Abstract
It has been proposed that glial cells may supply carbon fuel to neurons and also that there are fluxes of ammonium from neurons to glia. We have investigated both these proposals in Apis retinal slices, in which virtually all the mitochondria are in the photoreceptor neurons. Normally the superfusate contained no substrate of energy metabolism; addition of glucose or alanine did not increase oxygen consumption  , confirming that the neurons received adequate substrate from glycogen in the glia. 1,4-Dideoxy-1,4-imino-d-arabinitol (DAB, 100 μm), an inhibitor of glycogen phosphorylase, progressively decreased
, confirming that the neurons received adequate substrate from glycogen in the glia. 1,4-Dideoxy-1,4-imino-d-arabinitol (DAB, 100 μm), an inhibitor of glycogen phosphorylase, progressively decreased  . This decrease was reversed by alanine but not glucose. Ammonium-sensitive microelectrodes did not detect significant extracellular [NH4+] ([NH4+]e) in slices superfused with normal superfusate. Removal of Cl−, necessary for cotransport of NH4+ into the glia, increased [NH4+]e so that at the end of 2 min photostimulation mean [NH4+]e was 0.442 mm (s.e.m.= 0.082 mm, n = 16). In 0 Cl−, [NH4+]e was reduced by 2-(methylamino)isobutyrate (MeAIB) an inhibitor of alanine transport. MeAIB also blocked oxidation of alanine in the presence of DAB, but did not decrease
. This decrease was reversed by alanine but not glucose. Ammonium-sensitive microelectrodes did not detect significant extracellular [NH4+] ([NH4+]e) in slices superfused with normal superfusate. Removal of Cl−, necessary for cotransport of NH4+ into the glia, increased [NH4+]e so that at the end of 2 min photostimulation mean [NH4+]e was 0.442 mm (s.e.m.= 0.082 mm, n = 16). In 0 Cl−, [NH4+]e was reduced by 2-(methylamino)isobutyrate (MeAIB) an inhibitor of alanine transport. MeAIB also blocked oxidation of alanine in the presence of DAB, but did not decrease  in normal superfusate. Lactate (l and d) and pyruvate (but not glucose) increased
in normal superfusate. Lactate (l and d) and pyruvate (but not glucose) increased  in DAB and decreased [NH4+]e in 0 Cl−. These results strengthen the evidence that in superfused retinal slices, glucose is metabolized exclusively in the glia, which supply alanine to the neurons, and that ammonium returns to the glia. They also show that another fuel (perhaps lactate) can be supplied by the glia to the neurons.
in DAB and decreased [NH4+]e in 0 Cl−. These results strengthen the evidence that in superfused retinal slices, glucose is metabolized exclusively in the glia, which supply alanine to the neurons, and that ammonium returns to the glia. They also show that another fuel (perhaps lactate) can be supplied by the glia to the neurons.
Experiments with 2-[14C]deoxyglucose have suggested that about 50% of the glucose taken up by the brain of a freely moving rat is taken up by astrocytes (Nehlig et al. 2004) and yet experiments with 13C magnetic resonance spectroscopy suggest that only 29% of total brain oxidative metabolism takes place in the astrocytes (Hyder et al. 2006). This difference appears to imply that substrates of energy metabolism are transferred from glial cells to neurons in normoglycaemia. Glucose is taken up mainly by the glia in rat vagus nerve (Véga et al. 2003), and experiments on cells from guinea pig retina (Poitry-Yamate et al. 1995) also suggest glia–neuron transfer of energy substrate. In mammalian nervous tissue, the end product of glycolysis, pyruvate, is in part converted to lactate, which can be released to the extracellular medium (see Fillenz, 2005, for references). This observation and other circumstantial evidence have led to the proposal that lactate may be transferred from glia to neurons, but the proposal lacks strong direct experimental support and is controversial (Pellerin & Magistretti, 1994; Coles et al. 2000; Serres et al. 2003; Chih & Roberts, 2003; Fillenz, 2005; Nehlig & Coles, 2007; Pellerin et al. 2007). In mammals, pyruvate is also converted to alanine (Vrba, 1962; Larrabee, 1992).
The organization of the retina of the honey bee drone (male Apis mellifera) lends itself to investigation of glia–neuron metabolic exchange (Tsacopoulos & Magistretti, 1996). This nervous tissue has a structure of near-crystalline regularity (Fig. 2A) and shows extreme metabolic compartmentation: the neurons (which are all photoreceptors) contain many mitochondria, while the glial cells (‘outer pigment cells’) have virtually none, but are packed with glycogen (Perrelet, 1970; Dimitracos & Tsacopoulos, 1985). The photoreceptors in a retinal slice consume oxygen and respond electrically to light stimulation for many hours in the absence of exogenous glucose: this indicates that mechanisms exist for transferring products of glycogen breakdown from the glia to the neurons (Coles & Tsacopoulos, 1981; Tsacopoulos et al. 1987). Incubation of retinal slices in 2-[3H]deoxyglucose leads to labelling of the glia, but not the photoreceptors (Tsacopoulos et al. 1988), showing that glucose is not used directly by the photoreceptors. After incubation of a retinal slice in 14C glucose, radioactivity in the bathing solution is found mainly in alanine and trehalose, a dimer of glucose (Tsacopoulos et al. 1994). Alanine is released by isolated bundles of glial cells, and isoforms of alanine transaminase are present that should allow conversion of pyruvate to alanine in the glia and alanine to pyruvate in the neurons (Tsacopoulos et al. 1994). These reactions require the return of fixed N from the neurons to the glia. Retinal slices release ammonium (Tsacopoulos et al. 1997b), and the glial cells show Cl−-dependent specific uptake of NH4+ (Marcaggi et al. 1999, 2004; Marcaggi & Coles, 2000). It has therefore been proposed that the glia convert pyruvate not to lactate but to alanine, which is supplied to the neurons, and that ammonium is returned to the glia (Tsacopoulos et al. 1994; Tsacopoulos et al. 1997b).
Figure 2. Recordings of localPO2 in retinal slices.
A, schematic section of part of the superfused slice of drone retina. The rosettes represent the clusters of photoreceptor neurons behind each facet of the cornea. Glial cells and narrow extracellular clefts occupy almost all the remaining space. The precise location of the microelectrode tip was not known. B, light stimulation increased  but addition of glucose caused little further increase. The record shows PO2, initially in the dark. Onset of stimulation with light flashes at 1 Hz caused PO2 to fall (
but addition of glucose caused little further increase. The record shows PO2, initially in the dark. Onset of stimulation with light flashes at 1 Hz caused PO2 to fall ( to increase) to an approximately steady value. Switching to Cardinaud solution containing 10 mm glucose only slightly reduced PO2. (At solution changes, a bolus of solution not saturated with oxygen passed, often causing an artifactual downward deflexion.) The PO2 electrode was not calibrated until 40 min after the recording and the PO2 scale is approximate, as it is in nearly all PO2 records shown. C, like glucose, MeAIB had little effect on
to increase) to an approximately steady value. Switching to Cardinaud solution containing 10 mm glucose only slightly reduced PO2. (At solution changes, a bolus of solution not saturated with oxygen passed, often causing an artifactual downward deflexion.) The PO2 electrode was not calibrated until 40 min after the recording and the PO2 scale is approximate, as it is in nearly all PO2 records shown. C, like glucose, MeAIB had little effect on  . The upper trace includes an artifactual record of the light-induced field potentials, which appear fused on this slow time scale, and are filtered out on the lower trace. After the third train of light flashes, the electrode was withdrawn to the oxygenated bath and its response to reduced PO2 (‘air’, 20 kPa) recorded. At the arrow, the MeAIB solution was passed: after a transient fall (due to a bolus of unsaturated solution), the electrode current was unaffected. D, PO2 at a higher gain during continuous stimulation with light flashes. MeAIB did not change the slope of the (drifting) baseline. E, α-cyano-4-hydroxycinnamate (αCC) decreased
. The upper trace includes an artifactual record of the light-induced field potentials, which appear fused on this slow time scale, and are filtered out on the lower trace. After the third train of light flashes, the electrode was withdrawn to the oxygenated bath and its response to reduced PO2 (‘air’, 20 kPa) recorded. At the arrow, the MeAIB solution was passed: after a transient fall (due to a bolus of unsaturated solution), the electrode current was unaffected. D, PO2 at a higher gain during continuous stimulation with light flashes. MeAIB did not change the slope of the (drifting) baseline. E, α-cyano-4-hydroxycinnamate (αCC) decreased  . F, αCC (10 mm) plus MeAIB (10 mm) decreased
. F, αCC (10 mm) plus MeAIB (10 mm) decreased  about as much as did αCC alone. G, summary of results in normal Cardinaud solution. In each condition, the rate of change of PO2 (dPO2/dt) was measured in control and experimental conditions (5 min after the solution change) and the difference calculated and averaged. None of d-glucose (10 mm), l-alanine (25 mm, no record shown) and MeAIB (10 mm) significantly changed dPO2/dt. The integer at each column is the number of applications.
about as much as did αCC alone. G, summary of results in normal Cardinaud solution. In each condition, the rate of change of PO2 (dPO2/dt) was measured in control and experimental conditions (5 min after the solution change) and the difference calculated and averaged. None of d-glucose (10 mm), l-alanine (25 mm, no record shown) and MeAIB (10 mm) significantly changed dPO2/dt. The integer at each column is the number of applications.
Although the evidence for a glia–neuron alanine/ammonium shuttle in drone retina is extensive and generally coherent, at least two key questions had not been answered convincingly: is alanine an effective substrate for photoreceptor energy metabolism? and is there is a flux of ammonium from the photoreceptors to the glia? Given the current debate about possible glia–neuron exchange in mammalian energy metabolism, we thought it worthwhile to perform new kinds of experiments on drone retina, using tools not available to the earlier workers.
To look for effects of alanine and other candidate substrates on the oxidative metabolism of the photoreceptors, it was necessary first to reduce the supply from the glia. Saravelos & Tsacopoulos (1995) reported that the classical inhibitor of glycolysis, iodoacetate, is unusable for this experiment because it also inhibits photoreceptor metabolism. We found that 1,4-dideoxy-1,4-imino-d-arabinitol (DAB), an inhibitor of glycogen phosphorylase (Andersen et al. 1999), appears to reversibly reduce the supply of substrates from the glia without affecting the ability of the photoreceptors to use exogenous substrates.
Then, to test the hypothesis of a neuron–glia flux of ammonium, we used triple-barrelled ammoniumsensitive microelectrodes to measure [NH4+] in the extracellular clefts, and performed manoeuvres designed to interfere with either the glial uptake of ammonium or the production of ammonium in the photoreceptors.
Methods
Slices of bee retina
Honeybee drones and accompanying workers were purchased from La Ferme Apicole de l'Estrel, Frejus, or l'Abeille d'Or, Grenoble, and were maintained for up to 3 weeks at 30°C on 50% sucrose in water. A slice of the head 400–600 μm thick was made with a razor blade with the cut surfaces approximately parallel to the axes of the ommatidia. The slice was immediately placed at the bottom of a perfusion chamber with one retina over a groove in the floor so that it was superfused on both faces. The superfusate was oxygenated Cardinaud solution (see below) at room temperature (22–30°C); for the first 7–10 min, the superfusate contained additional Ca2+ to a total concentration of 16 mm. The photoreceptors could be stimulated with light flashes as previously described (Coles & Orkand, 1983; Coles et al. 1996). Solutions were gravity fed at 2.5–3.5 ml min−1 and selected, near the superfusion chamber, by mechanical and pneumatic valves.
Solutions and superfusion
The composition of the standard superfusate solution (Cardinaud solution) was based on the measured ionic concentrations and osmolarity in the extracellular space of retinas of living drones (mm): NaCl, 200; KCl, 10; MgCl2, 4; CaCl2, 2; d-(+)-saccharose, 240 (Cardinaud et al. 1994). pH was buffered with 10 mm MOPS, hemisodium salt, and adjusted with HCl (or methanesulphonic acid for chloride-free solutions) to 6.90, the value measured in vivo. The saccharose was added to match the osmolarity of the solution with that of the interstitial fluid in vivo (Cardinaud et al. 1994). No effect on oxygen consumption  was detected when saccharose was replaced by mannitol (n = 2, not shown). In chloride-free solutions, chloride salts were replaced by sodium glucuronate, and by gluconate salts of potassium, magnesium and calcium, the concentration of Ca(gluconate)2 being 4 mm following the Ca2+ electrode measurements by Coles et al. (1989). Glucuronate was used as the major anion because it has a lower affinity for Ca2+ than does gluconate; minor salts were gluconates because glucuronates were not readily available.
was detected when saccharose was replaced by mannitol (n = 2, not shown). In chloride-free solutions, chloride salts were replaced by sodium glucuronate, and by gluconate salts of potassium, magnesium and calcium, the concentration of Ca(gluconate)2 being 4 mm following the Ca2+ electrode measurements by Coles et al. (1989). Glucuronate was used as the major anion because it has a lower affinity for Ca2+ than does gluconate; minor salts were gluconates because glucuronates were not readily available.
When other compounds were included in the solution, sodium salts replaced equimolar amounts of NaCl (sodium pyruvate, sodium lactate, sodium 2-(methylamino)isobutyrate). Alanine, β-alanine, glucose and trehalose replaced equimolar amounts of sucrose. Chemicals were from Sigma-Aldrich apart from DAB, which was from Novo Nordisk, Bagsvaerd, DK.
Measurement of PO2
Oxygen microelectrodes each consisting of a tapered platinum (Pt) wire sealed into soda glass were used. The outer diameter of the glass tip was 8–16 μm and the Pt surface was in a recess 5–11.6 μm deep. A polarizing potential of −580 mV was applied, measured between the Pt wire and a KCl bridge bath electrode (either an agar bridge or a WPI Driref 2SH). This KCl electrode was clamped to system ground using an operational amplifier and an Ag/AgCl wire to pass current into the bath. The current flowing through the O2 electrode was measured with an operational amplifier (ad 549) with a 1010Ω feedback resistor (Eltec, Stäfa, Switzerland). We inserted PO2 electrodes a distance of 80–150 μm obliquely (at 54 deg to the vertical) into slices superfused with Cardinaud solution. PO2 at the electrode tip depends on  throughout the slice (Tsacopoulos et al. 1981); measurements were begun when PO2 was stable enough for changes to be observable and when there was a clear response to light stimulation. To increase
throughout the slice (Tsacopoulos et al. 1981); measurements were begun when PO2 was stable enough for changes to be observable and when there was a clear response to light stimulation. To increase  , the photoreceptors were stimulated with 50 ms light flashes, one per second. To minimize changes in oxygenation on switching perfusion solutions, a siphon tube jacketed with a larger tube continuously flushed with O2 was moved from one solution bottle to another. Signals were recorded on a paper chart and on a hard disk.
, the photoreceptors were stimulated with 50 ms light flashes, one per second. To minimize changes in oxygenation on switching perfusion solutions, a siphon tube jacketed with a larger tube continuously flushed with O2 was moved from one solution bottle to another. Signals were recorded on a paper chart and on a hard disk.
Ammonium microelectrodes
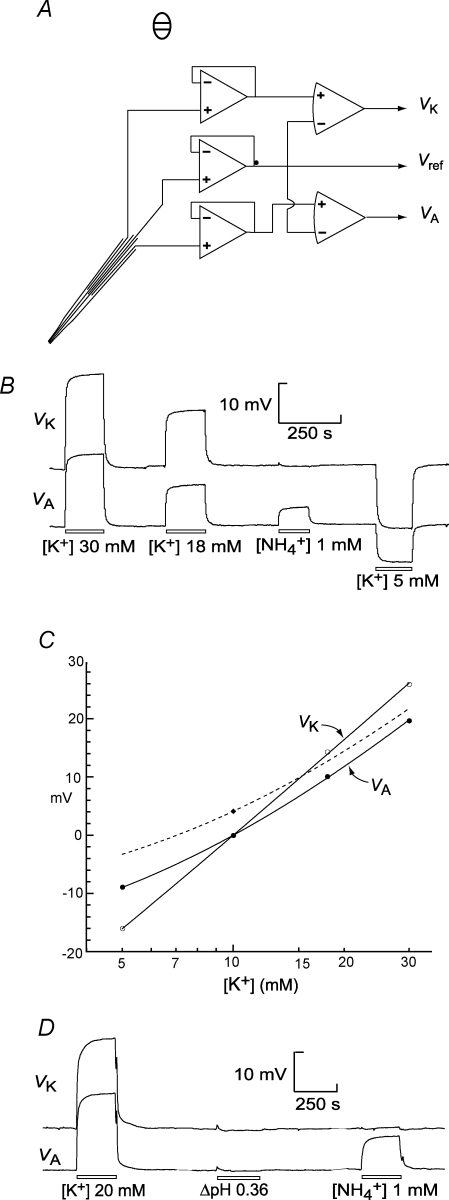
Ammonium concentration was measured with the ammonium sensor of Bührer et al. (1988) based on nonactin/monactin (Fluka 09877). This sensor also detects K+, so, since interstitial [K+] in the retinal extracellular space changes during light stimulation (Coles & Tsacopoulos, 1979), we made electrodes from triple-barrelled capillaries and measured the ‘ammonium’ signal and [K+] simultaneously (Provent et al. 2007). One outer barrel contained the ammonium sensor backed by Cardinaud solution to which was added 10 mm NH4Cl. The other outer barrel contained a K+ sensor based on valinomycin (Fluka 60403) with a ratio of valinomycin : potassium p-chlorophenylborate : solvent of 6 : 1: 93 by weight (Coles & Poulain, 1991); the solvent was either 1,2-dimethylnitrobenzene or 2-nitrophenyloctylether. This sensor was backed by Cardinaud solution. The central barrel measured the electrical potential; it was filled with Cardinaud solution, or, for experiments using 0 Cl− superfusate, with 0 Cl− Cardinaud solution to which was added about 10 mm NaCl. Tips were bevelled to 3–4.5 μm (in one case to 6.0 μm) on a miniature grindstone (De Marco, Meyrin, Switzerland). After analog subtraction of the reference potential, and amplification, the signals from the NH4+ barrel (VA) and the K+ barrel (VK) were digitized using a Handyscope 3 (TiePie Engineering, Sneek, the Netherlands) and stored on a hard disk for analysis off-line. After each recording in a retinal slice, the flow of the experimental solution (e.g. Cardinaud solution, or 0 Cl−+ MeAIB) was maintained while the electrode was withdrawn to the bath and a baseline (i.e. the calibration point for 10 mm[K+] and zero ammonium) established. Without interruption of the perfusion, the electrode was calibrated in variants of the experimental solution to which had been added KCl or NH4Cl to give concentrations of 15 or 25 mm K+ or 1 mm NH4+. With this procedure, any possible effect on any of the electrode potentials of removing Cl− or adding other compounds was automatically taken care of. (In some earlier experiments, the baseline was established in the experimental solution and the calibration performed in modified Cardinaud solutions or 0 Cl− solutions as appropriate.) VA and VK of some electrodes showed shifts of up to 2 mV on switching between 0 Cl− Cardinaud solution and 0 Cl− Cardinaud solution in which some glucuronate had been replaced by another charged compound (such as pyruvate or lactate) but no changes were seen when some of the sucrose was replaced by an uncharged molecule such as glucose and trehalose. However, any possible effect of glucuronate replacement on the calibration curve was beneath the accuracy of the measurements (including the preparation of the solutions). Switching from Cardinaud solution to 0 Cl− produces changes in potential difference at several points in the system (including between the bath and the bath electrode; see Coles et al. 1989). Therefore we never attempted to make measurements in Cardinaud solution and in 0 Cl− in the same experiment.
The changes in VK and VA in the calibration solutions (Fig. 1B) were measured on the Handyscope display and the Nikolsky equation was fitted to the calibration points:
| (1) |
| (2) |
where S, α and β are constants determined for each electrode, the subscripts K and A denoting the K+ and ammonium barrels. αA >> αK. A typical pair of calibration curves is shown in Fig. 1C. Ammonium concentration was calculated from:
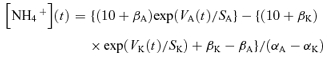 |
(3) |
These equations are equivalent to those given by Provent et al. (2007).
Figure 1. Methods.
A, triple barrelled microelectrode. B, example of a calibration made in the perfusion chamber with variants of Cardinaud solution. The baseline corresponds to 10 mm K+ and 0 NH4+. C, calibration points for VK (○) and VA (•) plotted against [K+] on a log scale. The points were fitted with eqns (1) and (2) for the responses in the absence of ammonium. VA in the presence of 1 mm NH4+ and 10 mm K+ is shown (♦); a dashed line has been constructed through this using eqn (2). D, record showing that the effect of a change in pH on VA and VK was small.
Since the calibration curves were determined for steady state values of VK and VA, eqn (3) may be inaccurate when VK and VA are changing rapidly compared to the response times of the two ion barrels. VK and VA do change rapidly at the onset and end of photostimulation (Fig. 5A) and therefore, at these times any difference in the response times of VK and VA leads to one signal lagging behind the other and generates artifactual positive or negative transients in [NH4+]e(t). These response times depend mainly on the electrical resistances of the ion barrels (Coles & Tsacopoulos, 1979) over which we had little control. We therefore confined the quantitative analysis of [NH4+]e to the nearly steady state conditions in the dark and at the end of the 120 s photostimulation. A few complete records selected from experiments made with microelectrodes whose ammonium and K+ barrels responded with approximately equal speed to changes in [K+] are shown in Fig. 5B.
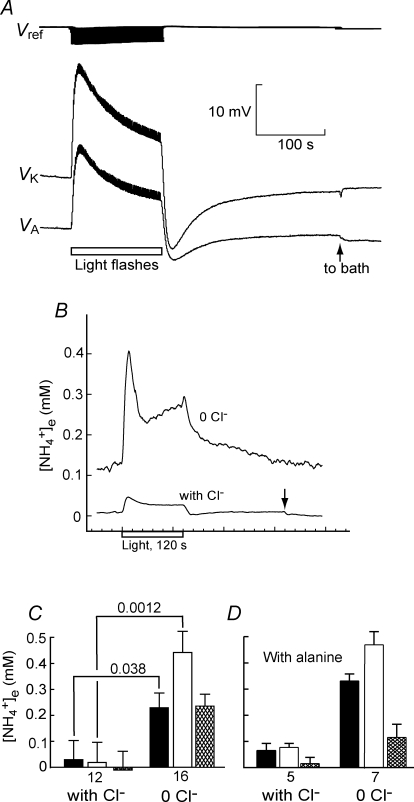
Figure 5.
A, measuring [NH4+]e with a triple-barrelled ion-selective microelectrode. The electrode barrels recorded the field potential (Vref, top trace), a K+ signal (VK, second trace), and a NH4+ signal contaminated by K+ (VA, third trace). Stimulation of the photoreceptors with light flashes at 1 Hz for 120 s caused both VK and VA to increase. About 4 min after the return to darkness, the electrode was withdrawn to the bath (arrow) and then promptly calibrated (see Fig. 1B). The superfusate for this record was 0 Cl− with 20 mm alanine. B, illustrative records of light-induced increases in [NH4+]e calculated using eqn (3). [NH4+]e in the dark was higher in 0 Cl− superfusate (‘0 Cl−’) than in normal superfusate (‘with Cl−’), and it increased more during light stimulation (rectangle). The arrow on the ‘with Cl−’ trace indicates withdrawal of the electrode to the bath. The 0 Cl− trace is the average of 3 records. C, mean values of [NH4+]e, in the dark, just before withdrawal of the microelectrode (filled columns), at the end of 120 s light stimulation (open columns) and the stimulation-induced increase (hatched columns). In Cardinaud solution (‘with Cl−’; left set of columns) no significant [NH4+]e was detected. In superfusate lacking Cl− (‘0 Cl−’; right set of columns), [NH4+]e was detectable (P = 0.0084 in the dark, P = 0.011 at the end of the light stimulation, P = 0.0022 for the mean stimulation-induced increase). D, including alanine in the superfusate did not significantly increase [NH4+]e whether or not the superfusate contained Cl−.
To make statistical comparisons between different superfusates, we also used a more transparent method of analysis that exploited the observation that in the slices NH4+ made only a small contribution to VA. The calibration points for VA and VK were plotted against log[K+] and fitted with second order polynomials. The second order coefficients were very small, as is clear from the near-linearity of the curves in Fig. 1C. VA and VK were measured at the two chosen times (the end of 120 s light stimulation and after subsequent recovery). [K+] was found from the VK calibration curve and [NH4+] was calculated using the approximation that NH4+ made a linear contribution to VA (Dionne, 1976).
The repetitive light stimulation that we used causes an increase in extracellular pH (pHe) of about 0.2 pH units (Coles et al. 1996). We therefore tested the effect of a change in pH on VA and VK. The effects were small (Fig. 1D). With electrode tips of 3.0–5.7 μm, the range of voltage deflexions per 0.1 pH unit was 0–0.094 mV for VA (n = 4 electrodes) and 0–0.044 mV for VK (n = 3). The light-induced ΔpHe is not significantly changed in 0 Cl− superfusate (Marcaggi et al. 1999). ΔpHe, like any change in pH, will change the proportions of ammonium in the NH4+ and NH3 forms. The fraction of total ammonium in the NH4+ form is (1 + 10(pH-pKa))−1 (see, e.g. Roos & Boron, 1981). In the present experiments, pKa≈ 9.1 and pHe≈ 6.9 so [NH4+]/[total ammonium]≈ 99.37%. Since the major interest of the results was comparison of [NH4+]e in different superfusates but similar pHe, it was not useful to try to correct for the small possible effects of changes in pHe on VA and VK, or for the very small fractional changes in [NH4+]/[total ammonium].
Ammonium recordings
NH4+ electrodes were inserted into slices as for the PO2 electrodes, while the photoreceptors were stimulated with light flashes. [K+]e was initially high, even when a negative-going electrical response of the reference barrel suggested that the tip was extracellular (Coles & Tsacopoulos, 1979) but, after 15–60 min, [K+]e fell to close to the 10 mm of the superfusate. For experiments with 0 Cl− solutions we switched first to 0 Cl− solution with no added substrate. Removal of Cl− caused the potential difference between the reference barrel and the bath electrode to shift (see Coles et al. 1989) and we waited until the signals had become steady. We then switched to the final experimental solution (e.g. 0 Cl− solution containing 10 mm MeAIB) for 5–8 min before making the measurement of [NH4+]e. This time is longer than the < 2 min required for a bath-applied drug to reach the photoreceptors at the depth of the recordings (observation on tetrodotoxin, Coles & Schneider-Picard, 1989). We then stimulated all the photoreceptors with a standard stimulation of a train of 120 light flashes, 50 ms duration, 1 s−1, 1016 photons cm−2 s−1. When VK had returned to a steady value (by about 4 min after the stimulation) the electrode was withdrawn from the slice to the bath, moved to the chamber inlet (the tip being kept submerged) and calibrated. We found no correlation between [NH4+]e and the time elapsed after the preparation of the slice (1–3 h). For a sample of 43 slices, the mean value of [K+]e immediately before the stimulation was 11.87 mm, s.d.= 1.92 mm, s.e.m.= 0.29 mm. That is, 1.87 mm above the value in the superfusate. For a sample of six conditions (different superfusates), we plotted graphs of [NH4+]e (at the end of stimulation) versus[K+]e. We found no significant correlation: the mean dependence of [NH4+]e on [K+]e was 0.0061, s.e.m. 0.014 (6 conditions, 43 retinas).
Unless otherwise stated, results are given as the mean ±s.e.m.P-values were determined by Student's two-tailed t test. Uncertainty in a ratio was calculated as: Δ(x/y) =Δx/y+xΔy/y2.
Results
The results fall into three sections: recording of PO2 in normal Cardinaud solution; recording of O2 in the presence of DAB; and the measurement of [NH4+]e, most often in the absence of Cl−.
Oxygen consumption was neither increased by glucose nor decreased by MeAIB
A retinal slice was superfused with oxygenated Cardinaud solution (containing no glucose) and an O2 microelectrode was advanced obliquely into the slice so that its tip was 70–100 μm below the surface (Fig. 2A). PO2 was lower than in the bath, and fell further when the photoreceptors were stimulated with light flashes as previously described (Tsacopoulos & Poitry, 1982), and illustrated in Fig. 2B. These decreases in PO2 are the result of consumption of O2 throughout the slice by the mitochondria, virtually all of which are in the photoreceptor neurons (Dimitracos & Tsacopoulos, 1985). Although the retina metabolizes glucose in vivo (Evêquoz et al. 1983), addition of 10 mm glucose to the superfusate did not markedly change  (Fig. 2B). This suggests that, in these slices, the supply of substrate to the photoreceptors from metabolic stores in the glia is almost sufficient for their needs.
(Fig. 2B). This suggests that, in these slices, the supply of substrate to the photoreceptors from metabolic stores in the glia is almost sufficient for their needs.
Considerable evidence points to alanine as a major energy substrate transferred from the glia to the photoreceptors (Tsacopoulos et al. 1994, 1997a). Uptake of alanine on some neutral amino acid transporters is inhibited by 2-(methylamino)isobutyric acid (MeAIB; Mackenzie et al. 2003). However, inclusion of 10 mm MeAIB in the Cardinaud solution had no marked effect on  for application times up to the maximum tested of 28 min (n = 3; Fig. 2C). Nor did the mean change in the amplitude of the light-induced decrease in PO2 change significantly (mean ratio 1.20 ± 0.12, n = 7). We found that a more sensitive way of detecting any change in
for application times up to the maximum tested of 28 min (n = 3; Fig. 2C). Nor did the mean change in the amplitude of the light-induced decrease in PO2 change significantly (mean ratio 1.20 ± 0.12, n = 7). We found that a more sensitive way of detecting any change in 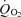 was to make the solution change during continuous stimulation with light flashes, as illustrated for MeAIB in Fig. 2D. We then measured the rate of change of
was to make the solution change during continuous stimulation with light flashes, as illustrated for MeAIB in Fig. 2D. We then measured the rate of change of  , 5 min after switching to the test solution. Any drift in baseline PO2 was corrected for by measuring
, 5 min after switching to the test solution. Any drift in baseline PO2 was corrected for by measuring  before and after the application, and subtracting the mean value from
before and after the application, and subtracting the mean value from  in the test solution. The mean change in
in the test solution. The mean change in  for 10 mm MeAIB was −1.7 ± 1.38 Pa s−1, n = 9, which is not significantly different from zero (P = 0.24; Fig. 2G). Nor did inclusion of 25 mm l-alanine or 10 mm d-glucose produce a significant change (Fig. 2G). In contrast, 10 mm 4-hydroxy-α-cyanocinnamate (αCC; an inhibitor of monocarboxylic acid cotransporters) did increase electrode current (P = 0.038) apparently by decreasing
for 10 mm MeAIB was −1.7 ± 1.38 Pa s−1, n = 9, which is not significantly different from zero (P = 0.24; Fig. 2G). Nor did inclusion of 25 mm l-alanine or 10 mm d-glucose produce a significant change (Fig. 2G). In contrast, 10 mm 4-hydroxy-α-cyanocinnamate (αCC; an inhibitor of monocarboxylic acid cotransporters) did increase electrode current (P = 0.038) apparently by decreasing  (Fig. 2E). However, we did not investigate whether it did this by inhibiting transport of a monocarboxylate across the plasma membrane, or across the mitochondrial membrane (Halestrap & Poole, 1989). Inclusion of both 10 mm MeAIB and 10 mmαCC in the superfusate decreased
(Fig. 2E). However, we did not investigate whether it did this by inhibiting transport of a monocarboxylate across the plasma membrane, or across the mitochondrial membrane (Halestrap & Poole, 1989). Inclusion of both 10 mm MeAIB and 10 mmαCC in the superfusate decreased  by no more than 10 mmαCC alone did (Fig. 2F and G).
by no more than 10 mmαCC alone did (Fig. 2F and G).
In the presence of DAB, alanine increased oxygen consumption
With the aim of reducing the supply of substrate from the glia to the photoreceptors, we used superfusate containing DAB, an inhibitor of glycogen phosphorylase (Andersen et al. 1999). Switching to DAB caused PO2 to increase ( to decrease) progressively (Fig. 3A). After a variable time of at least 1 h, the PO2 response to light stimulation was greatly reduced (not shown). Removal of DAB caused
to decrease) progressively (Fig. 3A). After a variable time of at least 1 h, the PO2 response to light stimulation was greatly reduced (not shown). Removal of DAB caused  to increase, in some cases after a delay of up to 8 min (Fig. 3B). The mean difference in
to increase, in some cases after a delay of up to 8 min (Fig. 3B). The mean difference in  with and without DAB was significant with P = 0.0032 (64 degrees of freedom). We tested the effect of including l-alanine in the superfusate at a concentration of 25 mm, as in the test made in the absence of DAB (Fig. 2G). This concentration is less than the 31 mm measured in interstitial fluid in vivo (Cardinaud et al. 1994). In the presence of DAB, l-alanine caused PO2 to decrease markedly, after a delay of less than 3 min (Fig. 3D). The mean change in
with and without DAB was significant with P = 0.0032 (64 degrees of freedom). We tested the effect of including l-alanine in the superfusate at a concentration of 25 mm, as in the test made in the absence of DAB (Fig. 2G). This concentration is less than the 31 mm measured in interstitial fluid in vivo (Cardinaud et al. 1994). In the presence of DAB, l-alanine caused PO2 to decrease markedly, after a delay of less than 3 min (Fig. 3D). The mean change in 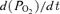 caused by l-alanine was −17.4 ± 2.5 Pa s−1 (n = 40; Fig. 3H). The effect of l-alanine was abolished by 10 mm MeAIB (Fig. 3E and H). d-alanine at 25 mm was about as effective as l-alanine at restoring
caused by l-alanine was −17.4 ± 2.5 Pa s−1 (n = 40; Fig. 3H). The effect of l-alanine was abolished by 10 mm MeAIB (Fig. 3E and H). d-alanine at 25 mm was about as effective as l-alanine at restoring  in the presence of DAB (Fig. 3G and H). However, 25 mmβ-alanine had no detectable effect on
in the presence of DAB (Fig. 3G and H). However, 25 mmβ-alanine had no detectable effect on 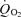 (Fig. 3F and H), showing that the effect of alanine was not a non-specific effect of amino acids on the electrode current.
(Fig. 3F and H), showing that the effect of alanine was not a non-specific effect of amino acids on the electrode current.
Figure 3. In the presence of 0.1 mm DAB, exogenous alanine increased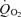 .
.
All records in this figure are of PO2 during continuous stimulation with light flashes. A, switching to DAB caused PO2 to increase steadily. At the arrow the light flashes were stopped for about 30 s and PO2 rose. Unlike the other records shown, this one was made from a scan of a chart recording. B, removal of DAB caused PO2 to decrease, after a delay. C, summary of the rates of change of PO2 in the absence and presence of DAB. D, 25 mm l-alanine caused PO2 to decrease ( to increase). E, application of 10 mm MeAIB at the same time as l-alanine blocked the increase in
to increase). E, application of 10 mm MeAIB at the same time as l-alanine blocked the increase in  . F, β-alanine (25 mm) had little effect on
. F, β-alanine (25 mm) had little effect on  . G, d-alanine (25 mm) was about as effective as l-alanine at increasing
. G, d-alanine (25 mm) was about as effective as l-alanine at increasing  . H, summary of effects of alanine and related compounds on dPO2/dt measured 5 min after the solution change and relative to the mean of the values before and after. Time bar applies to all records.
. H, summary of effects of alanine and related compounds on dPO2/dt measured 5 min after the solution change and relative to the mean of the values before and after. Time bar applies to all records.
These results confirm the proposal of Tsacopoulos et al. (1994) that alanine can act as a substrate of photoreceptor metabolism in superfused retinal slices.
The second major product of glucose metabolism that accumulates in the drone retinal slices, besides alanine, is trehalose (Tsacopoulos et al. 1994). We detected no effect of 5 mm trehalose on  in the presence of DAB (Fig. 4C and D). Proline is present in the interstitial fluid in vivo at 109 mm (Cardinaud et al. 1994); in the presence of DAB, 10 mml-proline significantly increased
in the presence of DAB (Fig. 4C and D). Proline is present in the interstitial fluid in vivo at 109 mm (Cardinaud et al. 1994); in the presence of DAB, 10 mml-proline significantly increased  (Fig. 4D). We will suggest in the Discussion that the effect is mainly anaplerotic.
(Fig. 4D). We will suggest in the Discussion that the effect is mainly anaplerotic.
Figure 4. Effects on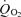 of other possible substrates of energy metabolism in the presence of 0.1 mm DAB and continuous stimulation with light flashes.
of other possible substrates of energy metabolism in the presence of 0.1 mm DAB and continuous stimulation with light flashes.
A, l-lactate (10 mm) decreased dPO2/dt relative to the rise caused by DAB. B, d-lactate (10 mm) also stopped PO2 from increasing, but was much less effective than 25 mm l-alanine. C, d-trehalose (5 mm) had little effect. D, mean changes in dPO2/dt caused by various compounds in the presence of DAB. Integers are numbers of applications. Asterisks indicate P-values for differences from zero: *P < 0.05, **P < 0.005, ***P < 0.0001. Pyruvate was applied at 10 mm (n = 4) and 20 mm (n = 4) and the results normalized to 10 mm. E, the results in D are shown scaled for equal concentrations of carbon atoms (e.g. 5 mm trehalose = 60 mEq l−1 carbon; 10 mm proline = 50 mEq l−1 carbon). Double asterisks indicate significantly different from l-alanine with P < 0.007.
Pyruvate and lactate, but not glucose, increased 
If alanine is used as a substrate for photoreceptor energy metabolism, it is presumably first deaminated to pyruvate (Tsacopoulos et al. 1994). In the presence of DAB, 10 mm pyruvate increased  : the mean decrease in dPO2/dt was 56 ± 18 Pa s−1 (n = 9, P = 0.003; Fig. 4D). When the magnitude of this effect was normalized to the same concentration as the alanine (by multiplying by 25/10), the means for the two compounds were not significantly different (Fig. 4E). Lactate is both produced and consumed through major metabolic fluxes in vertebrate nervous tissue (Larrabee, 1992; Poitry-Yamate et al. 1995; Véga et al. 1998; Pfeuffer et al. 1999; Nehlig & Coles, 2007). In drone retina, enzymatic tests for l-lactate had failed to detect it, although a peak was eluted by HPLC that corresponded to lactate (Cardinaud et al. 1994). In the presence of DAB, we found that 10 mm l-lactate decreased dPO2/dt (P = 0.012; Fig. 4A), and so did d-lactate (P = 0.0047; Fig. 4B). When the effects of l- and d-lactate were normalized to the concentration of alanine, the means were not significantly different from alanine (Fig. 4E). However, when the normalized data for pyruvate, and l- and d-lactate were pooled, their mean effect was significantly less than that of l-alanine (53.0 ± 7.6%, P = 0.0058, 56 degrees of freedom).
: the mean decrease in dPO2/dt was 56 ± 18 Pa s−1 (n = 9, P = 0.003; Fig. 4D). When the magnitude of this effect was normalized to the same concentration as the alanine (by multiplying by 25/10), the means for the two compounds were not significantly different (Fig. 4E). Lactate is both produced and consumed through major metabolic fluxes in vertebrate nervous tissue (Larrabee, 1992; Poitry-Yamate et al. 1995; Véga et al. 1998; Pfeuffer et al. 1999; Nehlig & Coles, 2007). In drone retina, enzymatic tests for l-lactate had failed to detect it, although a peak was eluted by HPLC that corresponded to lactate (Cardinaud et al. 1994). In the presence of DAB, we found that 10 mm l-lactate decreased dPO2/dt (P = 0.012; Fig. 4A), and so did d-lactate (P = 0.0047; Fig. 4B). When the effects of l- and d-lactate were normalized to the concentration of alanine, the means were not significantly different from alanine (Fig. 4E). However, when the normalized data for pyruvate, and l- and d-lactate were pooled, their mean effect was significantly less than that of l-alanine (53.0 ± 7.6%, P = 0.0058, 56 degrees of freedom).
d-Glucose had no significant effect on 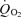 in the presence of DAB (Fig. 4D), showing that under these conditions the photoreceptors cannot use glucose as a substrate for energy metabolism. To compare the effects on
in the presence of DAB (Fig. 4D), showing that under these conditions the photoreceptors cannot use glucose as a substrate for energy metabolism. To compare the effects on  of compounds with different numbers of carbon atoms (e.g. trehalose has 12) we normalized the results to equal concentrations of C (Fig. 4E). d-Glucose and d-trehalose were significantly less effective than l-alanine.
of compounds with different numbers of carbon atoms (e.g. trehalose has 12) we normalized the results to equal concentrations of C (Fig. 4E). d-Glucose and d-trehalose were significantly less effective than l-alanine.
The effects of the various compounds were measured at the standard time of 5 min after application. Since glucose might have a delayed effect, via metabolism to alanine in the glia, we also made longer applications (4 in the range 16–20 min) but still saw no increase in 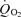 . This suggests that DAB interrupted the glial pathway leading from glucose to export of alanine.
. This suggests that DAB interrupted the glial pathway leading from glucose to export of alanine.
Release of ammonium to the interstitium
We measured [NH4+] with an ammonium-sensitive microelectrode in the interstitium of a retinal slice under conditions similar to those used for the measurements of PO2 (but without using DAB). Since the ammonium sensor was sensitive to K+, as well as to NH4+, we used a triple-barrelled microelectrode (see Methods). Light stimulation of all the photoreceptors in the slice increased [K+]e (Coles & Tsacopoulos, 1979) so the potentials on both sensor barrels, VA and VK, increased (Fig. 5A). Preliminary results had shown that [NH4+]e is small compared to [K+]e, both in the dark and when increased by photostimulation, so that its measurement was sensitive to small drifts in VA and VK. To minimize these effects, the protocol was to leave the electrode in the recording site until VA and VK had fallen to approximately steady values, stimulate with light for 120 s, and allow 4–5 min for VA and VK to recover in the dark. We then withdrew the microelectrode to the bath and immediately calibrated it (Fig. 1B). [NH4+]e(t) was calculated using eqns (1) (2) and (3).
In normal Cardinaud solution, mean [NH4+]e was not significantly different from zero in the dark or at the end of the 120 s photostimulation (Fig. 5C) and there was less than 5% probability that [NH4+]e exceeded 0.2 mm in either condition. In 0 Cl− superfusate, [K+]e increased more with light stimulation (Coles et al. 1989) but, in general, visual inspection of the records of VA and VK did not suggest any change in [NH4+]e. (The record in Fig. 5A, in 0 Cl− with 20 mm alanine, shows a small increase in VK and a decrease in VA on withdrawal to the bath. In this particular case it is obvious that a positive value for [NH4+]e in the dark would be calculated.) However, when [NH4+]e in 0 Cl− superfusate was calculated, the mean [NH4+]e in the dark was 0.227 ± 0.056 mm (n = 17), and at the end of 120 s photostimulation, 0.442 ± 0.082 mm (n = 16; Fig. 5C), in both cases significantly greater than in Cardinaud solution (P = 0.038 and 0.0012). The increase with photostimulation was significant with P = 0.00017, and the mean increase expressed as the ratio [NH4+]e(light)/[NH4+]e(dark) was 2.75 ± 0.40. The mean of three complete records in 0 Cl− is shown in Fig. 5B. [NH4+]e(t) appears to rise rapidly at the onset of photostimulation then fall with a half-time of about 11 s and rise slowly towards a plateau, much as does  (t) (Tsacopoulos et al. 1994). (Note that, in general, the time course of
(t) (Tsacopoulos et al. 1994). (Note that, in general, the time course of  does not mirror the time course of PO2; see Tsacopoulos & Poitry, 1982.) Figure 5B also shows a record of [NH4+]e(t) in Cardinaud solution that illustrates the markedly lower concentration of NH4+ in the presence of Cl−.
does not mirror the time course of PO2; see Tsacopoulos & Poitry, 1982.) Figure 5B also shows a record of [NH4+]e(t) in Cardinaud solution that illustrates the markedly lower concentration of NH4+ in the presence of Cl−.
To see if removal of Cl− from the Cardinaud solution (and its replacement by glucuronate and some gluconate) affected the rate of oxidative metabolism, recordings were made with a PO2 microelectrode inserted in the slice. When the superfusate was switched to 0 Cl− solution (n = 2, not shown) there was a small decrease in the electrode current, but tests in the bath suggested that this was an artifact due to the radical change in ionic composition. We conclude that the marked increase in [NH4+]e in 0 Cl− superfusate was not caused by a marked increase in  .
.
Exogenous alanine did not significantly increase ammonium release
Tsacopoulos et al. (1994) proposed that ammonium is produced in the photoreceptors by the conversion of alanine to pyruvate. It is known that retinal slices lose alanine to the bath (Tsacopoulos et al. 1994) so the extracellular alanine concentration in slices may be lower than in vivo. We therefore tested the effect of including l-alanine in the superfusate. Exogenous alanine (20 mm) did not significantly increase [NH4+]e in either the presence or the absence of Cl− (Fig. 5D). Since d-alanine was no more effective than l-alanine at increasing  in the presence of DAB (Fig. 3G) it was not tested.
in the presence of DAB (Fig. 3G) it was not tested.
[NH4+]e was reduced by 2-(methylamino)isobutyric acid
We have shown that, in the presence of DAB, MeAIB inhibits the effect of added alanine on  (Fig. 2C). Inclusion of 10 mm MeAIB in the 0 Cl− superfusate strikingly reduced mean [NH4+]e after 120 s light stimulation (P = 0.0001) to 18% of control (Fig. 6A). Since the effect of MeAIB on [NH4+]e was made in 0 Cl− superfusate, we tested whether MeAIB affected
(Fig. 2C). Inclusion of 10 mm MeAIB in the 0 Cl− superfusate strikingly reduced mean [NH4+]e after 120 s light stimulation (P = 0.0001) to 18% of control (Fig. 6A). Since the effect of MeAIB on [NH4+]e was made in 0 Cl− superfusate, we tested whether MeAIB affected  in 0 Cl− superfusate, and found no detectable effect on either baseline PO2 or the stimulus-induced decrease in PO2 (n = 2 retinas, Fig. 6B), as described above for the effect of MeAIB in normal Cardinaud solution (Fig. 2C and D). We conclude that, at least in the absence of Cl−, MeAIB decreases [NH4+]e without decreasing oxidative metabolism. Experiments to be described below suggest that the photoreceptors switched to using a substrate other than alanine.
in 0 Cl− superfusate, and found no detectable effect on either baseline PO2 or the stimulus-induced decrease in PO2 (n = 2 retinas, Fig. 6B), as described above for the effect of MeAIB in normal Cardinaud solution (Fig. 2C and D). We conclude that, at least in the absence of Cl−, MeAIB decreases [NH4+]e without decreasing oxidative metabolism. Experiments to be described below suggest that the photoreceptors switched to using a substrate other than alanine.
Figure 6. Effects of various compounds on [NH4+]e.
A, Measurements at the end of 120 s light stimulation in 0 Cl− superfusate. Inclusion in the superfusate of MeAIB, pyruvate, the two together, l-lactate or d-lactate, each at 10 mm, significantly reduced [NH4+]e (*P < 0.05, **P < 0.01). Propionate and d-glucose (10 mm) and d-trehalose (5 mm) did not significantly reduce [NH4+]e. B, recording of local PO2 as for Fig. 2B but in 0 Cl− superfusate. Large negative deflexions occurred when the photoreceptors were stimulated with trains of 20 light flashes at 1 Hz. Inclusion of 10 mm MeAIB in the superfusate had little effect on baseline PO2 or on the response to light.
[NH4+]e was reduced by pyruvate
To make a different test of whether [NH4+]e is related to the metabolism of alanine, we applied pyruvate, which, being the product, might slow the deamination of alanine to pyruvate. When 10 mm pyruvate was included in the 0 Cl− superfusate, mean [NH4+]e after 120 s light stimulation was reduced to 19%, from 0.442 mm to 0.088 ± 0.075 mm, n = 6 (P = 0.0012, Fig. 6A). Despite this reduction in [NH4+]e, pyruvate did not reduce the light-evoked field potentials even after 17 min, the longest application made (range 13–17 min, n = 6), and it increased in the presence of DAB (Fig. 4D). A combination of 10 mm MeAIB and 10 mm pyruvate also reduced [NH4+]e (Fig. 6A). This last result shows that the reductions caused by MeAIB and by pyruvate were not due to artifactual effects on the electrode; if this had been the case the combined effect of the two would have been an apparent negative value for [NH4+]e.
in the presence of DAB (Fig. 4D). A combination of 10 mm MeAIB and 10 mm pyruvate also reduced [NH4+]e (Fig. 6A). This last result shows that the reductions caused by MeAIB and by pyruvate were not due to artifactual effects on the electrode; if this had been the case the combined effect of the two would have been an apparent negative value for [NH4+]e.
Dissociation of [K+]e and [NH4+]e
Removing Cl−, which reduces K+ entry into the glia, did not detectably change  (see above) but it does increase the light-induced increase in [K+]e (Δ[K+]e; Coles et al. 1989). We asked whether the changes in [K+]e might in some way be responsible for the changes in [NH4+]e. In the present experiments, mean Δ[K+]e at the end of 120 s of light stimulation in the presence of Cl− was 3.65 ± 0.54 mm (n = 12). In the absence of Cl− (as in Fig. 5A), mean Δ[K+]e was 6.80 ± 0.45 mm (n = 17). The increase in Δ[K+]e is significant, with P = 0.0055. However, after recovery in the dark, the presence or absence of Cl− made no difference to [K+]e. Mean values of [K+]e were 10.60 ± 0.34 mm (n = 13) with Cl− and 11.35 ± 0.49 mm (n = 19) without Cl− (P for the difference = 0.32) Hence, the increase in [NH4+]e in 0 Cl− superfusate, at least in the dark, appears not to be associated with an increase in [K+]e. We also compared the effects on [K+]e and [NH4+]e of MeAIB, and of pyruvate or the two together. Mean [K+]e at the end of the 120 s light stimulation in 0 Cl− superfusate was 18.1 ± 1.2 mm (n = 17), and the mean of the pooled results for the three inhibitory conditions was [K+]e= 16.75 ± 0.71 mm (n = 20), which is not significantly different (P = 0.32). In contrast, mean [NH4+]e fell from 0.386 ± 0.064 mm (n = 15) to 0.087 ± 0.026 mm (n = 20), a factor of 4.4, the two means differing with P < 0.0001.
(see above) but it does increase the light-induced increase in [K+]e (Δ[K+]e; Coles et al. 1989). We asked whether the changes in [K+]e might in some way be responsible for the changes in [NH4+]e. In the present experiments, mean Δ[K+]e at the end of 120 s of light stimulation in the presence of Cl− was 3.65 ± 0.54 mm (n = 12). In the absence of Cl− (as in Fig. 5A), mean Δ[K+]e was 6.80 ± 0.45 mm (n = 17). The increase in Δ[K+]e is significant, with P = 0.0055. However, after recovery in the dark, the presence or absence of Cl− made no difference to [K+]e. Mean values of [K+]e were 10.60 ± 0.34 mm (n = 13) with Cl− and 11.35 ± 0.49 mm (n = 19) without Cl− (P for the difference = 0.32) Hence, the increase in [NH4+]e in 0 Cl− superfusate, at least in the dark, appears not to be associated with an increase in [K+]e. We also compared the effects on [K+]e and [NH4+]e of MeAIB, and of pyruvate or the two together. Mean [K+]e at the end of the 120 s light stimulation in 0 Cl− superfusate was 18.1 ± 1.2 mm (n = 17), and the mean of the pooled results for the three inhibitory conditions was [K+]e= 16.75 ± 0.71 mm (n = 20), which is not significantly different (P = 0.32). In contrast, mean [NH4+]e fell from 0.386 ± 0.064 mm (n = 15) to 0.087 ± 0.026 mm (n = 20), a factor of 4.4, the two means differing with P < 0.0001.
Lactate, but not propionate, reduced [NH4+]e
MeAIB reduced [NH4+]e (Fig. 6A) suggesting that less alanine was consumed, and it also prevented alanine from restoring  in the presence of DAB (Fig. 3E). However, MeAIB did not reduce
in the presence of DAB (Fig. 3E). However, MeAIB did not reduce  in either normal superfusate (Fig. 2C and D) or 0 Cl− superfusate (Fig. 6B). These results suggest that one or more energy substrates, other than alanine, can be supplied by the glia to the photoreceptors, and these substrates do not lead to release of NH4+. The measurements of PO2 in the presence of DAB showed that, in addition to alanine, the photoreceptors are capable of oxidizing pyruvate and l- and d-lactate. We have shown above that pyruvate reduced [NH4+]e. We also tested the effect of 10 mm l-lactate on [NH4+]e. l-Lactate at 10 mm was not seen to reduce the light-induced field potentials of the photoreceptors over times of 14–33 min (n = 5), and it increased
in either normal superfusate (Fig. 2C and D) or 0 Cl− superfusate (Fig. 6B). These results suggest that one or more energy substrates, other than alanine, can be supplied by the glia to the photoreceptors, and these substrates do not lead to release of NH4+. The measurements of PO2 in the presence of DAB showed that, in addition to alanine, the photoreceptors are capable of oxidizing pyruvate and l- and d-lactate. We have shown above that pyruvate reduced [NH4+]e. We also tested the effect of 10 mm l-lactate on [NH4+]e. l-Lactate at 10 mm was not seen to reduce the light-induced field potentials of the photoreceptors over times of 14–33 min (n = 5), and it increased in the presence of DAB. However, [NH4+]e at the end of 120 s light stimulation was reduced (P = 0.0038) to about 31% (Fig. 4C). This reduction is not significantly different from the reductions caused by MeAIB or by pyruvate (Fig. 6A). d-Lactate at 10 mm also reduced [NH4+]e (Fig. 6A).
in the presence of DAB. However, [NH4+]e at the end of 120 s light stimulation was reduced (P = 0.0038) to about 31% (Fig. 4C). This reduction is not significantly different from the reductions caused by MeAIB or by pyruvate (Fig. 6A). d-Lactate at 10 mm also reduced [NH4+]e (Fig. 6A).
Lactate (and also pyruvate) is transported across cell membranes in association with H+, either by diffusion through the lipid bilayer or by cotransport (Halestrap & Poole, 1989). Application of either lactate or pyruvate changes intracellular and extracellular pH in drone retina (J. A. Coles, unpublished observation), and changes in pH might affect [NH4+]e. We therefore tested a weak acid, propionate (pKa= 4.87) also known to change pH in drone retina (Fig. 8 in Coles et al. 1996). It is thought to do this by diffusion of the neutral, protonated form through the lipid membrane. However, propionate did not significantly reduce [NH4+]e (Fig. 6A). This suggests that the reduction of [NH4+]e by lactate and pyruvate involved their metabolism.
Neither glucose nor trehalose affect [NH4+]e
Neither 10 mm glucose nor 5 mm trehalose significantly reduced [NH4+]e (Fig. 6A). This agrees with their lack of effect on  in the presence of DAB (Fig. 4C, D and E).
in the presence of DAB (Fig. 4C, D and E).
Discussion
Effects of DAB on energy metabolism
When DAB was applied,  started to fall within a few minutes (Fig. 3A), showing that it readily enters cells.
started to fall within a few minutes (Fig. 3A), showing that it readily enters cells.  then continued to fall further over several tens of minutes. Local PO2 depends on
then continued to fall further over several tens of minutes. Local PO2 depends on  throughout the thickness of the slice (Tsacopoulos & Poitry, 1982), so one factor that might contribute to the slowness of complete inhibition in the slice is slow arrival of DAB in the deeper layers, diffusional transport through extracellular clefts being slowed by uptake of DAB into cells.
throughout the thickness of the slice (Tsacopoulos & Poitry, 1982), so one factor that might contribute to the slowness of complete inhibition in the slice is slow arrival of DAB in the deeper layers, diffusional transport through extracellular clefts being slowed by uptake of DAB into cells.
Glucose did not restore  in the presence of DAB. This confirms that glucose itself cannot be the substrate supplied by the glia, as predicted from the glial-specific uptake of 2-deoxyglucose (Tsacopoulos et al. 1988), and the presence of only a weak hexokinase activity in isolated photoreceptors (Veuthey et al. 1994). Unexpectedly, DAB also prevented exogenous glucose from being used by the glia to produce alanine, or another substrate (Figs 4D, 7A). In living drones, photostimulation increases both the turnover and the total quantity of glycogen in the retina (Evêquoz et al. 1983). These results suggest that glycogen metabolism may have a role in normal energy metabolism, as proposed for mammalian brain (Dienel et al. 2007).
in the presence of DAB. This confirms that glucose itself cannot be the substrate supplied by the glia, as predicted from the glial-specific uptake of 2-deoxyglucose (Tsacopoulos et al. 1988), and the presence of only a weak hexokinase activity in isolated photoreceptors (Veuthey et al. 1994). Unexpectedly, DAB also prevented exogenous glucose from being used by the glia to produce alanine, or another substrate (Figs 4D, 7A). In living drones, photostimulation increases both the turnover and the total quantity of glycogen in the retina (Evêquoz et al. 1983). These results suggest that glycogen metabolism may have a role in normal energy metabolism, as proposed for mammalian brain (Dienel et al. 2007).
Figure 7. Proposed interpretations of the main experimental results.
A, in DAB, the glia no longer supply adequate alanine. The photoreceptors can oxidize alanine, pyruvate, lactate and perhaps proline. The glial pathway from glucose to alanine is blocked. B, measurements of [NH4+]e show that MeAIB inhibits the alanine/ammonium shuttle. Another substrate, perhaps lactate, is supplied by the glia. C, suggested major metabolic traffic in vivo. The alanine/ammonium shuttle dominates. Proline and glycogen are involved in ways that are not fully understood.
Alanine was the most effective substrate of those tested, while d-trehalose, the other major compound formed from glucose (Tsacopoulos et al. 1994), had no significant effect (Figs 3F and H and 4C and E). d-Alanine was as effective as l-alanine (but β-alanine was ineffective). The concentration of d-alanine is less than micromolar in rat brain (Morikawa et al. 2003) but higher concentrations are present in at least some insects (Corrigan, 1969). We do not know which enantiomer predominates in drone retina. Further evidence in favour of alanine was provided by the measurements of [NH4+]e.
A neuron–glia flow of ammonium
Both  and [NH4+]e start to increase within seconds of the onset of light stimulation (Fig. 5B; Tsacopoulos et al. 1983). Dinitrophenol, which increases oxidative metabolism in mitochondria, increases both
and [NH4+]e start to increase within seconds of the onset of light stimulation (Fig. 5B; Tsacopoulos et al. 1983). Dinitrophenol, which increases oxidative metabolism in mitochondria, increases both  and ammonium release from the retina (Tsacopoulos et al. 1987; Coles et al. 1996; Tsacopoulos et al. 1997b), the mitochondria being located almost exclusively in the photoreceptors. These observations suggest that the source of ammonium is closely associated with photoreceptor energy metabolism.
and ammonium release from the retina (Tsacopoulos et al. 1987; Coles et al. 1996; Tsacopoulos et al. 1997b), the mitochondria being located almost exclusively in the photoreceptors. These observations suggest that the source of ammonium is closely associated with photoreceptor energy metabolism.
Elamari et al. (1997) used a fibre optic device to measure [NH4+]. The circular extremity of the probe (diameter more than 80 μm) was positioned on the surface of a retinal slice and they show a recording in which [NH4+] in the space between the probe and the retinal surface increased to over 0.1 mm[NH4+] during light stimulation. This is within the range of values we measured in the interstitium in normal Cardinaud solution, but the mean of our values was not significantly different from zero. We measured significant mean [NH4+]e only in 0 Cl− superfusate (Fig. 5B and C), and we suggest that this was because uptake of ammonium by the glial cells is inhibited when [Cl−]e is reduced (Marcaggi et al. 1999, 2004). Removal of Cl− did not increase  , showing that the marked increase in [NH4+]e was not the consequence of an increase in oxidative metabolism. The increase in [NH4+]e on removal of Cl− does not necessarily imply any decrease in the neuron–glia flux: the increase in the concentration gradient of NH4+ across the glial cell membrane might be sufficient to maintain an NH4+ flux adequate for the formation of alanine at the normal rate.
, showing that the marked increase in [NH4+]e was not the consequence of an increase in oxidative metabolism. The increase in [NH4+]e on removal of Cl− does not necessarily imply any decrease in the neuron–glia flux: the increase in the concentration gradient of NH4+ across the glial cell membrane might be sufficient to maintain an NH4+ flux adequate for the formation of alanine at the normal rate.
MeAIB generally inhibits uptake of neutral amino acids, e.g. on SNAT1 (Mackenzie et al. 2003) or on PAT2 (Kennedy et al. 2005). In the presence of DAB, MeAIB blocked oxidation of alanine by the photoreceptors (Fig. 3E). In the absence of DAB (and of Cl−), MeAIB reduced [NH4+]e (Fig. 6A). These results are strong evidence for the existence of an alanine/ammonium shuttle as proposed previously (Tsacopoulos et al. 1994, 1997b; Coles et al. 1996). The observations that neither  nor [NH4+]e was increased when the extracellular alanine concentration was increased above its normal level, but that [NH4+]e was reduced by pyruvate, which is expected to by-pass the deamination of alanine, suggest that alanine is the main, if not the only, substrate in slices superfused with Cardinaud solution.
nor [NH4+]e was increased when the extracellular alanine concentration was increased above its normal level, but that [NH4+]e was reduced by pyruvate, which is expected to by-pass the deamination of alanine, suggest that alanine is the main, if not the only, substrate in slices superfused with Cardinaud solution.
What carbon fuel in vivo?
In vivo, proline is present at a concentration of 109 mm in the interstitial fluid (Cardinaud et al. 1994), and, in retinal slices in the presence of DAB, exogenous proline increased the  of the photoreceptors (the applications lasting less than 10 min; Fig. 4D). However, in insect muscle, proline serves mainly for anaplerosis (Sacktor & Childress, 1967; Suarez et al. 2005), and the metabolic fate of proline in drone retinal slices suggest that this is also the case here (Fig. 1 in Tsacopoulos et al. 1997b). That is, exogenous proline stimulated the oxidation of carbon compounds already present (Sacktor & Childress, 1967), and perhaps increased protein synthesis.
of the photoreceptors (the applications lasting less than 10 min; Fig. 4D). However, in insect muscle, proline serves mainly for anaplerosis (Sacktor & Childress, 1967; Suarez et al. 2005), and the metabolic fate of proline in drone retinal slices suggest that this is also the case here (Fig. 1 in Tsacopoulos et al. 1997b). That is, exogenous proline stimulated the oxidation of carbon compounds already present (Sacktor & Childress, 1967), and perhaps increased protein synthesis.
MeAIB reduced [NH4+]e and prevented use of alanine in the presence of DAB, but did not reduce  in the absence of DAB (Fig. 2C and D). A possible explanation is that, in the absence of DAB, the photoreceptors switched to using a substrate whose metabolism did not lead to release of NH4+ (Fig. 7B). Pyruvate, l-lactate and d-lactate were all metabolized by the photoreceptors in the presence of DAB (Fig. 4A, B, D and E) and also reduced [NH4+]e (Fig. 6A). Previous work reported that no lactate was detectable in the haemolymph or in liquid in contact with a slice (Tsacopoulos et al. 1987; Cardinaud et al. 1994), but these measurements were made with an enzymatic assay specific for l-lactate. In contrast, a peak with an elution time corresponding to 2.7 mm lactate was found in HPLC analyses of interstitial fluid (Cardinaud et al. 1994), suggesting that d-lactate may be present. There are great variations between species and tissues in the metabolism of d-lactate. It is readily oxidized by rat liver mitochondria (de Bari et al. 2002), but inhibits use of l-lactate in mouse optic nerve (Tekkok et al. 2005); and in Octopus ocellatus it is a major metabolite (Fujisawa et al. 2005).
in the absence of DAB (Fig. 2C and D). A possible explanation is that, in the absence of DAB, the photoreceptors switched to using a substrate whose metabolism did not lead to release of NH4+ (Fig. 7B). Pyruvate, l-lactate and d-lactate were all metabolized by the photoreceptors in the presence of DAB (Fig. 4A, B, D and E) and also reduced [NH4+]e (Fig. 6A). Previous work reported that no lactate was detectable in the haemolymph or in liquid in contact with a slice (Tsacopoulos et al. 1987; Cardinaud et al. 1994), but these measurements were made with an enzymatic assay specific for l-lactate. In contrast, a peak with an elution time corresponding to 2.7 mm lactate was found in HPLC analyses of interstitial fluid (Cardinaud et al. 1994), suggesting that d-lactate may be present. There are great variations between species and tissues in the metabolism of d-lactate. It is readily oxidized by rat liver mitochondria (de Bari et al. 2002), but inhibits use of l-lactate in mouse optic nerve (Tekkok et al. 2005); and in Octopus ocellatus it is a major metabolite (Fujisawa et al. 2005).
It is therefore possible that some lactate (as the d enantiomer) and/or pyruvate is supplied by the glia. However, the interstitial concentration of alanine (31 mm) is much higher than those of pyruvate (0.47 mm) or the putative d-lactate (2.7 mm; Cardinaud et al. 1994). Hence, alanine is probably the principal fuel in vivo as well as in vitro (Fig. 7C). Nevertheless, mechanisms exist for the use of lactate, and probably for its generation, in drone retina, and lactate may make a small, but adjustable, contribution to the energy metabolism of the neurons.
Acknowledgments
The work was funded by an INSERM program grant to C. Segebarth. We thank Novo Nordisk for a gift of 1,4-dideoxy-1,4-imino-d-arabinitol, Jean-Luc Munoz for oxygen microelectrodes, and Pakan Marcaggi for critically reading an earlier version of the manuscript.
References
- Andersen B, Rassov A, Westergaard N, Lundgren K. Inhibition of glycogenolysis in primary rat hepatocytes by 1,4-dideoxy-1,4-imino-d-arabinitol. Biochem J. 1999;342:545–550. [PMC free article] [PubMed] [Google Scholar]
- Bührer T, Peter H, Simon W. NH4+ ion-selective microelectrode based on the antibiotics nonactin/monactin. Pflugers Arch. 1988;412:359–362. doi: 10.1007/BF01907552. [DOI] [PubMed] [Google Scholar]
- Cardinaud B, Coles JA, Perrottet P, Spencer AJ, Osborne AJ, Tsacopoulos M. The composition of the interstitial fluid in the retina of the honeybee drone: implications for the supply of substrates of energy metabolism from blood to neurons. Proc R Soc Lond B Biol Sci. 1994;257:49–58. [Google Scholar]
- Chih CP, Roberts EL., Jr Energy substrates for neurons during neural activity: a critical review of the astrocyteneuron lactate shuttle hypothesis. J Cereb Blood Flow Metab. 2003;23:1263–1281. doi: 10.1097/01.WCB.0000081369.51727.6F. [DOI] [PubMed] [Google Scholar]
- Coles JA, Marcaggi P, Véga C, Cotillon N. Effects of photoreceptor metabolism on interstitial and glial cell pH in bee retina: evidence of a role for NH4+ J Physiol. 1996;495:305–318. doi: 10.1113/jphysiol.1996.sp021595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles JA, Orkand RK. Modification of potassium movement through the retina of the drone (Apis mellifera ♂) by glial uptake. J Physiol. 1983;340:157–174. doi: 10.1113/jphysiol.1983.sp014756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles JA, Orkand RK, Yamate C. Chloride enters glial cells and photoreceptors in response to light stimulation in the retina of the honey bee drone. Glia. 1989;2:287–297. doi: 10.1002/glia.440020502. [DOI] [PubMed] [Google Scholar]
- Coles JA, Poulain DA. Extracellular K+ in the supraoptic nucleus of the rat during reflex bursting activity by oxytocin neurones. J Physiol. 1991;439:383–409. doi: 10.1113/jphysiol.1991.sp018672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles JA, Schneider-Picard G. Amplification of small signals by voltage-gated sodium channels in drone photoreceptors. J Comp Physiol. 1989;165:109–118. doi: 10.1007/BF00613804. [DOI] [PubMed] [Google Scholar]
- Coles JA, Tsacopoulos M. K+ activity in photoreceptors, glial cells and extracellular space in the drone retina: changes during photostimulation. J Physiol. 1979;290:525–549. doi: 10.1113/jphysiol.1979.sp012788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles JA, Tsacopoulos M. Ionic and possible metabolic interactions between sensory neurones and glial cells in the retina of the honeybee drone. J Exp Biol. 1981;95:75–92. doi: 10.1242/jeb.95.1.75. [DOI] [PubMed] [Google Scholar]
- Coles JA, Vega C, Marcaggi P. Metabolic trafficking between cells in nervous tissue. Prog Brain Res. 2000;125:241–254. doi: 10.1016/S0079-6123(00)25014-0. [DOI] [PubMed] [Google Scholar]
- Corrigan JJ. d-Amino acids in animals. Science. 1969;164:142–145. doi: 10.1126/science.164.3876.142. [DOI] [PubMed] [Google Scholar]
- de Bari L, Atlante A, Guaragnella N, Principato G, Passarella S. d-Lactate transport and metabolism in rat liver mitochondria. Biochem J. 2002;365:391–403. doi: 10.1042/BJ20020139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel GA, Ball KK, Cruz NF. A glycogen phosphorylase inhibitor selectively enhances local rates of glucose utilization in brain during sensory stimulation of conscious rats: implications for glycogen turnover. J Neurochem. 2007;102:466–478. doi: 10.1111/j.1471-4159.2007.04595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitracos SA, Tsacopoulos M. The recovery from a transient inhibition of the oxidative metabolism of the photoreceptors of the drone (Apis mellifera ♂) J Exp Biol. 1985;119:165–181. [Google Scholar]
- Dionne VE. Characterization of drug iontophoresis with a fast microassay technique. Biophys J. 1976;16:705–717. doi: 10.1016/S0006-3495(76)85723-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elamari A, Gisin N, Munoz JL, Poitry S, Tsacopoulos M, Zbinden H. Photon-counting optical-fiber sensor for the detection of ammonia in neurochemical applications. Sensors Actuators B. 1997;38–39:183–188. [Google Scholar]
- Evêquoz V, Stadelmann A, Tsacopoulos M. The effect of light on glycogen turnover in the retina of the intact honeybee drone (Apis mellifera) J Comp Physiol. 1983;150:69–75. [Google Scholar]
- Fillenz M. The role of lactate in brain metabolism. Neurochem Int. 2005;47:413–417. doi: 10.1016/j.neuint.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Fujisawa T, Akagi S, Kawase M, Yamamoto M, Ohmori S. d-Lactate metabolism in starved octopus ocellatus. J Exp Zool. 2005;303A:489–496. doi: 10.1002/jez.a.180. [DOI] [PubMed] [Google Scholar]
- Halestrap AP, Poole RC. The transport of pyruvate and lactate across mitochondrial and plasma membranes. In: Hamasaki NJM, editor. Anion Transport Protein of the Red Blood Cell Membrane. Amsterdam: Elsevier; 1989. pp. 73–86. [Google Scholar]
- Hyder F, Patel AB, Gjedde A, Rothman DL, Behar KL, Shulman RG. Neuronal-glial glucose oxidation and glutamatergic-GABAergic function. J Cereb Blood Flow Metab. 2006;26:865–877. doi: 10.1038/sj.jcbfm.9600263. [DOI] [PubMed] [Google Scholar]
- Kennedy DJ, Gatfield KM, Winpenny JP, Ganapathy V, Thwaites DT. Substrate specificity and functional characterisation of the H+/amino acid transporter rat PAT2 (Slc36a2) Br J Pharmacol. 2005;144:28–41. doi: 10.1038/sj.bjp.0706029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrabee MG. Extracellular intermediates of glucose metabolism: fluxes of endogenous lactate and alanine through extracellular pools in embryonic sympathetic ganglia. J Neurochem. 1992;59:1041–1052. doi: 10.1111/j.1471-4159.1992.tb08346.x. [DOI] [PubMed] [Google Scholar]
- Mackenzie B, Schafer MK, Erickson JD, Hediger MA, Weihe E, Varoqui H. Functional properties and cellular distribution of the system A glutamine transporter SNAT1 support specialized roles in central neurons. J Biol Chem. 2003;8:23720–23730. doi: 10.1074/jbc.M212718200. [DOI] [PubMed] [Google Scholar]
- Marcaggi P, Coles JA. A Cl− cotransporter selective for NH4+ over K+ in glial cells of bee retina. J Gen Physiol. 2000;116:125–141. doi: 10.1085/jgp.116.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcaggi P, Jeanne M, Coles JA. Neuron-glial trafficking of NH4+ and K+: separate routes of uptake into glial cells of bee retina. Eur J Neurosci. 2004;19:966–976. doi: 10.1111/j.0953-816x.2004.03165.x. [DOI] [PubMed] [Google Scholar]
- Marcaggi P, Thwaites D, Deitmer J, Coles JA. Chloride-dependent transport of NH4+ into bee retinal glial cells. Eur J Neurosci. 1999;11:167–177. doi: 10.1046/j.1460-9568.1999.00418.x. [DOI] [PubMed] [Google Scholar]
- Morikawa A, Hamase K, Zaitsu K. Determination of d-alanine in the rat central nervous system and periphery using column-switching high-performance liquid chromatography. Anal Biochem. 2003;312:66–72. doi: 10.1016/s0003-2697(02)00432-3. [DOI] [PubMed] [Google Scholar]
- Nehlig A, Coles JA. Cellular pathways of energy metabolism in the brain: Is glucose used by neurons or astrocytes? Glia. 2007;55:1238–1250. doi: 10.1002/glia.20376. [DOI] [PubMed] [Google Scholar]
- Nehlig A, Wittendorp-Rechenmann E, Lam CD. Selective uptake of [14C]2-deoxyglucose by neurons and astrocytes: high-resolution microautoradiographic imaging by cellular 14C-trajectography combined with immunohistochemistry. J Cereb Blood Flow Metab. 2004;24:1004–1014. doi: 10.1097/01.WCB.0000128533.84196.D8. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Bouzier-Sore AK, Aubert A, Serres S, Merle M, Costalat R, Magistretti PJ. Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia. 2007;55:1251–1262. doi: 10.1002/glia.20528. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrelet A. The fine structure of the retina of the honeybee drone. Z Zellforsch Mikrosk Anat. 1970;108:530–562. doi: 10.1007/BF00339658. [DOI] [PubMed] [Google Scholar]
- Pfeuffer J, Tkác I, Choi I-Y, Merkle H, Ugurbil K, Garwood M, Gruetter R. Localized in vivo1H NMR detection of neurotransmitter labeling in rat brain during infusion of [1-13C]d-glucose. Magn Reson Med. 1999;41:1077–1083. doi: 10.1002/(sici)1522-2594(199906)41:6<1077::aid-mrm1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Poitry-Yamate CL, Poitry S, Tsacopoulos M. Lactate released by Mller cells is metabolized by photoreceptors from mammalian retina. J Neurosci. 1995;15:5179–5191. doi: 10.1523/JNEUROSCI.15-07-05179.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provent P, Kickler N, Barbier EL, Bergerot ARF, Goury S, Marcaggi P, Segebarth C, Coles JA. The ammonium-induced increase in rat brain lactate concentration is rapid and reversible and is compatible with trafficking and signaling roles for ammonium. J Cereb Blood Flow Metab. 2007;27:1830–1840. doi: 10.1038/sj.jcbfm.9600480. [DOI] [PubMed] [Google Scholar]
- Roos A, Boron WF. Intracellular pH. Physiol Rev. 1981;61:296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Sacktor B, Childress CC. Metabolism of proline in insect flight muscle and its significance in stimulating the oxidation of pyruvate. Arch Biochem Biophys. 1967;120:583–588. [Google Scholar]
- Saravelos SG, Tsacopoulos M. Iodoacetate inhibits the biosynthesis of alanine in glial cells and its utilization in photoreceptors of the honeybee drone (Apis mellifera) retina. J Comp Physiol B. 1995;165:341–347. [Google Scholar]
- Serres S, Bouyer JJ, Bezancon E, Canioni P, Merle M. Involvement of brain lactate in neuronal metabolism. NMR Biomed. 2003;16:430–439. doi: 10.1002/nbm.838. [DOI] [PubMed] [Google Scholar]
- Suarez RK, Darveau CA, Welch KC, Jr, O'Brien DM, Roubik DW, Hochachka PW. Energy metabolism in orchid bee flight muscles: carbohydrate fuels all. J Exp Biol. 2005;208:3573–3579. doi: 10.1242/jeb.01775. [DOI] [PubMed] [Google Scholar]
- Tekkok SB, Brown AM, Westenbroek R, Pellerin L, Ransom BR. Transfer of glycogen-derived lactate from astrocytes to axons via specific monocarboxylate transporters supports mouse optic nerve activity. J Neurosci Res. 2005;81:644–652. doi: 10.1002/jnr.20573. [DOI] [PubMed] [Google Scholar]
- Tsacopoulos M, Coles JA, Van de Werve G. The supply of metabolic substrate from glia to photoreceptors in the retina of the honeybee drone. J Physiol (Paris) 1987;82:279–287. [PubMed] [Google Scholar]
- Tsacopoulos M, Evêquoz-Mercier V, Perrottet P, Buchner E. Honeybee retinal glial cells transform glucose and supply the neurons with metabolic substrate. Proc Natl Acad Sci U S A. 1988;85:8727–8731. doi: 10.1073/pnas.85.22.8727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsacopoulos M, Magistretti PJ. Metabolic coupling between glia and neurons. J Neurosci. 1996;16:877–885. doi: 10.1523/JNEUROSCI.16-03-00877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsacopoulos M, Orkand RK, Coles JA, Levy S, Poitry S. Oxygen uptake occurs faster than sodium pumping in bee retina after a light flash. Nature. 1983;301:604–606. doi: 10.1038/301604a0. [DOI] [PubMed] [Google Scholar]
- Tsacopoulos M, Poitry S. Kinetics of oxygen consumption after a single flash of light in photoreceptors of the drone (Apis mellifera) J Gen Physiol. 1982;80:19–55. doi: 10.1085/jgp.80.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsacopoulos M, Poitry S, Borsellino A. Diffusion and consumption of oxygen in the superfused retina of the drone (Apis mellifera) in darkness. J Gen Physiol. 1981;77:601–628. doi: 10.1085/jgp.77.6.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsacopoulos M, Poitry-Yamate CL, Poitry S. Ammonium and glutamate released by neurons are signals regulating the nutritive function of a glial cell. J Neurosci. 1997a;17:2383–2390. doi: 10.1523/JNEUROSCI.17-07-02383.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsacopoulos M, Poitry-Yamate CL, Poitry S, Perrottet P, Veuthey AL. The nutritive function of glia is regulated by signals released by neurons. Glia. 1997b;21:84–91. [PubMed] [Google Scholar]
- Tsacopoulos M, Veuthey AL, Saravelos G, Perrottet P, Tsoupras G. Glial cells transform glucose to alanine, which fuels the neurons in the honeybee retina. J Neurosci. 1994;14:1339–1351. doi: 10.1523/JNEUROSCI.14-03-01339.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Véga C, Martiel JL, Drouhault D, Burckhart MF, Coles JA. Uptake of locally applied deoxyglucose, glucose and lactate by axons and Schwann cells of rat vagus nerve. J Physiol. 2003;546:551–564. doi: 10.1113/jphysiol.2002.029751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Véga C, Poitry-Yamate CL, Jirounek P, Tsacopoulos M, Coles JA. Lactate is released and taken up by isolated rabbit vagus nerve during aerobic metabolism. J Neurochem. 1998;71:330–337. doi: 10.1046/j.1471-4159.1998.71010330.x. [DOI] [PubMed] [Google Scholar]
- Veuthey AL, Tsacopoulos M, Ruiz de Millan L, Perrottet P. Cellular and subcellular localization of hexokinase, glutamate dehydrogenase and alanine aminotransferase in the honeybee drone retina. J Neurochem. 1994;62:1939–1946. doi: 10.1046/j.1471-4159.1994.62051939.x. [DOI] [PubMed] [Google Scholar]
- Vrba R. Glucose metabolism in rat brain. Nature. 1962;195:663–665. doi: 10.1038/195663a0. [DOI] [PubMed] [Google Scholar]