyte adhesion. Circulating inflammatory cells can interact with tissues by the processes of adhesion and transmigration.28 Interestingly, leucocyte L‐selectin and the integrin β1 subunit have key roles in asthma,27 skin manifestations of allergy29,30 and atherosclerosis.31,32 There is mounting evidence that exercise can influence these processes even in healthy people. For example, heavy, prolonged exercise not only causes increases in circulating inflammatory cells but also recruitment of leucocytes to the airways even in healthy people without asthma.33,34,35 Our data suggest a mechanism that links (even brief) exercise to health effects in specific diseases—namely, the speculation that circulating lymphocytes with these adhesion molecules present on their surfaces eventually adhere to vessel walls and, ultimately, transmigrate into airways, skin or early atherosclerotic plaques.
We selected a comparison group of children with asthma because EIB is so common and disturbing a phenomenon in children, and seems to have roots in an altered inflammatory response to exercise. We showed that, as in the controls, the circulating immune response to brief exercise was robust and involved most leucocytes. Despite finding evidence of bronchoconstriction in the children with asthma, the increase in circulating leucocytes was quite similar to the controls. Unexpectedly, the only difference we found between children with asthma and controls was in the exercise response of circulating basophils and CD4+ CD45RO+ RA+ (figs 1 and 3), which failed to increase in participants with asthma despite an increase in the controls. We could not attribute this to factors such as fitness, which did not differ between the groups (table 2).
These observations could be explained by sequestration of basophils or CD4+ CD45RO+ RA+ lymphocytes in the lung or other marginal pools of participants with asthma. Similarly, circulating basophils have been found in previous studies to decrease in response to exercise in a pattern suggestive of pulmonary retention.36 This pattern has also been shown for eosinophils37 and myeloid dendritic cells38 after instillation of an allergen into the airways of participants, presumably because the cells found their way to the airways. Alternatively, children with asthma may not be able to effectively mobilise basophils, whether in peripheral blood or in the lung.
It is now known that a substantial number of basophils are present in the lumen of airways in fatal asthma,39 and it is clear that basophils have an active role in bronchial responsiveness in asthma40 (although a specific role for basophils in EIB has not yet been established). The migration of basophils to the airway lumen, or an inability in participants with asthma to mobilise basophils in the periphery, may explain why basophils did not increase in the circulation in children with asthma. Alternatively, degranulation of basophils (after which they would not be recognised by standard techniques) after exercise could also explain a blunted response.
Exercise led to a robust increase in T helper lymphocytes and subsets, including CD4+ CD62L+, CD4+ CD45RA+ and CD4+ CD45RO+ cells in both control and asthmatic groups (figs 3 and 5; tables A and B), but without a significant change for CD4+ CD45RO+ RA+ observed in the asthmatic group (fig 3). Memory T helper cells (CD4+ CD45RO+) have previously been shown to be increased in patients with asthma and after antigen challenge.41 Moreover, recent work shows that memory T cells originating in the circulation can migrate to the airways and cause bronchoconstriction even in the absence of eosinophils.42 Thus, the lower level of the memory T helper cell with up‐regulated adhesion molecule expression (CD4+ CD29+ CD45RO+) may indicate migration of this cell to areas of inflammation in the patients with asthma, such as the lung. Further, it is possible that the transition of lymphocytes from naive to memory T cells (CD4+ CD45RO+ RA+ is an early‐stage transitional cell43) in patients with asthma could also be sequestered in response to exercise, and this could explain the lack of increase that we observed. What remains enigmatic in our observations is why this particular transitional cell, and not the memory cells themselves, seemed to be most affected in children with asthma.
The robust increase in circulating inflammatory cells in both healthy children and children with asthma raises an intriguing question: why does everyone not wheeze with exercise? In healthy children, just 6 min of exercise was associated with a general leucocytosis and an increase in circulating eosinophils, such as typically seen in acute asthma.44 Moreover, the circulating blood in healthy children after exercise reflected a progression of T cells from naive to memory, also typically found in atopic participants and in participants with asthma.41 Although we did not measure intracellular cytokines and could not, therefore, gauge the shift in T helper 1 versus T helper 2 lymphocytes, the change in T helper cell numbers in general indicate the possibility that an altered balance of the pattern of these key cytokine‐producing lymphocytes might have occurred in circulation even in healthy children after exercise.
In summary, our study showed that relatively brief exercise in children and adolescents, at a level and duration commonly found naturally, led to a very robust increase in circulating inflammatory cells. Lymphocyte subtyping showed that the L‐selectin and integrin β1 subunit positive surface markers were increased, setting the stage, perhaps, for these cells to adhere to vascular endothelium and transmigrate to tissues, such as the respiratory airways, in which exercise seems to influence health. An intriguing observation was that, at least in some cases, the difference between healthy children and those with a history of inflammatory diseases, such as asthma, may be reflected by the absence of an exercise‐associated increase in circulating inflammatory cells, rather than an increase. What remains unknown is how levels of physical activity in children and adolescence, a critical period of growth and development, may ultimately condition leucocyte responses to exercise and, consequently, act to influence risk for diseases such as atherosclerosis and asthma.
What is known on this topic
Activity can influence health in children in both beneficial (eg, reduced long term risk of atherosclerosis) and adverse (eg exercise induced asthma) ways.
Habitual levels of physical activity and related factors, such as body composition and nutritional status, can alter peripheral leucocyte function.
Growing evidence in children suggests that prolonged exercise is associated with a substantial increase in circulating cytokines (such as interleukin 6) and leucocytes.
The mechanisms linking exercise and health are not known, but may rest, partly, on the ability of exercise to increase circulating immune cells.
Little is known of the effect of brief exercise on the immune cell responses.
What this study adds
Brief exercise in children and adolescents, at a level and duration commonly found naturally, robustly mobilises circulating immune cells.
Most circulating inflammatory cells increase similarly in both patients with asthma and controls without asthma in response to brief exercise.
Basophils and T helper lymphocyte memory transition cells did not increase in patients with asthma whereas they did increase in controls without asthma in response to brief exercise.
Supplementary Material
Abbreviations
EIB - execise‐induced bronchoconstriction
FEV1 - forced expiratory volume in 1 s
Footnotes
Funding: This work was supported in part by National Institutes of Health grants MO1‐RR00827, NICHD P01HD048721 and K23 ES014923‐01A1, and the ACAAI Young Faculty Support Award‐38675.
Competing interests: None declared.
References
- 1.Beaudouin E, Renaudin J M, Morisset M.et al Food‐dependent exercise‐induced anaphylaxis—update and current data. Allerg Immunol (Paris) 20063845–51. [PubMed] [Google Scholar]
- 2.Anderson S D. How does exercise cause asthma attacks? Curr Opin Allergy Clin Immunol 2006637–42. [DOI] [PubMed] [Google Scholar]
- 3.Dice J P. Physical urticaria. Immunol Allergy Clin North Am. 2004;24: 225–46, vi, [DOI] [PubMed]
- 4.Khallou‐Laschet J, Caligiuri G, Groyer E.et al The proatherogenic role of T cells requires cell division and is dependent on the stage of the disease. Arterioscler Thromb Vasc Biol 200626353–358. [DOI] [PubMed] [Google Scholar]
- 5.Zhou X, Robertson A K, Rudling M.et al Lesion development and response to immunization reveal a complex role for CD4 in atherosclerosis. Circ Res 200596427–434. [DOI] [PubMed] [Google Scholar]
- 6.Scheett T P, Mills P J, Ziegler M G.et al Effect of exercise on cytokines and growth mediators in prepubertal children. Pediatr Res 199946429–434. [DOI] [PubMed] [Google Scholar]
- 7.Timmons B W, Tarnopolsky M A, Bar‐Or O. immune responses to strenuous exercise and carbohydrate intake in boys and men. Pediatr Res 200456227–234. [DOI] [PubMed] [Google Scholar]
- 8.Ronsen O, Lea T, Bahr R.et al Enhanced plasma IL‐6 and IL‐1ra responses to repeated vs. single bouts of prolonged cycling in elite athletes. J Appl Physiol 2002922547–2553. [DOI] [PubMed] [Google Scholar]
- 9.Nieman D C, Henson D A, Smith L L.et al Cytokine changes after a marathon race. J Appl Physiol 200191109–114. [DOI] [PubMed] [Google Scholar]
- 10.Bailey R C, Olson J, Pepper S L.et al The level and tempo of children's physical activities: an observational study. Med Sci Sports Exerc 1995271033–1041. [DOI] [PubMed] [Google Scholar]
- 11.Zaldivar F, McMurray R G, Nemet D.et al Body fat and circulating leukocytes in children. Int J Obes (London) 200630906–911. [DOI] [PubMed] [Google Scholar]
- 12.Galassetti P R, Nemet D, Pescatello A.et al Exercise, caloric restriction, and systemic oxidative stress. J Invest Med 20065467–75. [DOI] [PubMed] [Google Scholar]
- 13.Hallstrand T S, Ault K A, Bates P W.et al Peripheral blood manifestations of T(H)2 lymphocyte activation in stable atopic asthma and during exercise‐induced bronchospasm. Ann Allergy Asthma Immunol 199880424–432. [DOI] [PubMed] [Google Scholar]
- 14.Lee T H, Nagy L, Nagakura T.et al Neutrophil chemotactic factor and exercise‐induced asthma. Agents Actions Suppl 19831335–42. [PubMed] [Google Scholar]
- 15.National Heart Lung and Blood Institute Guidelines for the diagnosis and management of asthma. Bethesda, MD: NHLBI, 1997 [PubMed]
- 16.Cooper D M, Springer C. Pulmonary function assessment in the laboratory during exercise. In: Chernick V, Boat TF, eds. Kendig's disorders of the respiratory tract in children. 6th ed. Philadelphia: W B Saunders, 1998214–237.
- 17.Cooper D M, Weiler‐Ravell D, Whipp B J.et al Aerobic parameters of exercise as a function of body size during growth in children. J Appl Physiol 198456628–634. [DOI] [PubMed] [Google Scholar]
- 18.Whitman S C, Rateri D L, Szilvassy S J.et al Depletion of natural killer cell function decreases atherosclerosis in low‐density lipoprotein receptor null mice. Arterioscler Thromb Vasc Biol 2004241049–1054. [DOI] [PubMed] [Google Scholar]
- 19.Martin‐Ventura J L, Blanco‐Colio L M, Munoz‐Garcia B.et al NF‐kappaB activation and Fas ligand overexpression in blood and plaques of patients with carotid atherosclerosis: potential implication in plaque instability. Stroke 200435458–463. [DOI] [PubMed] [Google Scholar]
- 20.Beck B R, Snow C M. Bone health across the lifespan—exercising our options. Exerc Sport Sci Rev 200331117–122. [DOI] [PubMed] [Google Scholar]
- 21.Kutuk O, Basaga H. Inflammation meets oxidation: NF‐kappaB as a mediator of initial lesion development in atherosclerosis. Trends Mol Med 20039549–557. [DOI] [PubMed] [Google Scholar]
- 22.Kim D J, Noh J H, Lee B W.et al A white blood cell count in the normal concentration range is independently related to cardiorespiratory fitness in apparently healthy Korean men. Metabolism 2005541448–1452. [DOI] [PubMed] [Google Scholar]
- 23.Hong S, Johnson T A, Farag N H.et al Attenuation of T‐lymphocyte demargination and adhesion molecule expression in response to moderate exercise in physically fit individuals. J Appl Physiol 2005981057–1063. [DOI] [PubMed] [Google Scholar]
- 24.McFarlin B K, Flynn M G, Phillips M D.et al Chronic resistance exercise training improves natural killer cell activity in older women. J Gerontol A: Biol Sci Med Sci 2005601315–1318. [DOI] [PubMed] [Google Scholar]
- 25.Dos Santos Cunha W D, Giampietro M V, De Souza D F.et al Exercise restores immune cell function in energy‐restricted rats. Med Sci Sports Exerc 2004362059–2064. [DOI] [PubMed] [Google Scholar]
- 26.Khan A I, Kubes P. L‐selectin: an emerging player in chemokine function. Microcirculation 200310351–358. [DOI] [PubMed] [Google Scholar]
- 27.Singh J, Adams S, Carter M B.et al Rational design of potent and selective VLA‐4 inhibitors and their utility in the treatment of asthma. Curr Top Med Chem 200441497–1507. [DOI] [PubMed] [Google Scholar]
- 28.Wagner J G, Roth R A. Neutrophil migration mechanisms, with an emphasis on the pulmonary vasculature. Pharmacol Rev 200052349–374. [PubMed] [Google Scholar]
- 29.Assing K, Bodtger U, Poulsen L K. Seasonal dynamics of chemokine receptors and CD62L in subjects with asymptomatic skin sensitization to birch and grass pollen. Allergy 200661759–768. [DOI] [PubMed] [Google Scholar]
- 30.Lugovic L, Cupic H, Lipozencic J.et al The role of adhesion molecules in atopic dermatitis. Acta Dermatovenerol Croat 2006142–7. [PubMed] [Google Scholar]
- 31.Galkina E, Kadl A, Sanders J.et al Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially L‐selectin dependent. J Exp Med 20062031273–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shih P T, Elices M J, Fang Z T.et al Minimally modified low‐density lipoprotein induces monocyte adhesion to endothelial connecting segment‐1 by activating beta1 integrin. J Clin Invest 1999103613–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonsignore M R, Morici G, Vignola A M.et al Increased airway inflammatory cells in endurance athletes: what do they mean? Clin Exp Allergy 20033314–21. [DOI] [PubMed] [Google Scholar]
- 34.Lavender J P, Goldman J M, Arnot R N.et al Kinetics of indium‐III labelled lymphocytes in normal subjects and patients with Hodgkin's disease. B M J 19772797–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonsignore M R, Morici G, Riccobono L.et al Airway inflammation in nonasthmatic amateur runners. Am J Physiol Lung Cell Mol Physiol 2001281L668–L676. [DOI] [PubMed] [Google Scholar]
- 36.Frampton M W, Stewart J C, Oberdorster G.et al Inhalation of ultrafine particles alters blood leukocyte expression of adhesion molecules in humans. Environ Health Perspect 200611451–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sklar L A, Tsuji H, Edwards B S.et al Eosinophil traffic in the circulation following allergen challenge. Allergy 200459596–605. [DOI] [PubMed] [Google Scholar]
- 38.Upham J W, Denburg J A, O'Byrne P M. Rapid response of circulating myeloid dendritic cells to inhaled allergen in asthmatic subjects. Clin Exp Allergy 200232818–823. [DOI] [PubMed] [Google Scholar]
- 39.Koshino T, Teshima S, Fukushima N.et al Identification of basophils by immunohistochemistry in the airways of post‐mortem cases of fatal asthma. Clin Exp Allergy 199323919–925. [DOI] [PubMed] [Google Scholar]
- 40.Marone G, Triggiani M, de Paulis A. Mast cells and basophils: friends as well as foes in bronchial asthma? Trends Immunol 20052625–31. [DOI] [PubMed] [Google Scholar]
- 41.Lara‐Marquez M L, Moan M J, Cartwright S.et al Atopic asthma: differential activation phenotypes among memory T helper cells. Clin Exp Allergy 2001311232–1241. [DOI] [PubMed] [Google Scholar]
- 42.Nakagome K, Dohi M, Okunishi K.et al Antigen‐sensitized CD4+CD62L low memory/effector T helper 2 cells can induce airway hyperresponsiveness in an antigen free setting. Respir Res 2005646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lara‐Marquez M L, Deykin A, Krinzman S.et al Analysis of T‐cell activation after bronchial allergen challenge in patients with atopic asthma. J Allergy Clin Immunol 1998101699–708. [DOI] [PubMed] [Google Scholar]
- 44.Koh Y I, Choi S. Blood eosinophil counts for the prediction of the severity of exercise‐induced bronchospasm in asthma. Respir Med 200296120–125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.