Abstract
Objective
To investigate factors associated with menstrual dysfunction, self‐reported bone stress injuries and energy balance in women runners.
Methods
613 runners were randomly sampled during the registration period for an endurance event. Demographic information, including self‐reported height and weight, training and injury history and menstrual history, was collected by questionnaire.
Results
Ultra‐marathon (ULTRA) participants (n = 276) were significantly older (mean (SD) 39 (8.2) vs 34 (10.5) years; p<0.001), lighter (58.2 (6.6) vs 59.6 (8.3) kg; p<0.05) and reported a higher training volume (p<0.001) than half‐marathon (HALF) participants (n = 337). Significantly more ULTRA subjects than HALF subjects reported a previous bone stress injury (21% vs 14%; p<0.05). There was no difference between the groups for menstrual status, but age at menarche was later (p<0.01) in the ULTRA group. Data were combined according to the absence (REG; n = 368/602 (61%)) or presence (IRREG; n = 234/602 (39%)) of a history of menstrual irregularity. Subject morphology was similar between groups, but the IRREG group had a higher self‐reported measure on the self‐loathing subscale (SLSS; p<0.01). The whole group was then classified according to current menstrual status, with 165 women being classified as currently irregular. (OLIGO/AMEN; 11.6%) and 445 women as currently regular (EUMEN; 88.4%). There were no morphological differences between the groups, however the OLIGO/AMEN group had a later age of menarche (p<0.01) than the EUMEN group. Further, women who reported a previous bone stress injury had higher SLSS scores than those who did not (2.91 (0.98) vs 2.68 (0.84); p<0.05).
Conclusions
There may be two independent mechanisms associated with energy balance, which are related to bone stress injuries, but may not necessarily be related to menstrual dysfunction.
Keywords: female, athlete, menstrual dysfunction, bone, energy balance
The female athlete triad is defined as a serious syndrome consisting of three inter‐related components—namely, disordered eating, amenorrhoea and osteoporosis,1 with people most at risk being those participating in sports in which success is determined by thinness and aesthetics. A paper by Khan et al2 suggests a revision of the definition of the triad to replace the term “osteoporosis” with “osteopenia” in order to ensure that the definition is more widely applicable and applies not specifically to athletes. Similarly, findings by Micklesfield et al,3 which show an association between oligomenorrhoea, as well as amenorrhoea, and reduced bone mineral density, suggest that the triad component, amenorrhoea, should also be revised. Less severe manifestations of the triad components, as proposed by Torstveit and Sundgot‐Borgen,4 should be considered when evaluating the prevalence of the triad in the athletic population. This may then result in a more accurate representation of this syndrome, its associated consequences and, ultimately, more effective management strategies.
The prevalence of menstrual dysfunction has been reported previously in elite athletes5,6 and in controls.6 Results are difficult to interpret as various definitions of menstrual function have been used and various sporting disciplines have been investigated. Wolman and Harries reported menstrual irregularity in 17–100% of athletes participating in various sports included in their study, with the highest prevalence (100%) occurring in the gymnasts.5
The link between current and past menstrual dysfunction and potential deleterious effects on bone mineral density has been thoroughly investigated in female athletes.3,7,8,9,10 Much of this work includes athletes characterised by high‐energy outputs, and the association is made more complex owing to the concomitant problem of disordered eating. Furthermore, whereas most longitudinal studies investigating the resumption of menses on bone, have shown increases in bone mineral density (BMD),11,12,13 the BMD of formerly amenorrhoeic/oligomenorrhoeic subjects is generally still lower than that of regularly menstruating women runners.9,14 Results of treatments for reversing bone loss in athletes with menstrual dysfunction, such as hormone replacement, have shown equivocal outcomes.15,16 However, the effectiveness of a reduced training load12 and improved nutrition13 on the resumption of regular menses, and the consequent increase in BMD, suggests that energy balance is also implicated in the relationship between compromised bone health and menstrual dysfunction. These findings imply that other mechanisms which are associated with energy balance need to be investigated, and appropriate treatment strategies adopted based on these findings. In addition, there is limited research investigating menstrual dysfunction in less competitive athletes.
Therefore, this study aimed at exploring the inter‐relationships between indirect indicators of disordered eating (as a proxy for energy intake), increased training load (a measure of energy expenditure), bone stress injuries, and menstrual dysfunction in female runners of various competitive levels, in order to define more clearly the factors associated with bone health in runners.
Methods
This study comprised a questionnaire survey conducted by trained interviewers during the 3‐day registration period for the Two Oceans ultra‐ (56 km) and half‐ (21.1 km) marathons.
Participants
Six hundred and fifty women (n = 650) were randomly selected to participate in the study. These women were recruited while registering for either the ultra‐marathon (ULTRA; 56 km) or the half‐marathon (HALF; 21.1 km) races in 2004. Seventeen hundred women completed the ultra‐marathon while 2718 women completed the half‐marathon in this year. Of the 650 women who were invited to participate in the study, only 37 (5.7%) women refused to take part, resulting in a sample of 613 women.
Questionnaire data
Demographic data and information, including self‐reported height and weight, training mileage, frequency and duration (volume), and history of bone stress injury or stress fractures, were collected. In addition, information on current and previous menstrual function, and reproductive history, including oral contraceptive use, number of pregnancies and hormone replacement therapy use, were collected. Lifestyle habits, including alcohol consumption and smoking data, were also collected.
In an effort to understand the potential body image disturbances in these athletes, an instrument known as the self‐loathing subscale (SLSS)17 was administered to all participants. This scale consists of four questions developed to reflect body (appearance) and self (performance) dissatisfaction, based on attitudes and experiences of people who exercise. This tool has been correlated with self‐reported eating disorder symptoms.18
This study was approved by the ethics and research committee of the University of Cape Town, and informed consent was obtained from the participants before completion of the questionnaire.
Statistical analyses
The Statsoft (Statistica v7, 2004) statistical package was used. Descriptive analyses were performed to determine mean values and standard deviations. Comparisons between HALF and ULTRA runners, and between runners with and without a current menstrual dysfunction or a history of menstrual dysfunction, were carried out using one‐way analysis of variance for continuous data and a χ2 test (Fisher's exact test) for categorical data.
Results
Subject characteristics
Table 1 shows the characteristics of the participants. Of the 613 female subjects who participated in the study, 337 completed the half‐marathon and 276 completed the ultra‐marathon. The ULTRA runners were significantly older (p<0.001) and lighter (p<0.05) than the HALF runners.
Table 1 Subject characteristics of 613 female runners in the Two Oceans.
| Characteristics | Half‐marathon (n = 337) Mean (SD) {range} | Ultra‐marathon (n = 276) Mean (SD) {range} |
|---|---|---|
| Age (years)*** | 34 (10.5) {16–62} | 39 (8.2) {22–61} |
| Weight (kg)* | 59.6 (8.3) {40–90} | 58.2 (6.6) {45–79} |
| Height (m) | 1.7 (0.1) {1.5–2} | 1.6 (0.1) {1.5–1.9} |
| BMI (kg/m2) | 21.9 (2.9) {16.0–30.7} | 21.6 (2.4) {14.7 (33.1} |
* p<0.05; ***p<0.001.
Training differences between the HALF and ULTRA runners
Table 2 shows differences between the HALF and ULTRA runners. Training frequency, duration and distance were significantly higher (p<0.001) in the ULTRA runners than in the HALF runners.
Table 2 Training data of 613 Two Oceans female runners.
| Training data | Half‐marathon (n = 337) (%) | Ultra‐marathon (n = 276) (%) |
|---|---|---|
| Training frequency (sessions/week)*** | ||
| 1–3 | 40.4 | 7.6 |
| 4–6 | 56.7 | 87 |
| >7 | 3 | 5.4 |
| Training duration (h/week)*** | ||
| 1–3 | 28.7 | 5.1 |
| 4–6 | 54.9 | 34.4 |
| 7–9 | 11.3 | 44.6 |
| >10 | 5.1 | 15.9 |
| Training distance (km/week)*** | ||
| <20 | 16.3 | 1.5 |
| 21–40 | 55.8 | 7.8 |
| 41–60 | 22.3 | 36.4 |
| 61–80 | 1.2 | 42.5 |
| >81 | 2.1 | 12.4 |
***p<0.001.
Reproductive status in HALF and ULTRA runners
The ULTRA runners were significantly older at menarche (14.1 (2.1) vs 13.6 (1.7) years; p<0.01) than the HALF runners. There were no significant differences in current menstrual status (cycles/year) between the groups, with 74.8% of the HALF runners reporting 10–13 cycles/year currently, and 70.7% of the ULTRA runners reporting current regular menses. Similarly, there were no significant differences in menstrual history between the ULTRA runners and the HALF runners, with 40.8% of the HALF runners and 36.4% of the ULTRA runners reporting a history of menstrual irregularity.
Injury history in HALF and ULTRA runners
Significantly more ULTRA runners (21%) reported a history of a bone stress injury than HALF runners (14%; p<0.05).
SLSS results in HALF and ULTRA runners
There was no difference in the SLSS score between the HALF (2.71 (0.80)) and the ULTRA (2.75 (0.95)) runners.
Menstrual patterns
Tables 3 and 4 show the menstrual pattern history and current menstrual problems, respectively.
Table 3 Menstrual pattern history of the Two Oceans female athletes.
| Characteristics | History of menstrual irregularity (n = 234) Mean (SD) | No history of menstrual irregularity (n = 368) Mean (SD) |
|---|---|---|
| Age (years) | 35.5 (10.4) | 37.1 (9.4) |
| Weight (kg) | 58.7 (8.2) | 59.1 (7.2) |
| Height (m) | 1.65 (0.07) | 1.64 (0.07) |
| BMI (kg/m2) | 21.6 (3) | 21.9 (2.5) |
| Age at menarche (years) | 13.9 (1.9) | 13.8 (1.9) |
| SLSS score** | 2.84 (0.91) | 2.65 (0.83) |
BMI, body mass index; SLSS, self‐loathing subscale.
**p<0.01.
Table 4 Current menstrual patterns of the Two Oceans female athletes.
| Characteristics | Current menstrual irregularity (n = 165) Mean (SD) | Current menstrual regularity (n = 445) Mean (SD) |
|---|---|---|
| Age (years) | 43.7 (10.1) | 33.8 (8.2) |
| Weight (kg) | 59.3 (8.0) | 58.9 (7.5) |
| Height (m) | 1.64 (0.07) | 1.65 (0.07) |
| BMI (kg/m2) | 21.9 (3.04) | 21.7 (2.6) |
| Age at menarche (years) | 13.9 (1.8) | 13.8 (1.9) |
| SLSS score | 2.71 (0.99) | 2.73 (0.82) |
BMI, body mass index; SLSS, self‐loathing subscale.
Two hundred and thirty‐four women reported a history of menstrual irregularity (IRREG), compared with 368 women who had always had regular menses (REG). There were no differences in age, weight, height, body mass index (BMI) or age at menarche between women with or without a history of menstrual irregularity. However, women with a history of menstrual irregularity had a significantly higher SLSS score than women with no history of menstrual irregularity (p<0.001).
The women who reported current oligomenorrhoea or amenorrhoea (OLIGO/AMEN; n = 165) were significantly older than the women who reported current regular menses (EUMEN; n = 445). There were no differences in weight, height, BMI, age at menarche or SLSS score between women with current menstrual irregularity compared with those who were currently regular. Nor were there any differences in training frequency, duration or distance between any of the groups. These results did not change significantly when the 154 (25%) women who reported current oral contraceptive use were excluded.
In the premenopausal subjects only (n = 497), BMI was significantly lower in the women who reported a history of menstrual dysfunction (n = 187; 21.3 (2.9) kg/m2) than in the women with no history of menstrual dysfunction (n = 300; 21.8 (2.5) kg/m2; p<0.05), and in the women who were currently irregular (n = 68; 21.0 (2.8) kg/m2) than in the women who had regular menses currently (n = 429; 21.7 (2.6) kg/m2; p = 0.05). When women were classified according to whether they had always had regular menstrual function (n = 280) or whether they had ever experienced menstrual irregularity, either currently or previously (n = 308), the difference between the groups was not significant (p = 0.08).
In the premenopausal subjects only, the SLSS score was significantly higher in the women with a history of menstrual dysfunction than in the women with no history of menstrual dysfunction (2.92 (0.88) vs 2.63 (0.80); p<0.001), however, it was not significantly different between the groups divided according to current menstrual status.
When the current and historical menstrual data were combined, the runners who reported menstrual dysfunction had a significantly higher SLSS score than women with no reported menstrual dysfunction (2.79 (0.92) vs 2.65 (0.80); p<0.05). When the women who reported current oral contraceptive use were excluded, the difference was still significant (p = 0.008).
SLSS scores and bone stress injury
A previous bone stress injury was reported by 17.3% of the runners. There was no difference in age, weight, BMI or menstrual function between the runners who did, and did not, report a bone stress injury. However the runners who did report a previous bone stress injury had a significantly higher SLSS score than runners with no history of a bone stress injury (2.91 (0.98) vs 2.68 (0.84); p<0.05).
Prevalence of the triad components
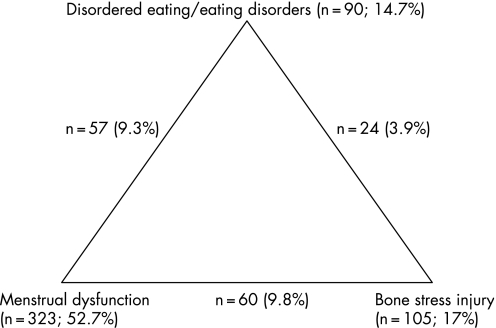
Figure 1 shows the prevalence of the triad components. Disordered eating patterns were positive if a subject scored ⩾3.67 on the SLSS, based on criteria established by Yates et al.18 Ninety (14.7%) subjects were categorised as positive for disordered eating. The existence of menstrual dysfunction was noted if the subject reported current menstrual dysfunction or a history of menstrual dysfunction (n = 323; 52.7%). For this study, a positive history of a bone stress injury (n = 105; 17%) was used as a proxy for low bone mineral density.19,20
Figure 1 Prevalence of the combination of two or three of the “proxy” criteria for the triad in female runners (n = 613).
Thirty‐six per cent (n = 219) of the subjects reported none of the three components, while 45.6% (n = 277) reported one component and 16.1% (n = 98) reported two of the triad components, as defined by this study. Fourteen athletes (2.3%) reported all three of the components of the triad, according to the criteria used in this study.
When comparing the prevalence of the three components between the ULTRA and HALF runners, there was a significant difference (p<0.05) between the groups for the number of runners reporting one (ULTRA: 40% vs HALF: 50%), two (ULTRA: 21% vs HALF: 12%) or three (ULTRA: 3.3% vs HALF: 1.5%) of the components.
Discussion
The findings of this study contribute to a greater understanding of the components traditionally described as the female athlete triad, their inter‐relationships, and the broader population of people who may be at risk for this syndrome. Our subjects were 613 runners who had entered an ultra‐marathon (56 km) or a half‐marathon (21.1 km). Fifty‐three per cent (52.7%) of the runners reported either current menstrual dysfunction or a history of menstrual dysfunction, while 17% reported a previous bone stress injury and 14.7% reported a score indicative of disordered eating patterns. Thirty‐six per cent of the athletes did not present with any of the triad components, as defined by our study, while 2.3% of the runners presented with all three components. The prevalence of all three components is lower than that reported by Torstveit and Sundgot‐Borgen4 for their sample of elite athletes (4.3%) and controls (3.4%). The higher prevalence of elite athletes in their sample than in our sample of predominantly recreational runners suggests that prevalence may be associated with training load. This is confirmed by the difference we found in the prevalence of the three triad components between the ULTRA and HALF runners (3.3 vs 1.5%). In addition, the criteria used for both studies were slightly different. Torstveit and Sundgot‐Borgen4 defined the triad as a combination of disordered eating and/or eating disorders (using the DSM‐IV criteria for anorexia nervosa, bulimia nervosa and eating disorders not otherwise specified), menstrual dysfunction (including amenorrhoea, oligomenorrhoea, and a short luteal phase) and a low BMD (Z‐score <−1.0).
What is already known on this topic?
Traditionally it has been accepted that the decrease in bone mineral density that accompanies menstrual dysfunction is due to chronic hypo‐oestrogenism.
However, the use of oral contraceptives and the restoration of regular menses in these athletes have not been successful in reversing this bone loss.
More recent research has suggested that there is a possible link between energy imbalance and reduced bone turnover, thereby proposing a different management plan for these athletes.
The prevalence of menstrual dysfunction (oligomenorrhoea and amenorrhoea) in our study was 52.7%, a similar finding to that of Wolman and Harries who studied elite athletes (52%). As previously reported,4,21 we confirmed an association between disordered eating patterns and menstrual dysfunction, as well as between disordered eating patterns and a history of bone stress injuries, but there was no association between menstrual dysfunction and a history of bone stress injuries. The higher prevalence of bone stress injuries in the ULTRA runners than in the HALF runners also implies a relationship between training load and bone mass. These findings suggest that there may be two independent mechanisms—namely, disordered eating and a high training load, which are both related to bone stress injuries, but may not necessarily occur through menstrual dysfunction.
What this study adds
This study suggests that the profile of the female athlete with menstrual dysfunction may be of one who is at an increased risk of low bone mass owing to disordered eating patterns and high training loads, rather than to menstrual dysfunction.
Our findings support previous reports which suggest that management of these athletes should focus on correcting the energy balance by increasing dietary energy intake rather than by altering exercise training.
Extensive reports confirm that female athletes who present with menstrual dysfunction have a lower BMD than eumenorrhoeic athletes.3,7,8 Traditionally, it has been accepted that the reason for the decrease in BMD that accompanies menstrual dysfunction is chronic hypo‐oestrogenism. However, the use of oral contraceptives and the restoration of regular menses in these athletes have not been successful in reversing this bone loss.9,14,22 In addition, Zanker and Swaine have shown that reduced bone formation, normally associated with undernutrition, rather than increased bone resorption as seen in hypo‐oestrogenic women, may be the mechanism of bone loss in female athletes.23 This suggests that another mechanism may be responsible for the bone loss seen in athletes with menstrual dysfunction, and as confirmed by the results in this study, which show a relationship between the occurrence of bone stress injuries and disordered eating patterns, as well as a high training load, this mechanism may be more related to energy balance than to hypo‐oestrogenism.
Although a history of menstrual dysfunction was associated with a higher score on the disordered eating scale, there was no association between menstrual dysfunction and training load. The components of energy balance—namely, energy intake and energy expenditure, have been investigated in female athletes and women with anorexia nervosa who present with menstrual dysfunction.23,24 In an anorexic population, in whom energy intake is inadequate, the prevalence of menstrual dysfunction is high, and recovery of regular menstrual function has been associated with an increase in body weight.10 When investigating the association between training load and menstrual dysfunction, several studies reported no association.6 These publications, together with our findings, suggest that management of these athletes should focus on correcting the energy balance by increasing dietary energy intake rather than by altering exercise training. This management strategy can be confirmed by the findings of Zanker and Swaine23 who measured bone turnover markers in a group of distance runners, and found that bone formation was reduced in their sample of amenorrhoeic runners, a similar profile of bone turnover to that seen in anorexic women, compared with the high bone turnover that is present in oestrogen‐deficient women.
We propose, based on the results of this study, that the profile of the female athlete with menstrual dysfunction may be of one who is at an increased risk of low bone mass owing to disordered eating patterns and high training loads, rather than to menstrual dysfunction, which has been so thoroughly reviewed in this population.
In summary, the findings of our study confirm the results of other studies suggesting that bone stress injuries are associated with increased energy expenditure, as measured by training load, and associated with inadequate nutrition, as measured by the proxy used for disordered eating patterns. We have not investigated the mechanisms responsible for these relationships; however, these relationships may be further confounded or exacerbated by menstrual dysfunction. Although it is unlikely that these mechanisms are wholly independent, these and other data suggest that treatment of compromised bone health should focus on nutrition, rather than solely on hormone replacement.
Abbreviations
BMD - bone mineral density
BMI - body mass index
HALF - half‐marathon
IRREG - presence of a history of menstrual irregularity
REG - absence of a history of menstrual irregularity
SLSS - self‐loathing subscale
ULTRA - ultra‐marathon
Footnotes
Conflict of interest: None declared.
References
- 1.Otis C L, Drinkwater B, Johnson M.et al American College of Sports Medicine position stand. The female athlete triad. Med Sci Sports Exerc. 1997;29: i–ix, [DOI] [PubMed]
- 2.Khan K M, Liu‐Ambrose T, Sran M M.et al New criteria for female athlete triad syndrome? As osteoporosis is rare, should osteopenia be among the criteria for defining the female athlete triad syndrome? Br J Sports Med 20023610–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Micklesfield L K, Lambert E V, Fataar A B.et al Bone mineral density in mature, premenopausal ultramarathon runners. Med Sci Sports Exerc 199527688–696. [PubMed] [Google Scholar]
- 4.Torstveit M K, Sundgot‐Borgen J. The female athlete triad exists in both elite athletes and controls. Med Sci Sports Exerc 2005371449–1459. [DOI] [PubMed] [Google Scholar]
- 5.Wolman R L, Harries M. Menstrual abnormalities in elite athletes. Clin Sports Med 1989195–100. [Google Scholar]
- 6.Torstveit M K, Sundgot‐Borgen J. Participation in leanness sports but not training volume is associated with menstrual dysfunction: a national survey of 1276 elite athletes and controls. Br J Sports Med 200539141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drinkwater B L, Nilson K, Chesnut CH I I I.et al Bone mineral content of amenorrheic and eumenorrheic athletes. N Engl J Med 1984311277–281. [DOI] [PubMed] [Google Scholar]
- 8.Marcus R, Cann C, Madvig P.et al Menstrual function and bone mass in elite women distance runners. Endocrine and metabolic features. Ann Intern Med 1985102158–163. [DOI] [PubMed] [Google Scholar]
- 9.Micklesfield L K, Reyneke L, Fataar A.et al Long‐term restoration of deficits in bone mineral density is inadequate in premenopausal women with prior menstrual irregularity. Clin J Sport Med 19988155–163. [DOI] [PubMed] [Google Scholar]
- 10.Miller K K, Lee E E, Lawson E A.et al Determinants of skeletal loss and recovery in Anorexia Nervosa. J Clin Endocrinol Metab 2006912931–2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drinkwater B L, Nilson K, Ott S.et al Bone mineral density after resumption of menses in amenorrheic athletes. JAMA 1986256380–382. [PubMed] [Google Scholar]
- 12.Lindberg J S, Powell M R, Hunt M M.et al Increased vertebral bone mineral in response to reduced exercise in amenorrheic runners. West J Med 198714639–42. [PMC free article] [PubMed] [Google Scholar]
- 13.Jonnavithula S, Warren M P, Fox R P.et al Bone density is compromised in amenorrheic women despite return of menses: a 2‐year study. Obstet Gynecol 199381669–674. [PubMed] [Google Scholar]
- 14.Keen A D, Drinkwater B L. Irreversible bone loss in former amenorrheic athletes. Osteoporos Int 19977311–315. [DOI] [PubMed] [Google Scholar]
- 15.Gibson J H, Mitchell A, Reeve J.et al Treatment of reduced bone mineral density in athletic amenorrhea: a pilot study. Osteoporos Int 199910284–289. [DOI] [PubMed] [Google Scholar]
- 16.Warren M P, Brooks‐Gunn J, Fox R P.et al Persistent osteopenia in ballet dancers with amenorrhea and delayed menarche despite hormone therapy: a longitudinal study. Fertil Steril 200380398–404. [DOI] [PubMed] [Google Scholar]
- 17.Yates A, Edman J D, Crago M.et al Using an exercise‐based instrument to detect signs of an eating disorder. Psychiatry Res 2001105231–241. [DOI] [PubMed] [Google Scholar]
- 18.Yates A, Edman J D, Crago M.et al Eating disorder symptoms in runners, cyclists, and paddlers. Addict Behav 2003281473–1480. [DOI] [PubMed] [Google Scholar]
- 19.Myburgh K H, Hutchins J, Fataar A B.et al Low bone density is an etiologic factor for stress fractures in athletes. Ann Intern Med 1990113754–759. [DOI] [PubMed] [Google Scholar]
- 20.Bennell K, Malcolm S A, Thomas S A.et al Risk factors for stress fractures in track and field athletes. Am J Sports Med 199624810–817. [DOI] [PubMed] [Google Scholar]
- 21.Cobb K L, Bachrach L K, Greendale G.et al Disordered eating, menstrual irregularity, and bone mineral density in female runners. Med Sci Sports Exerc 200335711–719. [DOI] [PubMed] [Google Scholar]
- 22.Mazess R B, Barden H S. Bone density in premenopausal women: effects of age, dietary intake, physical activity, smoking, and birth‐control pills. Am J Clin Nutr 199153132–142. [DOI] [PubMed] [Google Scholar]
- 23.Zanker C L, Swaine I L. Relation between bone turnover, oestradiol, and energy balance in women distance runners. Br J Sports Med 199832167–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zanker C L, Swaine I L. The relationship between serum oestradiol concentration and energy balance in young women distance runners. Int J Sports Med 199819104–108. [DOI] [PubMed] [Google Scholar]