The subthalamic nucleus (STN) is a member of the basal ganglia that has long been implicated in the expression of a variety of movement disorders. However, the importance of the STN in movement disorders has been reinforced by the recent acceptance of STN deep brain stimulation for the treatment of advanced Parkinson's disease. For many years it was hypothesized that increased neuronal activity in the STN contributed to the rigidity and bradykinesia seen in Parkinson's disease, whereas reduced STN output contributed to chorea and other hyperkinetic movements (DeLong, 1990). However, there has been a more recent appreciation that expression of symptoms of Parkinson's disease correlate better with excessive burst firing of action potentials in STN neurones, rather than an overall increase in firing rate (Bergman et al. 1994). In fact, excessive burst firing in basal ganglia such as the STN has become accepted as a physiological hallmark of parkinsonism (Bergman et al. 1998). Thus, much attention is now focused on firing pattern of STN neurones rather than absolute firing rate regarding the influence of basal ganglia output on manifestations of movement disorders.
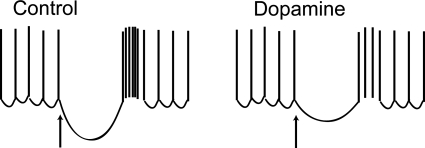
Because the classical symptoms of Parkinson's disease are caused by a loss of dopamine innervation, there has been much interest in characterizing the actions of dopamine in the STN. The prevailing hypothesis is that dopamine receptor stimulation attenuates Parkinson's disease symptoms because it acts to diminish burst firing in STN neurones. But how this might be accomplished is unclear. Stimulation of postsynaptic dopamine D2-like receptors has been shown to depolarize STN neurones, which takes membrane potential beyond the range at which burst firing can be sustained (Beurrier et al. 1999; Zhu et al. 2002). However, in this issue of The Journal of Physiology, Baufreton & Bevan (2008) highlight another mechanism by which dopamine may impair burst firing. Using perforated patch and whole-cell recording techniques in slices of rat brain, these authors show that dopamine, by stimulation of D2-like receptors, causes presynaptic inhibition of GABA-mediated inhibitory postsynaptic currents (IPSCs). Although this confirms previous studies (Shen & Johnson, 2000), the present work extends these studies by showing the consequence of IPSC inhibition on rebound firing patterns. Using the dynamic clamp technique to mimic GABA-mediated synaptic currents, Baufreton and Bevan show that an inhibitory synaptic current is followed by a rebound burst of action potentials. Previous studies have shown that the rebound burst is caused by de-inactivation of voltage-dependent calcium channels, of which STN neurones express in abundance (Song et al. 2000). By reducing the conductance associated with GABA-mediated synaptic currents, this disrupts rebound burst firing as well as the ability of the synaptic current to reset the spontaneous firing of action potentials (Fig. 1). Thus, the data presented by Baufreton and Bevan show that the dopamine-mediated suppression of synaptic inhibition may produce a paradoxical result that impairs rebound excitation. In this way, dopamine may act to suppress synaptically triggered burst firing.
Figure 1.
Schematic representation of spontaneous action potentials in an STN neuron in the presence and absence of dopamine Arrow signifies onset of GABA-mediated inhibitory postsynaptic potential. Note the reduction in rebound burst in the presence of dopamine.
Regulation of neuronal activity often is mediated by what may seem to be complex and indirect mechanisms. For example, neuronal disinhibition is a common mechanism for increasing neuronal output, in which inhibition of an inhibitory input results in neuronal excitation (Chevalier & Deniau, 1990). The paper by Baufreton and Bevan illustrates another paradoxical mechanism, in which a burst firing pattern may be suppressed by reducing the conductance associated with an inhibitory synaptic current. Thus, one must be careful not to assume that the only function of inhibitory input is to suppress excitability. In the case of the STN, suppression of GABA release by dopamine appears to exert a paradoxical action to reduce the burst firing that may be associated with symptoms of Parkinson's disease. As shown by Baufreton and Bevan, this illustrates the need to examine neurotransmitter actions in a dynamic context in order to fully understand the functional impact of neurotransmitter receptor activation in central neurones.
References
- Baufreton J, Bevan MD. J Physiol. 2008;586:2121–2142. doi: 10.1113/jphysiol.2008.151118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman H, Feingold A, Nini A, Raz A, Slovin H, Abeles M, Vaadia E. Trend Neurosci. 1998;21:32–38. doi: 10.1016/s0166-2236(97)01151-x. [DOI] [PubMed] [Google Scholar]
- Bergman H, Wichmann T, Karmon B, DeLong MR. J Neurophysiol. 1994;72:507–520. doi: 10.1152/jn.1994.72.2.507. [DOI] [PubMed] [Google Scholar]
- Beurrier C, Congar P, Bioulac B, Hammond C. J Neurosci. 1999;19:599–609. doi: 10.1523/JNEUROSCI.19-02-00599.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier G, Deniau JM. Trend Neurosci. 1990;13:277–280. doi: 10.1016/0166-2236(90)90109-n. [DOI] [PubMed] [Google Scholar]
- DeLong MR. Trend Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- Shen K-Z, Johnson SW. J Physiol. 2000;525:331–341. doi: 10.1111/j.1469-7793.2000.00331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W-J, Baba Y, Otsuka T, Murakami F. J Neurophysiol. 2000;84:2630–2637. doi: 10.1152/jn.2000.84.5.2630. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Bartol M, Shen K-Z, Johnson SW. Brain Res. 2002;945:31–40. doi: 10.1016/s0006-8993(02)02543-x. [DOI] [PubMed] [Google Scholar]