Abstract
The purpose of the study was to examine the influence of the difference between the recruitment threshold of a motor unit and the target force of the sustained contraction on the discharge of the motor unit at recruitment. The discharge characteristics of 53 motor units in biceps brachii were recorded after being recruited during a sustained contraction. Some motor units (n = 22) discharged action potentials tonically after being recruited, whereas others (n = 31) discharged intermittent trains of action potentials. The two groups of motor units were distinguished by the difference between the recruitment threshold of the motor unit and the target force for the sustained contraction: tonic, 5.9 ± 2.5%; intermittent, 10.7 ± 2.9%. Discharge rate for the tonic units decreased progressively (13.9 ± 2.7 to 11.7 ± 2.6 pulses s−1; P = 0.04) during the 99 ± 111 s contraction. Train rate, train duration and average discharge rate for the intermittent motor units did not change across 211 ± 153 s of intermittent discharge. The initial discharge rate at recruitment during the sustained contraction was lower for the intermittent motor units (11.0 ± 3.3 pulses s−1) than the tonic motor units (13.7 ± 3.3 pulses s−1; P = 0.005), and the coefficient of variation for interspike interval was higher for the intermittent motor units (34.6 ± 12.3%) than the tonic motor units (21.2 ± 9.4%) at recruitment (P = 0.001) and remained elevated for discharge duration (34.6 ± 9.2%versus 19.1 ± 11.7%, P < 0.001). In an additional experiment, 12 motor units were recorded at two different target forces below recruitment threshold (5.7 ± 1.9% and 10.5 ± 2.4%). Each motor unit exhibited the two discharge patterns (tonic and intermittent) as observed for the 53 motor units. The results suggest that newly recruited motor units with recruitment thresholds closer to the target force experienced less synaptic noise at the time of recruitment that resulted in them discharging action potentials at more regular and greater rates than motor units with recruitment thresholds further from the target force.
The decline in the force capacity of muscle during a submaximal fatiguing contraction is accompanied by a progressive increase in the drive to the motoneurone pool (Bigland-Ritchie et al. 1986; Garland et al. 1994; Adam & De Luca, 2003). When the target force for the contraction is less than the upper limit of recruitment, the increase in synaptic input recruits additional motor units but does not attenuate the decrease in discharge rate experienced by many motor units that were recruited at the onset of the contraction (Person & Kudina, 1972; Enoka et al. 1989; Garland et al. 1994; Garland et al. 1997). Some of the units active from the start of the contraction can even cease to discharge action potentials despite the continued synaptic input to the motoneurone pool (Freund et al. 1975; Grimby et al. 1981; Kato et al. 1981; Peters & Fuglevand, 1999; Carpentier et al. 2001). One explanation for these divergent adjustments is that the increase in excitation delivered to the motoneurone pool is countered by inhibition and the mechanisms that mediate adaptation in individual neurones (Kernell & Monster, 1982; Berg et al. 2007). However, Mottram et al. (2005) observed that the decrease in discharge rate of the same motor units differed with the type of load supported during the fatiguing contraction, which suggested a role for other factors besides intrinsic motoneurone properties in contributing to the decline in discharge rate.
Although some findings indicate that the concurrent decrease in discharge rate and recruitment of other units emerge progressively across the motor unit population during a fatiguing contraction, other observations document the intermittent discharge of action potentials by some motor units (Hannerz, 1974; Grimby & Hannerz, 1977; Kato et al. 1981; Maton & Gamet, 1989; Adam & De Luca, 2005). The three patterns of motor unit activity were observed by Carpentier et al. (2001) during a series of ramp-and-hold contractions with the first dorsal interosseus muscle. Low-threshold motor units that were recruited at the onset of the task exhibited a decrease in mean discharge rate, and motor units recruited during the task displayed an initial increase in discharge rate before experiencing a subsequent decline. In addition, the discharge of 18 motor units could not be analysed because they either discharged only during the ramp phase or the discharge during the hold phase was too irregular.
The irregular discharge of some motor units can be observed as bursts of activity in the surface EMG when an individual sustains a submaximal contraction for a long duration (Hunter et al. 2002; Rudroff et al. 2007; Riley et al. 2008). Given that the synaptic input to the motoneurone pool increases progressively during such contractions, it is uncertain why some newly recruited motor units discharge action potentials intermittently and why the discharge characteristics differ from those units that were active from the onset of the contraction. The purpose of the study was to determine the influence of the difference between the recruitment threshold of a motor unit and the target force of the sustained contraction on the discharge of the motor unit when it was recruited. Some of these data have been presented in abstract form (Riley & Enoka, 2007).
Methods
Eighteen healthy adults (16 men; 25.5 ± 6.2 years; range, 18–39 years) participated in the study. All subjects were moderately active and all reported being free of cardiovascular disease and neurological disorders. The Human Research Committee at the University of Colorado in Boulder approved the procedures and the experiments were performed in accordance with the Declaration of Helsinki. All subjects gave written informed consent prior to the study.
Each subject participated in one to six experimental sessions, with each session separated by at least 1 week. One to two motor units were recorded in a single session from either head of biceps brachii. The task involved subjects performing a single, sustained isometric contraction at a target force that was less than the recruitment threshold of an isolated motor unit. To be certain that the recordings were not influenced by displacement of the electrode, only those motor units that were discharging at the end of the contraction, or for which the recruitment threshold could be measured before and after the sustained contraction, are included in this report.
Experimental setup
Subjects were seated upright in an adjustable chair with the arm slightly abducted and the elbow resting on a support. The elbow was flexed to 1.57 rad and the forearm was kept in a neutral position midway between pronation and supination, and parallel to the floor. The hand and forearm were secured with a modified wrist–hand orthosis (Orthomerica; Newport Beach, CA, USA). The force exerted in a vertical direction was measured with a JR-3 force-moment sensor (900-N range, 89.7 N/V; JR-3, Woodland, CA, USA). The orthosis was attached to the transducer at the level of the wrist. The force was displayed on a 17 in monitor that was located at eye level ∼1.2 m in front of the subject.
Single motor unit recordings
Muscle fibre action potentials were recorded in biceps brachii with a branched bipolar electrode (stainless steel, 50 μm diameter; California Fine Wire) (Gydikov et al. 1986; Enoka et al. 1988; Mottram et al. 2005). The electrode consisted of three ∼1 mm areas of wire where insulation was removed; two sites on one wire, separated by 2–3 mm, and a single site on the other wire. A 25-gauge, 1.5 in disposable needle was used to insert the wires subcutaneously across the muscle belly without penetrating the muscle fascia and approximately perpendicular to the direction of the muscle fibres. The recording sites were adjusted to provide the highest signal-to-noise ratio during a brief, low-force contraction (5–10% maximal voluntary contraction (MVC)). A reference electrode was placed on the lateral epicondyle. Single motor unit recordings were amplified (1000–5000 times), band-pass filtered (0.3–8 kHz), displayed on an oscilloscope, and stored on a computer. The single motor unit potentials were later identified off-line using Spike2 software (v.5.16, CED, Cambridge, UK).
Surface EMG recordings
Surface EMG activity of the biceps brachii was recorded with a pair of bipolar electrodes (8 mm diameter; silver–silver chloride) placed directly above the branched bipolar electrode. The electrode pair was always at least 1 cm from the septum separating the short and long heads of biceps brachii. Additionally, one pair of surface electrodes was attached over triceps brachii. Reference electrodes were placed over the acromion. The surface EMG signals were amplified (1000 times) and band-pass filtered (20–800 Hz; Coulbourn Instruments, Allenstown, PA, USA) prior to data acquisition.
Protocol
The protocol comprised ramp contractions and a sustained isometric contraction with the elbow flexor muscles of the left arm. Each experimental session consisted of six tasks: (1) assessment of the maximal voluntary contraction (MVC) force for the elbow flexor and extensor muscles; (2) identification of a single motor unit; (3) measurement of the recruitment and de-recruitment thresholds of the motor unit; (4) performance of the sustained isometric contraction at 5–10% below the recruitment threshold of the motor unit; (5) evaluation of the recruitment and de-recruitment thresholds of the motor unit immediately after the sustained contraction; and (6) completion of another MVC with the elbow flexor muscles. In additional experiments, some subjects performed brief, sustained contractions at both 5% and 10% below the recruitment threshold of the motor unit. To determine the influence of the sustained contraction on MVC force, five subjects repeated the protocol but without identifying a motor unit. The sustained contraction was performed at the same target force and contraction duration as the session in which the discharge of a motor unit was recorded, and the final MVC was performed immediately after the sustained contraction.
The experimental session began with a minimum of two isometric MVCs in the flexion direction and at least one in the extension direction. The MVC task involved increasing the force from zero to maximum over 3 s and then holding the maximum for 3 s. To minimize fatigue, subjects rested for 90–120 s between trials. If the MVC forces for the two elbow flexor trials were within 5% of each other, the larger of the two values was recorded as the maximum and used as a reference for the recruitment threshold of the motor unit. Otherwise, additional trials were performed until the 5% criterion was achieved.
Motor units were identified in the subcutaneous recording as the subject slowly increased the force to about 50% of MVC force over ∼10 s. Once a potential unit was identified, a target line was set at ∼2 times the force at which the motor unit began discharging action potentials. Subjects then performed three to five ramp contractions up to the target line and back down to zero, with each contraction lasting 5–8 s but requiring a similar rate of change in force. The minimal force at which the motor unit began discharging action potentials was estimated, averaged across all ramp contractions, and recorded.
The target force for the sustained contraction was 5–10% MVC below the recruitment threshold of the motor unit. A line was set at the target force and the subject was asked to increase the force up to the line gradually (over ∼10 s) and to maintain the target force as steadily as possible until told by the investigator to relax. Each subject was instructed to exert torque only in the vertical direction with the elbow flexors during the contraction and to avoid movement of the upper body. The task was terminated when either the isolated motor unit began discharging action potentials tonically for > 60 s or when the force exerted by the subject fluctuated by greater than ± 4% MVC force from the target force. Pilot measurements indicated that motor units could be recruited and de-recruited when the fluctuations in force exceeded ± 4% MVC force.
Data analysis
Recruitment threshold was quantified by advancing a 500 ms window in 1 ms steps across the discharge times of the motor unit until the coefficient of variation for interspike interval in the window was < 50% (Moritz et al. 2005). The force corresponding to the time of the first discharge in the window was taken as the recruitment threshold of the unit. The same method was applied to determine the de-recruitment threshold of the motor unit. The minimal discharge rate and the coefficient of variation for interspike interval were determined for a 0.5 s window at recruitment.
Single motor unit potentials for each trial were discriminated using the template-matching feature of Spike2. Motor units were classified as displaying either tonic or intermittent activity. Motor units (n = 22) that discharged action potentials continuously for at least 60 s after being recruited were classified as tonic motor units. The discharge rates of the tonic motor units were averaged for each 20% of the discharge duration and the coefficient of variation for interspike interval was calculated from the first five intervals in each 20% epoch of discharge duration. Motor units (n = 31) that discharged trains of action potentials after being recruited were classified as intermittent motor units. The classification of units as either tonic or intermittent refers to the discharge characteristics of the motor unit only, and to no other property. The activity of the intermittent motor units was characterized by determining the number of trains of action potentials, the number of trains per minute, train duration, discharge rates within each train, and the coefficient of variation for the first five interspike intervals in each train. For comparison with the tonic units, the discharge rates and coefficients of variation in each train for the intermittent units were averaged across each 20% epoch of discharge duration. The minimal number of action potentials in a train was set to four and the maximal acceptable duration between consecutive action potentials was 1 s. There were only 11 occasions where a motor unit discharged less than four action potentials when activated.
In an additional experiment, 12 motor units were recorded during brief, sustained contractions at 5% and 10% below the recruitment threshold of the motor unit. The trials were counterbalanced for target force and the contractions were ended at ∼40 s after the first action potential. After a brief rest period (3–4 min), the contraction at the other target force was performed. Discharge rate and coefficient of variation for the first five interspike intervals when the motor unit became active were analysed as well as the time to recruitment.
Surface EMG from the biceps and triceps brachii are reported as the root-mean-square (RMS) of the signal normalized to the RMS of a 0.5 s epoch from the peak EMG during the MVC.
Statistics
The two groups of motor units (tonic and intermittent) were compared by examining recruitment and de-recruitment thresholds, target force, the difference between the recruitment threshold and target forces, and the time from the start of the contraction to the recruitment of the motor unit with independent sample t tests. The minimal discharge rate at recruitment during the ramp threshold task was compared with the initial discharge rate of the first five interspike intervals during the sustained contraction with paired sample t tests for both groups of motor units. The same comparison was used to analyse the coefficient of variation for interspike interval at recruitment during the two tasks. A Pearson correlation was used to determine the association between the initial discharge rate during the sustained contraction with the difference between the recruitment threshold and the target force.
MVC force for the intermittent units was compared before and after the sustained contraction with a paired sample t test. The mean discharge rate and coefficient of variation for interspike interval in each 20% epoch of discharge duration were compared with repeated-measures two-way ANOVAs (group × time). The number of trains of action potentials, train frequency, and train duration for each 20% of discharge duration during the intermittent discharge were evaluated with one-way ANOVAs. The coefficient of variation for force was analysed for each third of discharge duration. The same statistical analysis was used to compare the RMS amplitude of biceps and triceps surface EMG for each one-third and the last 5% of the contraction duration.
Recruitment threshold, the difference between the recruitment threshold and target forces, and the time to motor unit recruitment were all examined with paired samples t tests for the 12 motor units recorded at two different target forces. Paired sample t tests were also used to compare the initial discharge rate and coefficient of variation for the first five interspike intervals when the motor unit was recruited in the two conditions.
Tukey's post hoc tests were used to identify differences when appropriate. A significance level of P < 0.05 was used to identify statistical significance. All statistical analyses were performed in SPSS v. 15 (Chicago, IL, USA). Data are presented in the text as means ± standard deviations (s.d.) and in figures as means ± standard error of the mean (s.e.m.).
Results
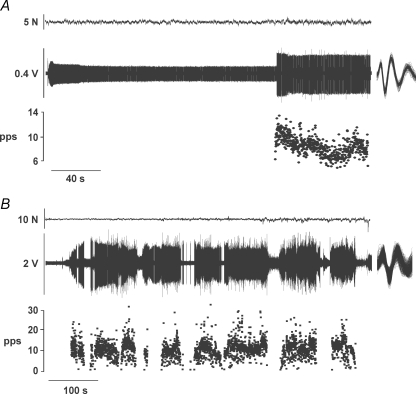
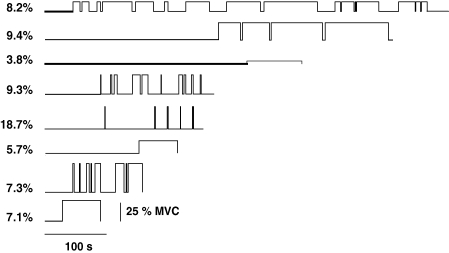
Twenty-two of the 53 motor units discharged action potentials tonically once recruited, whereas the other 31 units discharged intermittently (Table 1). The two behaviours were observed in motor units recorded from both heads of biceps brachii. An example of a tonic motor unit that was recruited at 139 s into the contraction is displayed in Fig. 1A, and an intermittent motor unit that was recruited at 49.4 s into the contraction is shown in Fig. 1B. There was a range of times from the start of the sustained contraction to the recruitment of the tonic and intermittent motor units and also in the number and frequency of trains of action potentials discharged by the intermittent motor units (see Fig. 2). Only 5 of the 31 intermittent units were active for more than 4 s immediately before the task was terminated; for example, the unit shown in the second trace from the bottom in Fig. 2.
Table 1.
Motor unit and task characteristics
| Tonic (n = 22) | Intermittent (n = 31) | |
|---|---|---|
| Recruitment threshold (% MVC) | 23.1 ± 13.1 (7.6–57.7) | 36.7 ± 8.0 (22.0–55.0) |
| De-recruitment threshold (% MVC) | 8.4 ± 13.6 (2.6–53.6) | 35.0 ± 11.2 (14.1–53.9 |
| Target force (% MVC) | ||
| Relative to MVC force | 17.2 ± 11.7 (4.1–47.1) | 26.1 ± 7.8 (13.5–44.6) |
| Below recruitment threshold | 5.9 ± 2.5 (3.1–10.6) | 10.7 ± 2.9 (6.8–21.3) |
| Contraction time (s) | ||
| Task duration | 167 ± 155 (70–538) | 313 ± 256 (46–1365) |
| Time to recruitment | 67 ± 88 (1–294) | 103 ± 158 (3–815) |
Values are mean ±s.d. (range).
Figure 1.
Representative tonic and intermittent discharge patterns A, the subject held a constant force at 71 N (top trace), which was 5.7% below the recruitment threshold of the motor unit. The times at which a single motor unit discharged action potentials continuously (tonic motor unit) after being recruited (second trace) were used to estimate its instantaneous discharge rate in pulses per second (pps; third trace). The 566 discriminated potentials are superimposed to the right of the second trace. B, another subject held a constant force at 89 N (top trace), which was 8.2% below the recruitment threshold of the motor unit. Once recruited, the motor unit discharged trains of action potentials (intermittent motor unit) that were used to estimate its instantaneous discharge rate. The 3321 discriminated potentials are superimposed to the right of the second trace.
Figure 2.
Representative discharge patterns of motor units The on–off times for eight motor units that indicate the range of discharge patterns observed. The value to the left of each trace indicates the percentage that the target force was set below the recruitment threshold of the unit. The traces are scaled (see bottom trace) to absolute times (s) and to contraction force (% MVC).
There was no difference between the average recruitment and de-recruitment thresholds for each group of units (Tables 1, P > 0.25), but both thresholds for the tonic motor units (23.1 ± 13.1 and 18.4 ± 13.6%, respectively) were significantly lower (P < 0.001) than those for the intermittent units (36.7 ± 8.0 and 35.0 ± 11.2% MVC force; Table 1). However, the tonic group included units with recruitment thresholds up to 57.7% MVC force, which was similar to the range for the intermittent group (55.0% MVC force). The target force for the sustained contraction was also significantly (P < 0.004) less for the tonic units (17.2 ± 11.7% MVC force) than that for the intermittent units (26.1 ± 7.8% MVC force), and the difference between the recruitment threshold and target force was less (P < 0.001) for the tonic units (5.9 ± 2.5% MVC force) compared with the intermittent units (10.7 ± 2.9% MVC force).
Despite the lesser difference between recruitment threshold and the target force for the tonic motor units, the time to recruitment did not differ statistically (P = 0.3) for the tonic (67 ± 88 s) and intermittent units (103 ± 158 s). The sustained contraction was terminated at 99 ± 111 s after the tonic motor units were recruited and discharged action potentials continuously, and 211 ± 153 s after the intermittent units were recruited and discharged trains of action potentials. One tonic motor unit was recruited 1 s into the sustained contraction, but the difference between target force and recruitment threshold was only 4% of MVC force. An intermittent motor unit began discharging 3 s into the contraction, presumably due to a relatively fast ramp to the target force (18.9% MVC s−1). The rates of change in force during the threshold task did not differ for the tonic and intermittent motor units for the recruitment (tonic: 10.8 ± 3.9% MVC s−1; intermittent: 11.0 ± 4.5% MVC s−1; P = 0.8) and de-recruitment (tonic: −11.4 ± 3.8% MVC s−1; intermittent: −11.2 ± 4.2% MVC s−1, respectively; P = 0.8) ramps.
Tonic motor units
The sustained contraction when the motor units discharged tonically, which lasted for 167 ± 155 s at a target force of 17.2 ± 11.7% MVC force, did not involve a change in either the coefficient of variation for force (first third: 1.4 ± 0.5%; last third; 1.9 ± 1.5%; P = 0.5) or the amplitude of the surface EMG for biceps brachii (P = 0.7) and triceps brachii (P = 0.8). The EMG for the last 5% of contraction duration only reached 15.9 ± 13.2% of the peak EMG during the initial MVC.
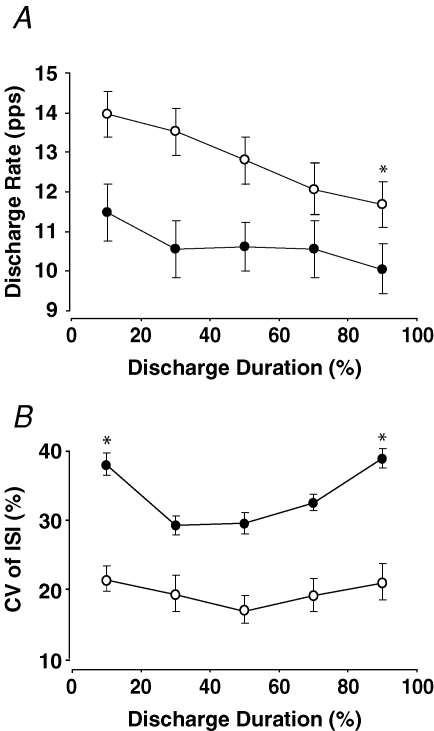
Mean discharge rate of the tonic motor units decreased from the first 20% (13.9 ± 2.7 pulses s−1) to the last 20% (11.7 ± 2.6 pulses s−1) epoch of discharge duration (P = 0.04; Fig. 3A, open circles). The change in discharge rate did not depend on the initial discharge rate (r = 0.32, P = 0.2). The average coefficient of variation for interspike interval (19.1 ± 11.7%) did not change across the discharge duration (P = 0.5; Fig. 3B open circles).
Figure 3.
Changes in average discharge rate and coefficient of variation for interspike interval for tonic and intermittent motor units A, mean discharge rate (pps) of the 22 tonic motor units (○) for each 20% of discharge duration. Discharge rate decreased significantly from the first 20% to the last 20% of discharge duration (*P = 0.04). Mean discharge rate of the 31 intermittent motor units (•) did not change across discharge duration. B, coefficient of variation for interspike interval (ISI; averages of five ISIs) for each 20% of discharge duration for the tonic (○) and intermittent (•) motor units. The average coefficient of variation was less for the tonic units (P < 0.001) and it was greater for the first and last 20% of discharge duration (*) compared with the middle time points for the intermittent motor units. Values are means ± standard error of the mean (s.e.m.).
The initial discharge rate for the first five interspike intervals (13.7 ± 3.3 pulses s−1) during the sustained contraction was significantly higher than the minimal discharge rate at recruitment during the ramp threshold task (11.9 ± 2.7 pulses s−1; P = 0.04; Table 2). The coefficient of variation for interspike interval for the initial discharge rate at recruitment during the sustained contraction (21.2 ± 9.4%) was less than that during the ramp threshold task (28.0 ± 9.2%; P = 0.02).
Table 2.
Average discharge rate and coefficient of variation (CV) for interspike interval (ISI) for the two groups of motor units during the two tasks
| Discharge rate (pps) | CV for ISI (%) | |||
|---|---|---|---|---|
| Tonic | Intermittent | Tonic | Intermittent | |
| Ramp contraction | 11.9 ± 2.7 | 13.4 ± 2.7 | 28.0 ± 9.2 | 27.4 ± 10.4 |
| Sustained contraction | 13.7 ± 3.3* | 11.0 ± 3.3*† | 21.2 ± 9.4* | 34.6 ± 12.3† |
Mean ±s.d. for the first five ISIs at recruitment during the ramp contraction and during the sustained contraction.
P < 0.05 compared with the ramp contraction (recruitment threshold task).
P < 0.05 compared with the tonic motor units.
Intermittent motor units
The sustained contraction for the intermittent units lasted for 313 ± 256 s at a target force of 26.1 ± 7.8% MVC force. These contractions were maintained until failure (± 4% MVC force) to determine if the motor unit would begin discharging tonically. When the five subjects performed the abbreviated protocol without the motor unit recording, MVC force declined significantly (P < 0.001) from 288 ± 85 N to 206 ± 26 N after the sustained contraction (−28.7 ± 12.9%). The coefficient of variation for force increased from the first-third (1.3 ± 0.3%) to the middle-third (1.7 ± 0.4%, P = 0.004) of the contraction, and from the middle-third to the last-third (3.0 ± 0.8%, P < 0.001). However, there was no association between the coefficient of variation for force at each one-third of the contraction and the number of trains of action potentials discharged by the intermittent units (r = −0.13−0.36, P≥ 0.1). The amplitude of the surface EMG for biceps brachii increased from the first-third (19.3 ± 10.9% MVC) to the last-third (28.3 ± 12.8% MVC, P = 0.03) and from the middle-third (22.2 ± 11.3% MVC) to the last 5% (33.1 ± 15.7% MVC, P = 0.005) of the discharge duration. Despite the progressive increase in EMG amplitude, the value during the final 5% of the contraction corresponded to only 33.1 ± 15.7% of the peak EMG during the initial MVCs and 42.9 ± 30.1% of the peak for the final MVCs. The amplitude of the surface EMG for triceps brachii (6.6 ± 5.5%) did not change (P = 0.54) during the fatiguing contraction.
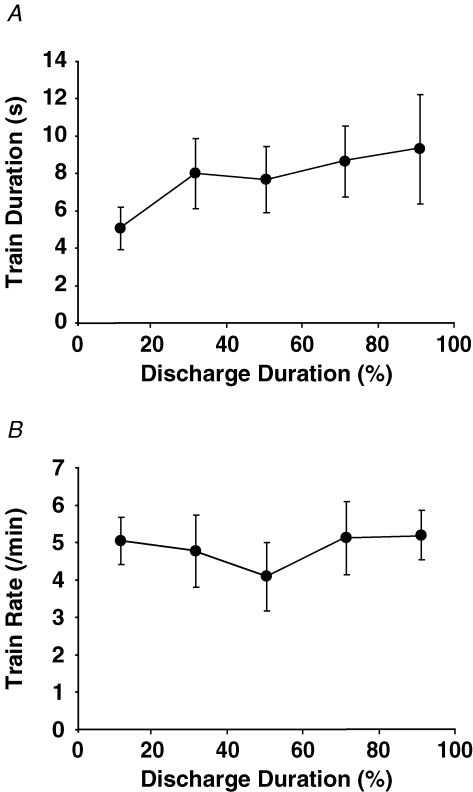
The intermittent units discharged an average of 12.2 ± 6.7 trains of action potentials (4.4 ± 2.2 trains min−1) with average train durations of 8.2 ± 10.2 s and an average discharge rate was 10.8 ± 2.2 pulses s−1. There were no associations between the target force relative to recruitment threshold and either the total number of trains (r = −0.09, P = 0.6), the number of trains per minute (r = 0.06, P = 0.7), train duration (r = −0.04, P = 0.8), or discharge rate (r = 0.17, P = 0.4). The total number of trains in a trial was positively correlated with motor unit discharge duration (r = 0.41, P = 0.02) for the 31 intermittent units. Discharge rates did not change across discharge duration (P = 0.63; Fig. 3A, filled circles), and were, on average, lower for the intermittent motor units (10.8 ± 2.2 pulses s−1) than for the tonic motor units (12.8 ± 2.8 pulses s−1, P = 0.01). The coefficient of variation for interspike interval was higher for the first (39.1 ± 9.4%) and last (40.0 ± 8.2%) of the 20% epochs of discharge duration than for the middle time points (29.5 ± 8.2%, 29.9 ± 7.9% and 33.0 ± 6.3%; P < 0.05; Fig. 3B, filled circles). Furthermore, the average coefficient of variation for interspike interval for the entire discharge duration was significantly higher for the intermittent motor units (34.6 ± 9.2%) compared with the tonic motor units (19.1 ± 11.7%, P < 0.001). Average train duration (P = 0.64; Fig. 4A), and train rate (P = 0.79; Fig. 4B) did not change across discharge duration.
Figure 4.
Intermittent discharge train duration and train rate Average train duration (A) and average train rate (B) did not change across discharge duration. Values are means ±s.e.m.
In contrast to the tonic motor units, the discharge rate during the first five interspike intervals (11.0 ± 3.3 pulses s−1) was significantly lower than the minimal discharge rate at recruitment during the threshold task (13.4 ± 2.7 pulses s−1; P = 0.003; Table 2). The coefficient of variation for interspike interval for the initial discharge rate at recruitment during the sustained contraction (34.6 ± 12.3%) was not statistically different from that during the ramp threshold task (27.4 ± 10.4%; P = 0.08). Consequently, initial discharge rate for the first five interspike intervals during the sustained contraction was significantly greater for the tonic motor units than for the intermittent motor units (P = 0.005), whereas the coefficient of variation for interspike interval was greater for the intermittent motor units (P = 0.001; Table 2).
Tonic and intermittent motor units
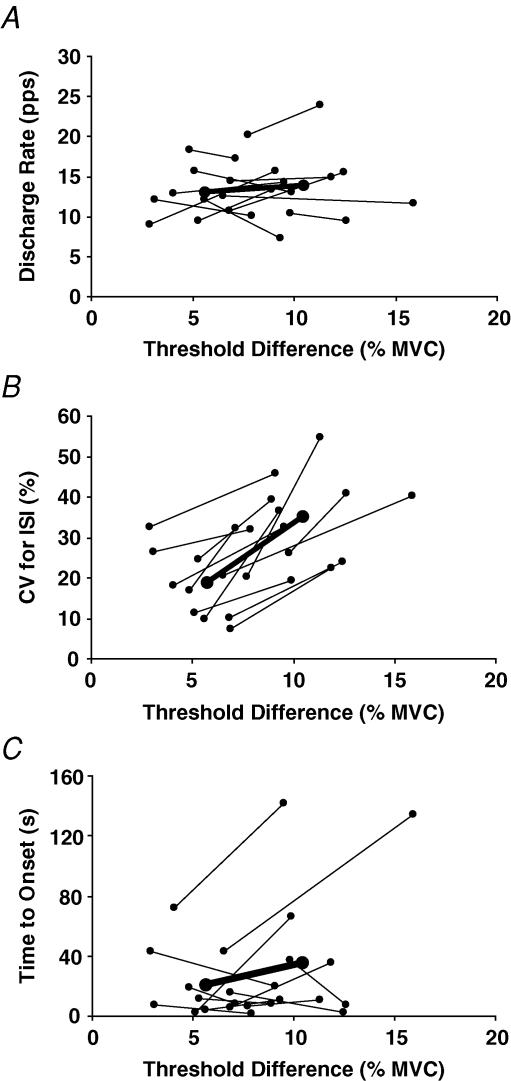
Twelve motor units were recorded during brief contractions (63.2 ± 27.9 s) at two different forces below recruitment threshold. The 12 motor units discharged tonically when the target force was 5.7 ± 1.9% below recruitment threshold, and intermittently when target force was 10.5 ± 2.4% MVC force below recruitment threshold (P < 0.001). The average recruitment threshold for these motor units was 32.8 ± 9.6% MVC force (21.7–53.9% MVC force), and discharge rate at recruitment during the threshold task was 12.7 ± 2.4 pulses s−1. There were no differences in discharge rate of the 12 motor units for the first five interspike intervals at recruitment during the sustained contractions (Fig. 5A; P = 0.5). However, the coefficient of variation for interspike interval was significantly higher when the motor units discharged intermittently (35.0 ± 10.2%) compared with the tonic discharge (18.7 ± 7.9%; P = 0.001; Fig. 5B). There was no statistical difference in the time to recruitment for the two conditions (tonic: 22.5 ± 21.7 s; intermittent: 37.1 ± 50.4 s; P = 0.4; Fig. 5C).
Figure 5.
Discharge characteristics and time to onset for motor units recorded at two different target forces Discharge characteristics of 12 motor units that discharged tonically when the target force was closer (5.7 ± 1.9% MVC force) to recruitment threshold and intermittently when the target force was further (10.5 ± 2.4% MVC force) from recruitment threshold. A, mean discharge rate for the first 5 interspike intervals was not statistically different for the tonic and intermittent discharge patterns. B, coefficient of variation for interspike interval was statistically greater for the intermittent discharge (P < 0.001). C, time to recruitment was variable and not statistically different for the two discharge patterns. Thin lines indicate individual responses and the thick line is the mean for all 12 motor units.
Discussion
The purpose of the study was to determine the influence of the difference between the recruitment threshold of a motor unit and the target force during a sustained contraction on the discharge of the motor unit when it was recruited. The main finding was that the discharge characteristics of the motor unit depended on the extent to which the target force was set below the recruitment threshold of the unit. The difference between the recruitment threshold of the motor unit and the target force influenced the discharge pattern during the sustained contraction and the initial discharge rate of the unit at the time it was recruited. The progressive increase in synaptic input to the motoneurone pool during a sustained contraction with the elbow flexor muscles caused motor units that were closer to threshold to discharge tonically at recruitment, whereas motor units further from threshold discharged intermittent trains of action potentials, presumably due to greater fluctuations in membrane potential.
Discharge pattern
When the target force for the sustained contraction was set at an average of 5.9% MVC force (range: 3.1–10.6% MVC force) below the recruitment threshold of a motor unit, it discharged action potentials tonically once it was recruited (Garland et al. 1997; Adam & De Luca, 2005; Mottram et al. 2005). In contrast, a target force of 10.9% (range: 6.8–21.3% MVC force) below the recruitment threshold resulted in the motor unit discharging trains of action potentials once it was recruited. The divergent behaviour cannot be explained by a difference in the type of motor unit because the 12 motor units recorded at the two target forces displayed both tonic and intermittent discharge characteristics, and these motor units had a similar range of recruitment thresholds (21.7–53.9% MVC force) to the larger sample of tonic and intermittent motor units.
As there was no consistent difference in the time to recruitment of the tonic (67 ± 88 s) and intermittent (103 ± 158 s) units as well as for the 12 motor units that exhibited both discharge patterns, the different discharge characteristics at recruitment cannot be explained by adjustments that accumulated with the increase in contraction time. The two discharge patterns, however, do suggest a difference in the adjustments experienced by the motoneurones. Whereas mean discharge rate declined progressively for the tonic units (Fig. 3A) after only 99 ± 111 s of discharging action potentials, there was no change in mean discharge rate for the intermittent units (Fig. 3A). Presumably, the trains of action potentials discharged by the intermittent units were too brief (8.2 ± 10.2 s) for the mechanisms that are responsible for the decrease in discharge rate to become activated. Furthermore, the discharge rates for each train were highly variable, and the first few discharges of each train were not elevated as would be expected with the early adaptation of motoneurone discharge rates (Sawczuk et al. 1995). The candidate mechanisms for the decrease in discharge rate exhibited by the tonic motor units are late adaptation and a reduction in the net excitatory input to the motoneurone (Carpentier et al. 2001; Butler et al. 2003; Nordstrom et al. 2007). Assuming that the change in synaptic input to the motoneurone pool was qualitatively similar for the two discharge patterns, the decrease in discharge rate exhibited by the tonic units was probably caused by motoneurone adaptation rather than a reduction in net excitatory input.
In addition to an absence of adaptation, the intermittent units did not appear to receive synaptic input that evoked persistent inward currents capable of sustaining the discharge of the motoneurones. A persistent inward current is a sustained depolarization mediated by voltage-gated, inward currents that persist after the synaptic excitation has been either reduced or removed (Schwindt & Crill, 1980; Hounsgaard & Kiehn, 1989; Lee & Heckman, 1998). The existence of persistent inward currents in human motoneurones has been inferred from the discharge of single motor units and the force exerted by muscle in a variety of protocols (Collins et al. 2002; Gorassini et al. 2002; Hornby et al. 2003; Nickolls et al. 2004; Kamen et al. 2006). The discharge of trains of action potentials by some motor units during a progressive increase in the synaptic input to the motoneurone pool suggests that either the motor units included in the sample did not express plateau potentials (recruitment thresholds: 22–55% MVC force), the persistent inward currents were not activated during this task, or the inward currents were suppressed by inhibitory input to the motoneurone pool. Although persistent inward currents are observed more often in low-threshold motoneurones (Heckman et al. 2005; Nordstrom et al. 2007), the presumed activation of these currents has been reported for motor units in soleus with recruitment thresholds up to about 40% MVC force (Gorassini et al. 2002) and the force exerted by tibialis anterior increased by 42% MVC force after high-frequency electrical stimulation engaged central mechanisms that may have included persistent inward currents (Collins et al. 2002). In contrast to the tonic units that discharged action potentials repetitively once recruited, the intermittent units exhibited pauses in the discharge of action potentials that is not consistent with the activation of mechanisms that produce a self-sustained discharge of action potentials. Also there was no evidence that discharge rate accelerated to denote the onset of a persistent inward current.
The decrease in discharge rate exhibited by the tonic motor units also suggests that persistent inward currents were not activated during this task. Based on a comparison of the discharge evoked in spinal motoneurones of cats in various states of descending monoaminergic drive, Nordstrom et al. (2007) concluded that the activation of persistent inward currents should be able to attenuate late adaptation. Since the mean discharge rate of the tonic motor units in the current study declined during the sustained contraction and this adjustment may have been caused by late adaptation, presumably there was no functionally significant sustained depolarization produced by persistent inward currents.
Initial discharge rate
Another distinction between the two discharge patterns was the discharge rate of the motor units when they were first recruited during the sustained contraction. Compared with the discharge characteristics during the ramp contractions to assess recruitment threshold, the mean discharge rate was lower and the coefficient of variation for interspike interval was greater for the intermittent units, but not the tonic units, at recruitment during the sustained contraction (Table 2). There was a trend (P = 0.052) for the initial discharge rate during the ramp contractions to be less for the tonic units (11.9 ± 2.7 pulses s−1) than for the intermittent units (13.4 ± 2.7 pulses s−1), which is consistent with the difference in recruitment threshold for the two groups of units (Moritz et al. 2005). Nonetheless, the coefficient of variation at recruitment during the ramp contractions did not differ (P = 0.8) for the two groups of units (tonic, 28.0 ± 9.2%; intermittent, 27.4 ± 10.4%). Given the association between synaptic noise and discharge variability (Calvin & Stevens, 1968; Matthews, 1996; Stein et al. 2005), these results suggest that the two groups of units received qualitatively similar synaptic inputs at the time of recruitment during the ramp contractions.
In contrast, the synaptic inputs delivered to the two groups of motor units differed at the time of recruitment during the sustained contraction. Initial discharge rate at recruitment during the sustained contraction was greater (P = 0.005) for the tonic units (13.7 ± 3.3 pulses s−1) compared with the intermittent units (11.0 ± 3.3 pulses s−1). Furthermore, the coefficient of variation for the first five interspike intervals at recruitment was much higher for the intermittent units (34.6 ± 12.3%) compared with the tonic units (21.2 ± 9.4%), and it remained elevated across discharge duration (Fig. 3B). A potential confounding factor is that the coefficient of variation for interspike interval is inversely related to discharge rate (Moritz et al. 2005), and the greater variability in discharge for the intermittent units could have been influenced by the slower discharge rates at recruitment. However, there was no difference in initial discharge rate at recruitment for the 12 motor units that exhibited both discharge patterns (Fig. 5A), yet the coefficient of variation for interspike interval was still greater when the units discharged intermittently (Fig. 5B). When compared with the coefficients of variation observed during brief isometric contractions to various target forces around recruitment thresholds of motor units (Moritz et al. 2005; Barry et al. 2007), the current findings indicate that the coefficient of variation for the tonic discharge was less than the control values whereas it was elevated for the intermittent discharge at the time of recruitment.
The differences in mean discharge rate and discharge variability at recruitment are consistent with greater amounts of excitatory and inhibitory input being received by the motoneurones that discharged action potentials intermittently after being recruited (Berg et al. 2007). As the quantity of synaptic input increases and the fluctuations in membrane potential are augmented, action potentials are triggered by brief depolarizing transients that produce irregular trains of action potentials. Because the recruitment threshold of the intermittent units was further from the target force, the motoneurones had to receive a greater amount of synaptic input to be recruited, which increased the fluctuations in membrane trajectory and the accompanying variability in discharge times. The intermittent units remained in this state for the duration of the contraction, whereas the tonic units discharged a single more regular train of action potentials.
In a condition where there is very little synaptic noise, and a progressive increase in synaptic input to the motoneuron pool, a two-fold increase in the difference between recruitment threshold and target force should result in the motor unit consistently being recruited later in the contraction. However, the findings from the experiments on the two samples of motor units as well as the experiments on the same motor unit (Fig. 5C) demonstrate that the time to recruitment can vary substantially. This further supports the interpretation that the motor units were recruited by synaptic noise that caused fluctuations in membrane potential, and that the difference from threshold to the target force was responsible for the pattern of discharge at recruitment and for the remainder of the contraction.
In summary, the discharge characteristics of motor units in biceps brachii that were recruited during a sustained contraction depended on the difference between the target force for the sustained contraction and the recruitment threshold of the motor unit. A relatively small difference between the two forces was associated with motor units discharging action potentials continuously after being recruited (tonic motor units). In contrast, a greater difference between the two forces resulted in the recruited motor unit discharging trains of action potentials (intermittent motor units). Discharge rate during the sustained contraction declined for the tonic units, but not the intermittent units. At the time of recruitment, the coefficient of variation for interspike interval was reduced for the tonic units during the sustained contraction compared with the ramp contraction of the threshold task, and compared with the intermittent units during the sustained contraction. These results suggest that the increase in synaptic input caused motor units with recruitment thresholds closer to the target force to discharge action potentials at more regular rates when recruited, whereas the input resulted in greater synaptic noise when the target force was further from recruitment threshold and this caused the motor units to discharge action potentials intermittently.
Acknowledgments
The work was supported by an award (NS043275) from the National Institute of Neurological Disorders and Stroke to R.M.E. We thank Professor Jacques Duchateau for comments on a draft of the manuscript.
References
- Adam A, De Luca CJ. Recruitment order of motor units in human vastus lateralis muscle is maintained during fatiguing contractions. J Neurophysiol. 2003;90:2919–2927. doi: 10.1152/jn.00179.2003. [DOI] [PubMed] [Google Scholar]
- Adam A, De Luca CJ. Firing rates of motor units in human vastus lateralis muscle during fatiguing isometric contractions. J Appl Physiol. 2005;99:268–280. doi: 10.1152/japplphysiol.01344.2004. [DOI] [PubMed] [Google Scholar]
- Barry BK, Pascoe MA, Jesunathadas M, Enoka RM. Rate coding is compressed but variability is unaltered for motor units in a hand muscle of old adults. J Neurophysiol. 2007;97:3206–3218. doi: 10.1152/jn.01280.2006. [DOI] [PubMed] [Google Scholar]
- Berg RW, Alaburda A, Hounsgaard J. Balanced inhibition and excitation drive spike activity in spinal half-centers. Science. 2007;315:390–393. doi: 10.1126/science.1134960. [DOI] [PubMed] [Google Scholar]
- Bigland-Ritchie B, Furbush F, Woods JJ. Fatigue of intermittent submaximal voluntary contractions: central and peripheral factors. J Appl Physiol. 1986;61:421–429. doi: 10.1152/jappl.1986.61.2.421. [DOI] [PubMed] [Google Scholar]
- Butler JE, Taylor JL, Gandevia SC. Responses of human motoneurons to corticospinal stimulation during maximal voluntary contractions and ischemia. J Neurosci. 2003;23:10224–10230. doi: 10.1523/JNEUROSCI.23-32-10224.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvin WH, Stevens CF. Synaptic noise and other sources of randomness in motoneuron interspike intervals. J Neurophysiol. 1968;31:574–587. doi: 10.1152/jn.1968.31.4.574. [DOI] [PubMed] [Google Scholar]
- Carpentier A, Duchateau J, Hainaut K. Motor unit behaviour and contractile changes during fatigue in the human first dorsal interosseus. J Physiol. 2001;534:903–912. doi: 10.1111/j.1469-7793.2001.00903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DF, Burke D, Gandevia SC. Sustained contractions produced by plateau-like behaviour in human motoneurones. J Physiol. 2002;538:289–301. doi: 10.1113/jphysiol.2001.012825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoka RM, Robinson GA, Kossev AR. A stable, selective electrode for recording single motor-unit potentials in humans. Exp Neurol. 1988;99:761–764. doi: 10.1016/0014-4886(88)90189-6. [DOI] [PubMed] [Google Scholar]
- Enoka RM, Robinson GA, Kossev AR. Task and fatigue effects on low-threshold motor units in human hand muscle. J Neurophysiol. 1989;62:1344–1359. doi: 10.1152/jn.1989.62.6.1344. [DOI] [PubMed] [Google Scholar]
- Freund HJ, Budingen HJ, Dietz V. Activity of single motor units from human forearm muscles during voluntary isometric contractions. J Neurophysiol. 1975;38:933–946. doi: 10.1152/jn.1975.38.4.933. [DOI] [PubMed] [Google Scholar]
- Garland SJ, Enoka RM, Serrano LP, Robinson GA. Behavior of motor units in human biceps brachii during a submaximal fatiguing contraction. J Appl Physiol. 1994;76:2411–2419. doi: 10.1152/jappl.1994.76.6.2411. [DOI] [PubMed] [Google Scholar]
- Garland SJ, Griffin L, Ivanova T. Motor unit discharge rate is not associated with muscle relaxation time in sustained submaximal contractions in humans. Neurosci Lett. 1997;239:25–28. doi: 10.1016/s0304-3940(97)00885-9. [DOI] [PubMed] [Google Scholar]
- Gorassini M, Yang JF, Siu M, Bennett DJ. Intrinsic activation of human motoneurons: possible contribution to motor unit excitation. J Neurophysiol. 2002;87:1850–1858. doi: 10.1152/jn.00024.2001. [DOI] [PubMed] [Google Scholar]
- Grimby L, Hannerz J. Firing rate and recruitment order of toe extensor motor units in different modes of voluntary conraction. J Physiol. 1977;264:865–879. doi: 10.1113/jphysiol.1977.sp011699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimby L, Hannerz J, Borg J, Hedman B. Firing properties of single human motor units on maintained maximal voluntary effort. Ciba Found Symp. 1981;82:157–177. doi: 10.1002/9780470715420.ch10. [DOI] [PubMed] [Google Scholar]
- Gydikov A, Kossev A, Trayanova N, Radicheva N. Selective recording of motor unit potentials. Electromyogr Clin Neurophysiol. 1986;26:273–281. [PubMed] [Google Scholar]
- Hannerz J. Discharge properties of motor units in relation to recruitment order in voluntary contraction. Acta Physiol Scand. 1974;91:374–385. doi: 10.1111/j.1748-1716.1974.tb05692.x. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Gorassini MA, Bennett DJ. Persistent inward currents in motoneuron dendrites: implications for motor output. Muscle Nerve. 2005;31:135–156. doi: 10.1002/mus.20261. [DOI] [PubMed] [Google Scholar]
- Hornby TG, Rymer WZ, Benz EN, Schmit BD. Windup of flexion reflexes in chronic human spinal cord injury: a marker for neuronal plateau potentials? J Neurophysiol. 2003;89:416–426. doi: 10.1152/jn.00979.2001. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O. Serotonin-induced bistability of turtle motoneurones caused by a nifedipine-sensitive calcium plateau potential. J Physiol. 1989;414:265–282. doi: 10.1113/jphysiol.1989.sp017687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter SK, Ryan DL, Ortega JD, Enoka RM. Task differences with the same load torque alter the endurance time of submaximal fatiguing contractions in humans. J Neurophysiol. 2002;88:3087–3096. doi: 10.1152/jn.00232.2002. [DOI] [PubMed] [Google Scholar]
- Kamen G, Sullivan R, Rubinstein S, Christie A. Evidence of self-sustained motoneuron firing in young and older adults. J Electromyogr Kinesiol. 2006;16:25–31. doi: 10.1016/j.jelekin.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Kato M, Murakami S, Takahashi K, Hirayama H. Motor unit activities during maintained voluntary muscle contraction at constant levels in man. Neurosci Lett. 1981;25:149–154. doi: 10.1016/0304-3940(81)90323-2. [DOI] [PubMed] [Google Scholar]
- Kernell D, Monster AW. Time course and properties of late adaptation in spinal motoneurones of the cat. Exp Brain Res. 1982;46:191–196. doi: 10.1007/BF00237176. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in persistent inward currents. J Neurophysiol. 1998;80:583–593. doi: 10.1152/jn.1998.80.2.583. [DOI] [PubMed] [Google Scholar]
- Maton B, Gamet D. The fatigability of two agonistic muscles in human isometric voluntary submaximal contraction: an EMG study. II. Motor unit firing rate and recruitment. Eur J Appl Physiol Occup Physiol. 1989;58:369–374. doi: 10.1007/BF00643511. [DOI] [PubMed] [Google Scholar]
- Matthews PB. Relationship of firing intervals of human motor units to the trajectory of post-spike after-hyperpolarization and synaptic noise. J Physiol. 1996;492:597–628. doi: 10.1113/jphysiol.1996.sp021332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz CT, Barry BK, Pascoe MA, Enoka RM. Discharge rate variability influences the variation in force fluctuations across the working range of a hand muscle. J Neurophysiol. 2005;93:2449–2459. doi: 10.1152/jn.01122.2004. [DOI] [PubMed] [Google Scholar]
- Mottram CJ, Jakobi JM, Semmler JG, Enoka RM. Motor-unit activity differs with load type during a fatiguing contraction. J Neurophysiol. 2005;93:1381–1392. doi: 10.1152/jn.00837.2004. [DOI] [PubMed] [Google Scholar]
- Nickolls P, Collins DF, Gorman RB, Burke D, Gandevia SC. Forces consistent with plateau-like behaviour of spinal neurons evoked in patients with spinal cord injuries. Brain. 2004;127:660–670. doi: 10.1093/brain/awh073. [DOI] [PubMed] [Google Scholar]
- Nordstrom MA, Gorman RB, Laouris Y, Spielmann JM, Stuart DG. Does motoneuron adaptation contribute to muscle fatigue? Muscle Nerve. 2007;35:135–158. doi: 10.1002/mus.20712. [DOI] [PubMed] [Google Scholar]
- Person RS, Kudina LP. Discharge frequency and discharge pattern of human motor units during voluntary contraction of muscle. Electroencephalogr Clin Neurophysiol. 1972;32:471–483. doi: 10.1016/0013-4694(72)90058-2. [DOI] [PubMed] [Google Scholar]
- Peters EJ, Fuglevand AJ. Cessation of human motor unit discharge during sustained maximal voluntary contraction. Neurosci Lett. 1999;274:66–70. doi: 10.1016/s0304-3940(99)00666-7. [DOI] [PubMed] [Google Scholar]
- Riley ZA, Enoka RM. Adjustments in motor unit activity during sustained submaximal contractions. IBRO World Congress of Neuroscience Satellite Meeting titled Motor Control of the Top End.2007. [Google Scholar]
- Riley ZA, Terry ME, Mendez-Villanueva A, Litsey JC, Enoka RM. Motor unit recruitment and bursts of activity in the surface electromyogram during a sustained contraction. Muscle Nerve. 2008 doi: 10.1002/mus.20978. DOI: 10.1002/mus.20978. [DOI] [PubMed] [Google Scholar]
- Rudroff T, Christou EA, Poston B, Bojsen-Moller J, Enoka RM. Time to failure of a sustained contraction is predicted by target torque and initial electromyographic bursts in elbow flexor muscles. Muscle Nerve. 2007;35:657–666. doi: 10.1002/mus.20752. [DOI] [PubMed] [Google Scholar]
- Sawczuk A, Powers RK, Binder MD. Spike frequency adaptation studied in hypoglossal motoneurons of the rat. J Neurophysiol. 1995;73:1799–1810. doi: 10.1152/jn.1995.73.5.1799. [DOI] [PubMed] [Google Scholar]
- Schwindt PC, Crill WE. Properties of a persistent inward current in normal and TEA-injected motoneurons. J Neurophysiol. 1980;43:1700–1724. doi: 10.1152/jn.1980.43.6.1700. [DOI] [PubMed] [Google Scholar]
- Stein RB, Gossen ER, Jones KE. Neuronal variability: noise or part of the signal? Nat Rev Neurosci. 2005;6:389–397. doi: 10.1038/nrn1668. [DOI] [PubMed] [Google Scholar]