Abstract
The effect of β-adrenergic stimulation on endogenous G-protein-activated K+ (GIRK) current has been investigated in atrial myocytes from hearts of adult rats. β-Adrenergic stimulation (10 μm isoprenaline, Iso) had no effect on activation kinetics, peak current or steady-state current but resulted in slowing of deactivation upon washout of acetylcholine (ACh), the time constant (τd) being increased by a factor of about 2.5. The effect of Iso could be mimicked by inclusion of cAMP (500 μm) in the filling solution of the patch clamp pipette. The Iso-induced increase in τd was blocked by the selective β1 receptor antagonist CGP-20112A (2 μm) and by the PKA inhibitor H9 (100 μm included in the pipette solution). A candidate for mediating these effects is RGS10, one of the regulators of G-protein signalling (RGS) species expressed in cardiac myocytes. Overexpression of RGS10 by adenoviral gene transfer resulted in a reduction in τd of 60%. Sensitivity of τd to Iso remained in cells overexpressing RGS10. Overexpression of RGS4 caused a comparable reduction in τd, which became insensitive to Iso. Expression of an RGS10 carrying a mutation (RGS10-S168A), which deletes a PKA phosphorylation site, caused a decrease in τd comparable to overexpression of wild-type RGS10. Sensitivity of τd to Iso was lost in RGS10-S168A-expressing myocytes. Silencing of RGS10 by means of adenovirus-mediated transcription of a short hairpin RNA did not affect basal τd but removed sensitivity to Iso. These data suggest that endogenous RGS10 has GTPase-activating protein (GAP) activity on the G-protein species that mediates activation of atrial GIRK channels. Moreover, RGS10, via PKA-dependent phosphorylation, enables a crosstalk between β-adrenergic and muscarinic cholinergic signalling.
G-protein gated inwardly rectifying K+ (GIRK) channels expressed in the heart, in neurons and in endocrine cells contribute to physiological vagal bradycardia, generation of slow IPSPs in the brain and to regulation of hormone secretion. These channels are activated by direct interaction with βγ subunits released from pertussis toxin (PTX)-sensitive heterotrimeric G-proteins upon agonist stimulation of appropriate G-protein coupled receptors (GPCRs) (see Stanfield et al. 2002; Sadja et al. 2003 for reviews).
Because of their direct coupling to G-protein activation, i.e. without intermediate signalling molecules, GIRK channels provide a high-fidelity online assay for activity of PTX-sensitive G-proteins with unrivalled time resolution. In this context they have been frequently used in expression systems to study the role of accessory proteins that modulate receptor–G-protein pathways (e.g. Zhang et al. 2002; Benians et al. 2005; Fowler et al. 2007).
Signalling via heterotrimeric G-proteins containing either PTX-sensitive αi/o subunits or αq subunits is modulated by RGS (regulators of G-protein signalling) proteins, representing a family comprising about 25 members that serve various functions. Their common motif is a domain of 120–125 residues (RGS domain) that catalyses an acceleration of the endogenous GTPase activity, preferentially of Gi/o but also Gq, analogous to GTPase-activating proteins (GAPs) for small G-proteins. Apart from this function, members of the RGS family act as scaffolds for organizing signalling pathways by means of a variety of protein interaction modules (see Hollinger & Hepler, 2002; Siderovski & Willard, 2005; Sato et al. 2006 for reviews).
Receptor-dependent gating, particularly deactivation of GIRK current upon washout of the activating agonist, has been found to be significantly slower in heterologous expression systems (Xenopus oocytes or mammalian cell lines) as compared to GIRK channels in their native environment (cardiac myocytes and neurons). This parameter could be tuned into a physiological range by co-expression of RGS proteins (Doupnik et al. 1997; Saitoh et al. 1997). In atrial myocytes, evidence for expression of about 10 different RGS species has been provided (Kardestuncer et al. 1998; Ho & Murrell-Lagnado, 1999). This includes RGS10, the smallest member of this family, which corresponds to an RGS domain devoid of other interaction domains. To date only sparse information is available on the impact of individual members of this family on signalling via GPCRs in general and G-protein kinetics in particular in cardiac myocytes. Using Xenopus oocytes as expression system, it had been demonstrated that deactivation of GIRK current carried by expressed Kir3.1/Kir3.2 channels is accelerated by co-expressed RGS10. This effect could be removed by PKA-dependent phosphorylation, which results in translocation of RGS10 to the nucleus, where it might serve functions yet to be determined (Burgon et al. 2001).
In the present study we have investigated the role of endogenous RGS10 on GIRK current deactivation in atrial myocytes at physiological expression levels of the signalling components and upon overexpression or siRNA-mediated knockdown of RGS10 or expression of a PKA phosphorylation-deficient RGS10 mutant. Our data suggest a contribution of this RGS species to deactivation of GIRK current or signalling via Gi/o in more general terms. Moreover the results suggest a crosstalk between β-adrenergic (Gs) and Gi/o-mediated signalling in atrial myocytes via RGS10.
Methods
Isolation and culture of atrial myocytes
Rats were killed following protocols approved by the local authorities for the regulation of animal welfare (Regierungspräsident) in accordance with the guidelines of the European Community (86/609/EEC). Wistar Kyoto rats of either sex, aged between 8 and 10 weeks, were anaesthetized by i.v. injection of urethane (1 g kg−1). The chest was opened and the heart was removed and mounted on the cannula of a sterile Langendorff apparatus for coronary perfusion at constant flow. The method of enzymatic isolation of rat myocytes and serum-free culture conditions have been described in detail elsewhere (Wellner-Kienitz et al. 2000). Endogenous expression of RGS10 was verified by PCR (forward primer: 5′-AGT TTC ACC CGC GCC GT-3′; reverse 5′-TTA TGT GTT GTA GAT TCT GGA AG-3′) after reverse transcription of RNA isolated from rat atrial myocytes.
Solutions and chemicals
For whole-cell measurements of membrane currents an extracellular solution of the following composition was used (mm): NaCl 122; KCl 20; CaCl2 0.5; MgCl2 1.0; Hepes/NaOH 10.0, pH 7.4. The pipette solution contained (mm): potassium aspartate 100; KCl 40; NaCl 5.0, MgCl2 2.0; Na2ATP 5.0; EGTA 2.0; GTP 0.025; Hepes/KOH 20.0, pH 7.4. The K+ reversal potential under these conditions was calculated as −48 mV. Standard chemicals were from Merck (Darmstadt, Germany). EGTA, Hepes, MgATP, GTP, acetylcholine-chloride, isoprenaline 3′-5′cyclic AMP (cAMP) and H9 were from Sigma-Aldrich (Taufkirchen, Germany). CGP-207112A was kindly provided by Novartis Pharma (Basel, Switzerland).
Current measurement
Membrane currents were measured at ambient temperature (22–24°C) using whole-cell patch clamp as described in detail previously (Wellner-Kienitz et al. 2000). Cells were voltage-clamped at a holding potential of −90 mV, i.e. negative to EK, resulting in inward K+ currents. Rapid exposure to agonist-containing solutions was performed by means of a custom-made solenoid-operated flow system permitting a change of solution around an individual cell with a half-time of about 100 ms.
Adenovirus constructs and gene transfer in atrial myocytes
The pAd-Easy1 plasmid encoding for the adenovirus type 5 and pAd-Track-CMV were kindly provided by Dr B. Vogelstein (Johns Hopkins University, Baltimore, MD, USA). Production and purification of the recombinant virus were performed as described in detail previously (He et al. 1998). Briefly, the cDNAs of human RGS10, RGS10 mutant (S168A) and rat RGS4 were subcloned into pAd-Track-CMV shuttle vector, using Xba I and Kpn I to yield corresponding pAd-Track vectors. Adenovirus recombinant plasmids were generated by homologous recombination between pAd-Track and pAd-Easy1 in E. coli to produce the recombinant viruses. The recombinant viruses were propagated in HEK293 cells and recovered after several freezing–thawing cycles. Virus titres were estimated by serial dilution and infection of myocyte cultures. For infection, cells were incubated with 1 ml culture medium containing 105 infectious particles (gene transforming units). cDNAs were kindly provided by: Dr T. Chatterjee (University of Iowa), human RGS10; Dr P. Burgon (Harvard Medical School), human RGS10-S168A; and Dr H. A. Lester (Caltech), rat RGS4.
RNA interference
A detailed description of the experimental conditions and methodology for RNAi in adult cardiac myocytes using adenovirus-driven transcription of RNA hairpins has been published elsewhere (Rinne et al. 2006). Briefly, pShuttle was used to insert the murine U6 promoter and the anti-RGS10 directed siRNA as double stranded oligonucleotide using Xho I and Kpn I restriction sites. The vector pmU6pro containing the murine U6snRNA promoter, which served as template, was kindly provided by Dr D. G. Turner (University of Michigan, Ann Arbor, USA).
To produce siRNA-hairpin-encoding vectors the U6-promotor and the hairpin construct were fused using a universal 5′U6 primer (5′-GGAAGA-TCTGATCCGACGCCGCCATC-3′) (Castanotto et al. 2002) and a 3′-primer including the siRNA (bold) and complementary U6 promoter sequences (italic): 5′-GGG-GTACCAAAAAA AGAGATTCAGGGAATTTCTTCTCTTGAAAAGAAATTCCCTGAATCTCTAAACAAGGCTT-TTCTCCAAG-3′. The underlined sequence corresponds to the hairpin loop.
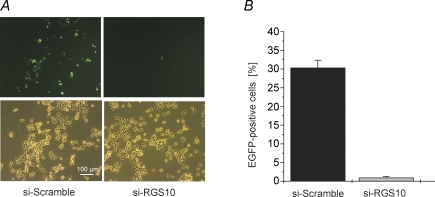
A randomised sequence (siScramble) based on the above sequence served as control. The recombinant (Ad-siRGS10; Ad-siScramble) adenoviruses were generated by homologous recombination between pshuttlesiRGS10 (pshuttle-siScramble) and pAd-Easy1 in E. coli. Efficient silencing of RGS10 by the above siRNA sequence was verified in cultures of HEK293 cells transfected with a plasmid encoding for a C-terminal fusion construct with GFP. Efficiency of silencing was evaluated by counting the fraction of fluorescent cells in an optical field as illustrated in Fig. 1A. The summarized data (Fig. 1B) demonstrate that expression of the fusion protein was almost completely inhibited by the above silencing construct.
Figure 1. Silencing of RGS10–GFP fusion protein in HEK293 cells by siRNA targeting RGS10.
A, representative fluorescence (top) and phase contrast micrographs from HEK293 cultures transfected with a plasmid encoding for RGS10 C-terminally fused to EGFP plus either the hairpin sequence directed against RGS10 (shown in RNA interference in Methods) or an equivalent construct containing the 20-meric siRNA in a scrambled order. B, summarized data based on counting 7 fields as in A each from 3 different cultures for both conditions. Cell counts were done about 48 h after transfection.
Statistical analysis
Wherever possible, data are presented as mean ±s.e.m. and were analysed using Student's unpaired t test. P < 0.05 was considered significant and indicated by an asterisk in figures.
Results
Reproducibility of ACh-activated current in individual cells
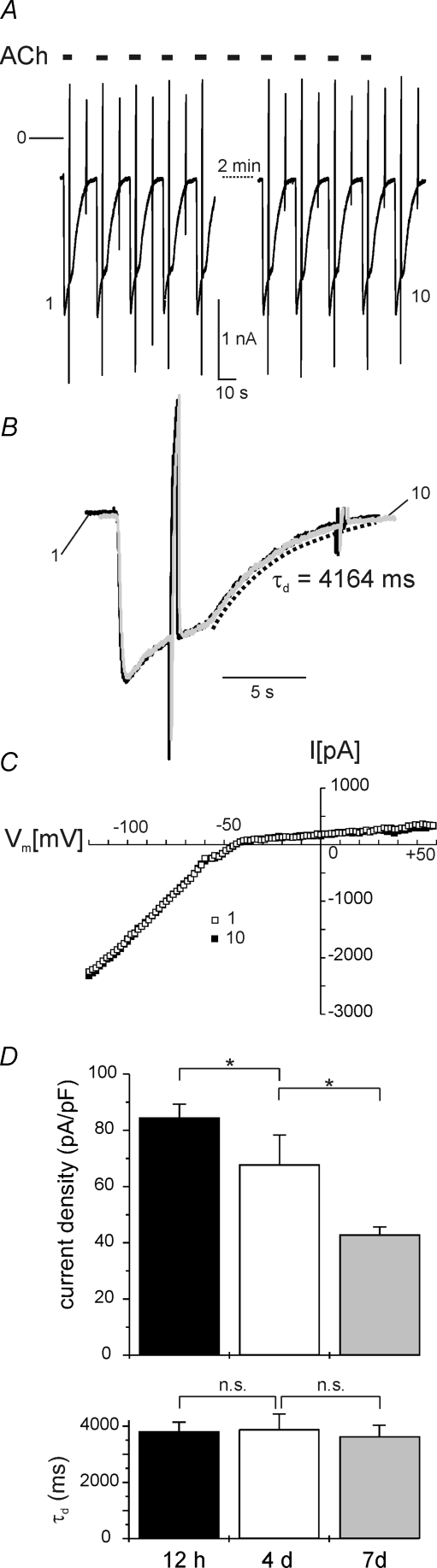
In the present study acetylcholine-induced GIRK current, with particular focus on deactivation following washout of the agonist, has been used as an online readout for GPCR-induced free Gβγ under various experimental conditions. In a given cell at constant conditions, such an assay should provide signals of high reproducibility and little time-dependent changes such as rundown within the time frame of an acute experiment. As illustrated in Fig. 2A, the amplitude of the current activated by ACh (2 μm, applied for 5 s at a time) remained largely constant for a period of about 5 min in this representative recording. In Fig. 2B the first and the last current transients of this series have been superimposed on an expanded time scale. Both are virtually identical for kinetics of activation, acute desensitization and deactivation. The latter (trace 1) has been fitted using a single exponential with a time constant (τd) of 4.16 s (dotted curve), which yields a perfect fit also for the trace labelled 10. Likewise, voltage dependence of IK(ACh), determined by ramp-shaped changes in membrane potential from −120 to +60 mV, remained constant throughout an experiment as illustrated by the superimposed I–V curves in Fig. 2C. This demonstrates that IK(ACh) represents a highly stable signal with unprecedented time resolution for detecting experimentally evoked changes in GPCR–G-protein signalling properties. In the culture conditions of the present study there was a significant reduction of IK(ACh) density with time, as illustrated in Fig. 2D. However, no change in τd, the major parameter of interest, was found between myocytes cultured for about 12 h and 7 days (Fig. 2D). Because of the adequate formal fit of the deactivation time course by a single exponential, this was routinely used in the following to extract τd under various conditions.
Figure 2. Stability of IK(ACh).
A, membrane current was recorded from an atrial myocyte after 4 days in vitro. Holding potential in this and subsequent figures was −90 mV. ACh (2 μm) was repetitively applied for 5 s as indicated. Rapid deflections in this and most subsequent figures result from ramp-shaped changes in membrane potential from −120 to +60 mV within 500 ms to generate current–voltage relations. Zero current level in this and subsequent figures has been marked ‘0’. B, superimposed traces labelled 1 and 10 in A on an expanded time scale. The dotted line represents a least-square fit to the deactivation phase of trace 1 using a single exponential decay with a time constant of 4164 ms. C, current–voltage relations of ACh-activated currents (background-subtracted) labelled 1 and 10 in panel A. D, density of IK(ACh) is reduced with time in culture (top panel), whereas τd was not different in myocytes between 12 h after isolation (this group represents a period of time from about 10 h to 15 h after plating) and 7 days in culture. Each bar represents 12–14 different cells.
Deactivation kinetics of IK(ACh) is independent of the concentration of ACh
The kinetics of GIRK current deactivation upon switching to an agonist-free solution can be determined by diffusion of the ligand from its receptor, if unbinding is slow (Benians et al. 2003b). Alternatively for a ligand rapidly dissociating from its receptor, the deactivation time course is likely to be determined by re-association of Gβγ with Gα, which in turn is dictated by the GTPase activity of the Gα subunit (Benians et al. 2003b). In case of a fast (low affinity) ligand, therefore, τd should not be affected by the ligand concentration.
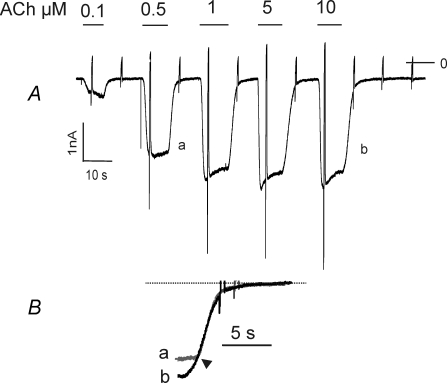
In Fig. 3A GIRK currents evoked by five different concentrations of ACh are shown. Whereas in line with previous studies (Bünemann et al. 1996), activation kinetics shows a strong dependence on the concentration of ACh, the time course of deactivation was not affected by the concentration of the agonist. This is demonstrated in more detail in Fig. 3B, showing superimposed deactivation phases of the currents evoked by 0.5 μm and 10 μm ACh. Apart from an increase in a lag phase after switching from 10 μm, a saturating concentration of ACh, to ACh-free solution, the traces are perfectly superimposable. This confirms that the time course of deactivation in this case is not determined by dissociation of the agonist from the receptor. This had been demonstrated for other GPCR–agonist combinations in cell lines heterologously expressing the respective receptor species and functional GIRK channels (Benians et al. 2003b)
Figure 3. Deactivation of IK(ACh) is independent of the concentration of ACh.
A, recording of membrane current from an atrial myocyte (2 days in vitro). ACh at various concentrations was applied as indicated. B, deactivation phases labelled a and b in A on expanded timescale. Traces were superimposed with regard to the point of time, when the exponential time course started, as indicated by the arrowhead.
Deactivation of IK(ACh) is slowed by β-adrenergic stimulation
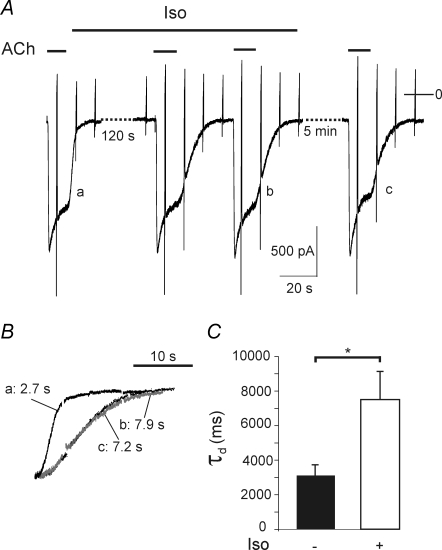
The β-adrenergic agonist isoprenaline (Iso), at a concentration (10 μm) that is highly effective in stimulating the Gs-coupled signalling pathway in the type of cell under study (Wellner-Kienitz et al. 2001), did not affect baseline current, nor did it affect the amplitude of the current activated by 2 μm ACh, as illustrated in Fig. 4A. However, Iso caused a marked increase in the time constant of deactivation upon washout of ACh (Fig. 4A–C). In the representative myocyte τd was increased from 2.7 s to 7.9 s. This effect of β-adrenergic stimulation was not reversible during the time course of an acute experiment (Fig. 4A and C). The mean increase in τd in this series of experiments was about 2.5-fold (Fig. 4C).
Figure 4. Slowing of deactivation by isoprenaline.
A, recording of membrane current from an atrial myocyte (4 days in vivo). ACh and Iso (10 μm) were applied as indicated. B, deactivation phases of current transients labelled a to c in A on expanded time scale. The figures indicate τd for each trace. C, summarized data (n = 11).
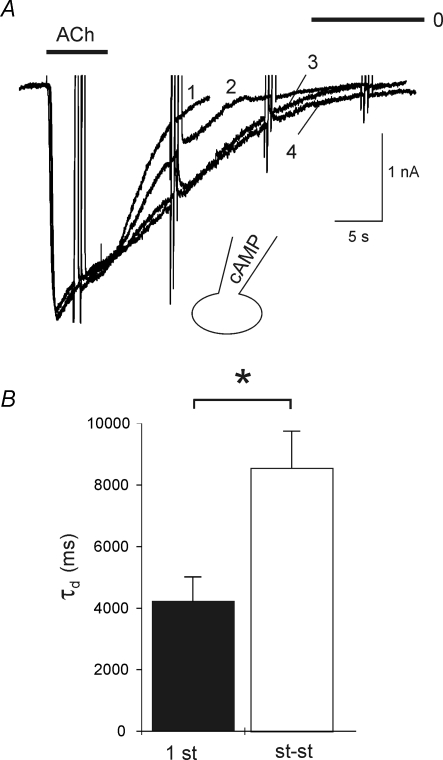
An increase in τd comparable to the effect of β-adrenergic stimulation was recorded when the pipette filling solution was supplemented with 3′-5′-cAMP (500 μm). Figure 5A shows superimposed ACh-induced currents recorded within 10 s of obtaining whole-cell mode (1) and at various times thereafter (2–4) As above, neither activation kinetics nor current amplitude were affected by loading of the cell with the cyclic nucleotide, whereas deactivation was substantially slowed. In this representative cell τd was increased from 4.2 s (first ACh exposure) to 11.8 s in the steady state, i.e. after ≥ 6 min of dialysing the cell with cAMP. On average, loading of cells with the cyclic nucleotide caused about a 2-fold significant increase in τd (Fig. 5B), analogous to the effect of β-adrenergic stimulation.
Figure 5. Slowing of deactivation by intracellular cAMP.
A, superimposed recordings of IK(ACh) induced by 10 μm ACh. The pipette filling solution was supplemented with 500 μm cAMP. The first current transient, labelled 1 was recorded at about 10 s after getting into the whole-cell mode. The traces labelled 2–4 were recorded about 2, 4 and 6 min later. B, summarized τd values from 7 measurements analogous to A. The labelling ‘1st’ refers to ACh-evoked current elicited with ≤ 10 s after rupturing the membrane patch, whereas st-st refers to steady-state, i.e. after ≥ 4 min of cAMP loading.
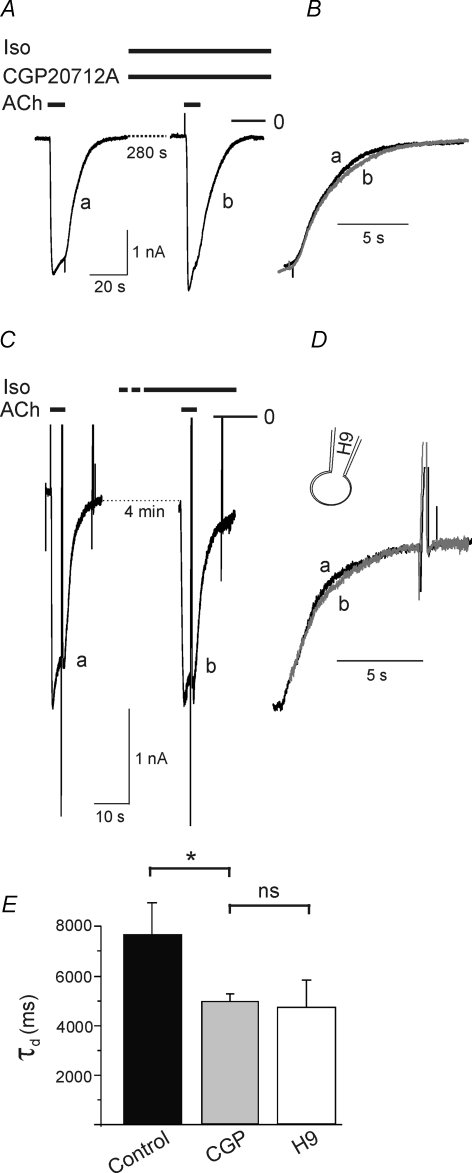
CGP-20112A, a β1-selective receptor antagonist, significantly reduced the Iso-induced increase in τd, while leaving this parameter unaffected in the control condition (Fig. 6A, B and E), suggesting that the Iso-induced prolongation results from selective stimulation of β1 receptors. The compound ICI118,551, a standard β2-selective antagonist, in line with previous observations, at 1 μm acts as an effective direct GIRK channel blocker (Wellner-Kienitz et al. 2001) and therefore was unsuitable for further experiments aiming to delineate the β-adrenergic receptor subtype. The effect of Iso was significantly antagonized by H9, an inhibitor of PKA, applied intracellularly at 100 μm via the pipette filling solution (Fig. 6C–E).
Figure 6. Isoprenaline-induced increase in τd is suppressed by CGP-20712A, a β1-selective antagonist and by H9, a PKA inhibitor.
A, IK(ACh) recordings in the presence of CGP (2 μm) without Iso and after about 5 min exposure to Iso. B, superimposed deactivation phases on expanded timescale demonstrate complete insensitivity to Iso. C, recordings of IK(ACh) in a myocyte loaded with H9 (100 μm) included in the pipette filling solution. D, superimposed deactivation phases on expanded timescale demonstrate insensitivity of τd to Iso. E, summarized data (τd in the presence of Iso (controls) n = 11; Iso plus H9, n = 8; Iso plus CGP n = 4).
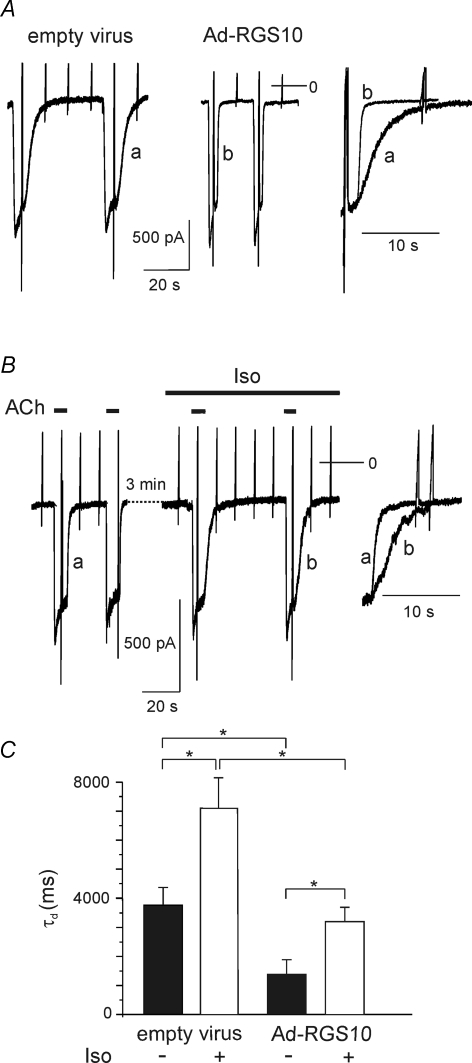
Since agonist dissociation from the receptor in the present condition is not limiting the rate of deactivation, a likely mechanism underlying the observed cAMP-sensitive slowing of deactivation is a reduction in GTPase activity of the Gα subunit(s) relevant to signalling via GIRK channels. A conceivable candidate for mediating this effect is one of the RGS proteins expressed in atrial myocytes (Kardestuncer et al. 1998; Doupnik et al. 2001). Phosphorylation by PKA has been described for RGS4, RGS14 and RGS10. Whereas PKA-dependent phosphorylation of the former two RGS species has been demonstrated to result in an increase in their GAP activity (Hollinger et al. 2003; Huang et al. 2007), phosphorylation of RGS10, when expressed in Xenopus oocytes, has been demonstrated to abolish its effect on GTPase activity of Gαi/o (Hunt et al. 1996; Burgon et al. 2001). To study if RGS10 contributes to the PKA-mediated effects described above, at first we overexpressed this protein using adenoviral gene transfer. As illustrated in Fig. 7, in myocytes infected with Ad-RGS10, basal τd was reduced by about 60% on average as compared to native or mock-infected myocytes (Fig. 7A and C). Exposure to Iso resulted in slowing of deactivation by about the same factor as in normal or mock-infected myocytes. The absolute value of τd under β-adrenergic stimulation, however, was significantly smaller than in mock-infected or native cells (Fig. 7B and C; cf. Fig. 2).
Figure 7. Reduction in τd by overexpression of RGS10.
A, comparison of ACh-evoked currents in myocytes infected either with empty virus (left) or Ad-RGS10 (middle). Currents were recorded from sister cultures on day 4 after infection. The right panel shows superimposed deactivation traces on expanded timescale. B, effect of Iso on IK(ACh) in a myocyte overexpressing RGS10 (day 5 in vitro). C, summarized data comparing τd in cells infected with empty virus or AdRGS10, respectively (n = 15).
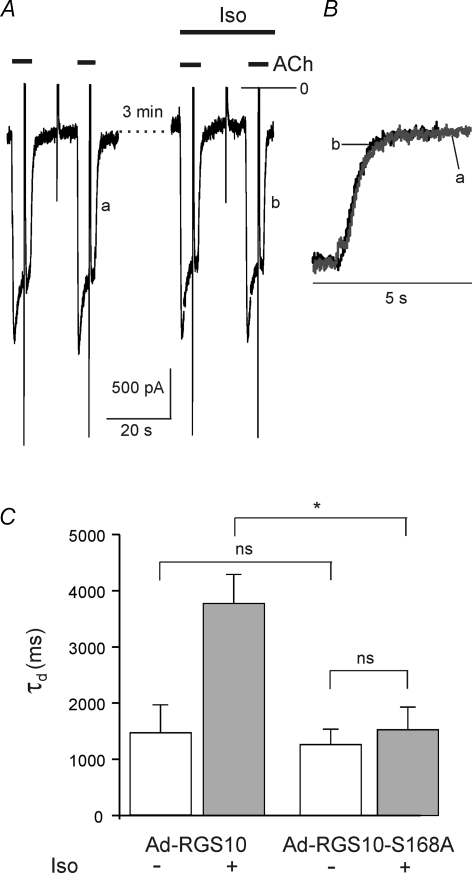
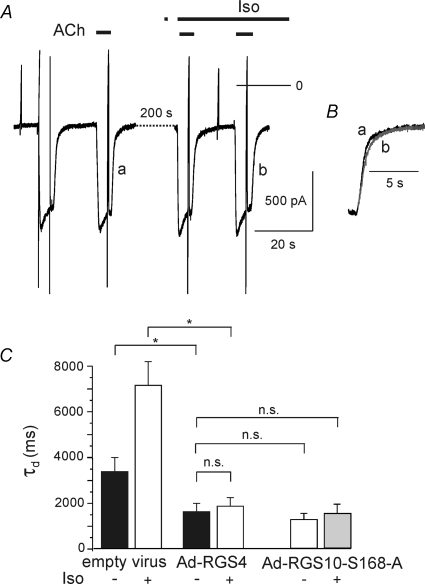
Human RGS10 is phosphorylated by PKA at Serine 168. Expression of a phosphorylation-deficient mutant (RGS10-S168A, Burgon et al. 2001) caused a comparable reduction in τd as overexpression of wild-type (wt) RGS10. However, β-adrenergic stimulation failed to cause an increase in τd, as illustrated in Fig. 8. These data clearly suggest that the slowing of GIRK current deactivation by β-adrenergic stimulation is mediated by phosphorylation of endogenous RGS10. In order to address the question of whether RGS10 is required for maintaining the basal deactivation properties, we used adenovirus-mediated RNA interference to reduce the expression of RGS10.
Figure 8. Expression of mutant RGS10-S168A.
A, ACh-induced currents recorded from an Ad-RGS10-S168A-infected myocyte. Superimposed deactivation phases (B) demonstrate complete insensitivity to Iso. C, summarized data comparing myocytes overexpressing wt RGS10 (same data as in Fig. 7) or expressing the PKA-insensitive mutant (n = 12)
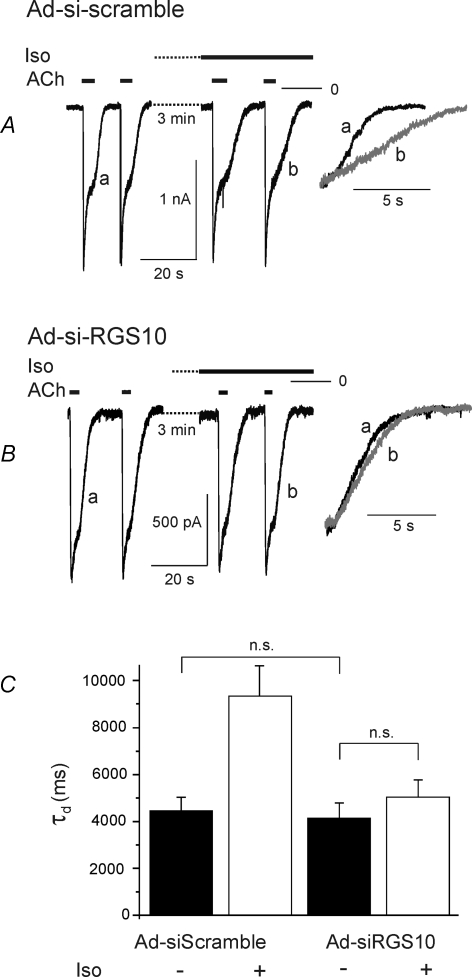
Surprisingly, in Ad-siRGS10-infected myocytes, basal τd was not significantly different from control (native) myocytes or myocytes exposed to virus encoding for a scrambled shRNA sequence (Fig. 9). However, in RGS10-silenced myocytes, τd was not significantly affected by β-adrenergic stimulation (Fig. 9B and C). Thus, despite a clear contribution of RGS10 to terminating Gi/o-mediated signals in atrial myocytes, it appears to be not essential for this process. This could be due to a promiscuous interaction of different RGS species expressed in atrial myocytes (Doupnik et al. 2001) with heterotrimeric G-proteins or preformed signalling complexes (Abramow-Newerly et al. 2006; Fowler et al. 2007), depending on their expression levels. We therefore overexpressed RGS4, as an example of another RGS species that is natively expressed in atrial myocytes (Zhang et al. 1998; Mittmann et al. 2002). Analogous to overexpression of RGS10, wild-type or S168A mutant, this resulted in a substantial reduction in τd by about 50% as compared to mock-infected myocytes (Fig. 10). As in cells expressing the mutant RGS10-S168A, β-adrenergic stimulation failed to affect τd in RGS4-overexpressing cells.
Figure 9. Sensitivity of τd to β-adrenergic stimulation is abolished by silencing RGS10.
A, representative recordings of ACh-induced currents from a myocyte infected with Ad-siScramble demonstrating normal increase in τd by Iso. B, current recordings from a representative myocyte infected with Ad-siRGS10 demonstrate a loss in sensitivity of τd to Iso. C, summarized data (n = 7 for scrambled sequence; n = 13 for Ad-siRGS10). Measurements were performed on day 7 after exposure to the adenoviral constructs.
Figure 10. Overexpression of RGS4.
A, current recording from a myocyte infected with Ad-RGS4: ACh and Iso were applied as indicated. B, superimposed deactivation phases of ACh-induced currents labelled a and b in A on expanded timescale. C, summarized data comparing τd in the absence and presence of Iso in mock-infected (empty virus) and Ad-RGS4-infected myocytes (n = 9). For comparison the bars representing the τd values from myocytes expressing the RGS10-S168A mutant (from Fig. 8) have been included.
Discussion
Kir3 channels represent the classical targets activated by binding of Gβγ subunits that are either released by dissociation or by a spatial rearrangement of the heterotrimeric complex upon stimulation of appropriate GPCRs (Dascal, 2001; Sadja et al. 2003; Bünemann et al. 2003). It is generally accepted that activation of atrial Kir3 channels at physiological expression levels of the endogenous set of GPCRs is limited to Gβγ released from G-protein trimeric complexes containing α subunits of the pertussis toxin-sensitive class (Gi/o) (Wellner-Kienitz et al. 2001, 2002). In the heart, activation of Kir3 channels contributes to vagally or metabolically induced physiological bradycardia and atrial action potential shortening (Wesley et al. 1986, Yamada, 2002). In the nervous system, activation of these channels represents a major mechanism of generating slow IPSPs (Koyrakh et al. 2005).
Apart from their physiological roles, Kir3 channel currents provide an online assay of free Gβγ in intact cells with temporal resolution only matched by measurements of protein–protein interactions in the pathway under study using optical methods such as dynamic FRET (fluorescence resonance energy transfer; Hein et al. 2005; Mintert et al. 2007). Because of this striking feature, GIRK currents have been frequently used as Gβγ sensors in studies investigating various aspects of GPCR–G-protein signalling including functions of RGS proteins (Saitoh et al. 1999; Keren-Raifman et al. 2001; Ishii et al. 2002; Fowler et al. 2007).
When Kir3 channels and appropriate GPCRs are reconstituted in Xenopus oocytes or in mammalian cell lines, the kinetics of onset and offset of current upon application and washout of a receptor agonist have been found to be much slower than in atrial myocytes or neurons. Co-expression of various RGS proteins resulted in activation and deactivation kinetics comparable to atrial myocytes (Doupnik et al. 1997; Saitoh et al. 1997). From these and related studies it became evident that endogenous atrial and neuronal GIRK currents and other targets of Gi/o and Gq proteins are under the control of RGS proteins (Zhang et al. 2005). Consequently, RGS proteins are considered as important modulators of cardiac frequency and force of contraction by virtue of their multiple roles in cellular signalling, and they are discussed as potential pharmacological targets for cardiovascular diseases (Wieland & Mittmann, 2003; Fu et al. 2006). Although it is commonly accepted that RGS proteins are important modulators of GPCR signalling, including regulation of Kir3 channels, the endogenous cardiac RGS protein(s) that contribute to regulating kinetics of GIRK currents are currently not known. This is mainly due to the fact that functional studies related to this issue in cardiac myocytes are sparse. There are, however, a few exceptions. An important role for RGS4 in regulating voltage-dependent relaxation of atrial GIRK currents has been identified and thoroughly investigated in cardiac myocytes (Ishii et al. 2002). These authors demonstrated that Ca2+–calmodulin facilitates GTPase activity of RGS4 by removing inhibition by PIP3 and thus decreases the available channel number on depolarization. On hyperpolarization, RGS4 is inhibited, resulting in an increase in Kir3 current (current relaxation). These studies and others (Benians et al. 2003a; Clancy et al. 2005; Fowler et al. 2007) suggest a close association of the signalling proteins in terms of pre-coupled ternary or higher order signalling complexes (GPCR, G-protein, RGS, Kir3 channel tetramer). However, this issue is not undisputed. Several authors who addressed this question using FRET measurements between different tagged proteins in the GPCR–Kir3 signalling pathway came to different conclusions (e.g. Hein et al. 2005; Fowler et al. 2007).
The major finding of the present study is a slowing of GIRK current deactivation in atrial myocytes by manoeuvres that stimulate PKA. The pharmacology of the β-adrenergic effect is in line with being mediated by the canonical β1-receptor–cAMP–PKA pathway. A potential PKA target in the system under study is the Kir3 channel complex itself or either of its subunits (Kim, 1990; Müllner et al. 2000, 2003). In these studies, either a direct activation of Kir3 channels by β-adrenergic stimulation or facilitatory effects on Kir3 channel activation by muscarinic receptors have been described. These β-adrenergic actions were interpreted as resulting from phosphorylation of Kir3 subunits (Müllner et al. 2000, 2003), or a protein yet to be identified. Our present data and previous work are in conflict with these publications (Wellner-Kienitz et al. 2001) in that we never detected any indication of a direct activation of atrial GIRK channels by stimulating the endogenous PKA pathway at physiological expression levels of β-adrenergic receptors. Only when either β1 or β2 receptors were overexpressed in atrial myocytes did isoprenaline cause activation of Kir3 current. This was, however, mediated by βγ subunits released from Gs or Gi/o, respectively, depending on the subtype of β-receptor (Wellner-Kienitz et al. 2001).
The experiments using (i) a phosphorylation-deficient mutant of RGS10, (ii) overexpression of wt RGS10 and (iii) silencing of RGS10 clearly support the notion that the β-adrenergic effects on deactivation of IK(ACh) are mediated via RGS10. RGS10 is a member of the D/R12 subfamily of RGS proteins. It has been shown to have GAP activity for Gαo, Gαi3 and Gαz (Hunt et al. 1996). It lacks an N-terminal ampiphatic helix that localizes other RGS species to the cell membrane. Membrane targeting of RGS10 has been shown to result from palmitoylation of a conserved cysteine within the RGS domain (Tu et al. 1999), which appears to be conditional for its GAP activity.
In the first functional study of RGS10 it was demonstrated that phosphorylation by PKA results in translocation from the plasma membrane and cytosol to the nucleus (Burgon et al. 2001). The GAP activity as such, measured in a cell-free system, was not affected by phosphorylation. Apart from its GAP activity on Gαo, Gαi3 and Gαz, it has been demonstrated to interfere with cAMP production by a G-protein-independent mechanism under certain conditions (Ghavami et al. 2004).
In conjunction with the present study, expression of RGS10 fused N- or C-terminally to GFP yielded divergent results as to localization (data not shown). Since in myocytes expressing these constructs the β-adrenergic effect on current deactivation was lost, we assume that the normal function of RGS was compromised in the fusion proteins.
Our results demonstrate substantial changes in deactivation kinetics of IK(ACh) without concomitant changes in activation kinetics or current amplitude, either by stimulation of the PKA pathway or by manipulating expression of functional RGS10. In previous studies on different RGS species in reconstituted systems using Kir3 current as readout, an increase in deactivation rate was usually parallelled by an increase in the rate of activation (e.g. Herlitze et al. 1999; Saitoh et al. 1999; Keren-Raifman et al. 2001). Since an increase in the off-rate alone should result in a reduction in steady state free Gβγ, this served as an explanation for the lack of effects on steady-state current previously described. There are, however, examples of isolated effects of RGS on deactivation without concomitant reduction in equilibrium amplitudes of GPCR-mediated signals. Benians et al. (2005) found that RGS8 accelerated activation of Kir3 currents via GABAB receptors, but not via A1 or M2 receptors, whereas deactivation was accelerated for all three GPCR species. In murine rod photoreceptors transgenic overexpression of a photoreceptor RGS complex (RGS9-1.Gbeta5L.R9AP) resulted in a considerable acceleration of deactivation of the light response, without an effect on rate of activation and peak or steady-state response (Krispel et al. 2006). These findings support the notion that RGS proteins are not just negative regulators of signalling via heterotrimeric G-proteins but contribute to regulation of signalling in the time domain in a more complex way. It is not understood at present, why acceleration of GTPase activity alone does not necessarily result in a reduction in peak or steady-state signalling.
In most types of cells including atrial myocytes, a number of different RGS proteins have been identified (Kardestuncer et al. 1998; Doupnik et al. 2001). Modulation of the GPCR–GIRK current pathway in atrial myocytes has been demonstrated for RGS4 (Ishii et al. 2002) and RGS10 (present study). Other RGS species expressed in atrial myocytes thus far have not been investigated with regard to this pathway. Our data suggest some promiscuity at least between RGS10 and RGS4. To understand the contributions of different RGS species endogenously expressed in myocytes to regulation of Kir3 channels or in more general terms to Gi/o signalling, further work on native myocytes is required.
Acknowledgments
We thank Anke Galhoff and Gabriele Reimus for unfailing technical assistance. This work was supported by the Deutsche Forschungsgemeinschaft (Po212/12-1) and the International Graduate School of Neuroscience NRW (M.T.)
References
- Abramow-Newerly M, Roy AA, Nunn C, Chidiac P. RGS proteins have a signalling complex: interactions between RGS proteins and GPCRs, effectors, and auxiliary proteins. Cell Signal. 2006;18:579–591. doi: 10.1016/j.cellsig.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Benians A, Leaney JL, Milligan G, Tinker A. The dynamics of formation and action of the ternary complex revealed in living cells using a G-protein-gated K+ channel as a biosensor. J Biol Chem. 2003a;278:10851–10858. doi: 10.1074/jbc.M212299200. [DOI] [PubMed] [Google Scholar]
- Benians A, Leaney JL, Tinker A. Agonist unbinding from receptor dictates the nature of deactivation kinetics of G protein-gated K+ channels. Proc Nat Acad Sci U S A. 2003b;100:6239–6244. doi: 10.1073/pnas.1037595100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benians A, Nobles M, Hosny S, Tinker A. Regulators of G-protein signalling form a quaternary complex with the agonist, receptor, and G-protein. A novel explanation for the acceleration of signalling activation kinetics. J Biol Chem. 2005;280:13383–13394. doi: 10.1074/jbc.M410163200. [DOI] [PubMed] [Google Scholar]
- Bünemann M, Brandts B, Pott L. Downregulation of muscarinic M2 receptors linked to K+ current in cultured guinea-pig atrial myocytes. J Physiol. 1996;492:351–362. doi: 10.1113/jphysiol.1996.sp021497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bünemann M, Frank M, Lohse MJ. Gi protein activation in intact cells involves subunit rearrangement rather than dissociation. Proc Natl Acad Sci U S A. 2003;100:16077–16082. doi: 10.1073/pnas.2536719100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgon PG, Lee WL, Nixon AB, Peralta EG, Casey PJ. Phosphorylation and nuclear translocation of a regulator of G protein signalling (RGS10) J Biol Chem. 2001;276:32828–32834. doi: 10.1074/jbc.M100960200. [DOI] [PubMed] [Google Scholar]
- Castanotto D, Li H, Rossi JJ. Functional siRNA expression from transfected PCR products. RNA. 2002;8:1454–1460. doi: 10.1017/s1355838202021362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy SM, Fowler CE, Finley M, Suen KF, Arrabit C, Berton F, Kosaza T, Casey PJ, Slesinger PA. Pertussis-toxin-sensitive Gα subunits selectively bind to C-terminal domain of neuronal GIRK channels: evidence for a heterotrimeric G-protein-channel complex. Mol Cell Neurosci. 2005;28:375–389. doi: 10.1016/j.mcn.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Dascal N. Ion-channel regulation by G proteins. Trends Endocrinol Metab. 2001;12:391–398. doi: 10.1016/s1043-2760(01)00475-1. [DOI] [PubMed] [Google Scholar]
- Doupnik CA, Davidson N, Lester HA, Kofuji P. RGS proteins reconstitute the rapid gating kinetics of Gβy-activated inwardly rectifying K+ channels. Proc Natl Acad Sci U S A. 1997;94:10461–10466. doi: 10.1073/pnas.94.19.10461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupnik CA, Xu T, Shinaman JM. Profile of RGS expression in single rat atrial myocytes. Biochim Biophys Acta. 2001;1522:97–107. doi: 10.1016/s0167-4781(01)00342-6. [DOI] [PubMed] [Google Scholar]
- Fowler CE, Aryal P, Suen KF, Slesinger PA. Evidence for association of GABAB receptors with Kir3 channels and regulators of G protein signalling (RGS4) proteins. J Physiol. 2007;580:51–65. doi: 10.1113/jphysiol.2006.123216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Huang X, Zhong H, Mortensen RM, D'Alecy LG, Neubig RR. Endogenous RGS proteins and Ga subtypes differentially control muscarinic and adenosine-mediated chronotropic effects. Circ Res. 2006;98:659–666. doi: 10.1161/01.RES.0000207497.50477.60. [DOI] [PubMed] [Google Scholar]
- Ghavami A, Hunt RA, Olsen MA, Zhang J, Smith DL, Kalgaonkar S, Rahman Z, Young KH. Differential effects of regulator of G protein signalling (RGS) proteins on serotonin 5-HT1A, 5-HT2A, and dopamine D2 receptor-mediated signalling and adenylyl cyclase activity. Cell Signal. 2004;16:711–721. doi: 10.1016/j.cellsig.2003.11.006. [DOI] [PubMed] [Google Scholar]
- He TC, Zhou SB, Da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein P, Frank M, Hoffmann C, Lohse MJ, Bünemann M. Dynamics of receptor/G protein coupling in living cells. EMBO J. 2005;24:4106–4114. doi: 10.1038/sj.emboj.7600870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlitze S, Ruppersberg JP, Mark MD. New roles for RGS2, 5 and 8 on the ratio-dependent modulation of recombinant GIRK channels expressed in Xenopus oocytes. J Physiol. 1999;517:341–352. doi: 10.1111/j.1469-7793.1999.0341t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho IH, Murrell-Lagnado RD. Molecular determinants for sodium-dependent activation of G protein-gated K+ channels. J Biol Chem. 1999;274:8639–8648. doi: 10.1074/jbc.274.13.8639. [DOI] [PubMed] [Google Scholar]
- Hollinger S, Hepler JR. Cellular regulation of RGS proteins: modulators and integrators of G protein signalling. Pharmacol Rev. 2002;54:527–559. doi: 10.1124/pr.54.3.527. [DOI] [PubMed] [Google Scholar]
- Hollinger S, Ramineni S, Hepler JR. Phosphorylation of RGS14 by protein kinase A potentiates its activity toward Gαi. Biochemistry. 2003;42:811–819. doi: 10.1021/bi026664y. [DOI] [PubMed] [Google Scholar]
- Huang J, Zhou H, Mahavadi S, Sriwai W, Murthy KS. Inhibition of Gαq-dependent PLC-β1 activity by PKG and PKA is mediated by phosphorylation of RGS4 and GRK2. Am J Physiol Cell Physiol. 2007;292:C200–C208. doi: 10.1152/ajpcell.00103.2006. [DOI] [PubMed] [Google Scholar]
- Hunt TW, Fields TA, Casey PJ, Peralta EG. RGS10 is a selective activator of Gαi GTPase activity. Nature. 1996;383:175–177. doi: 10.1038/383175a0. [DOI] [PubMed] [Google Scholar]
- Ishii M, Inanobe A, Kurachi Y. PIP3 inhibition of RGS protein and its reversal by Ca2+/calmodulin mediate voltage-dependent control of the G protein cycle in a cardiac K+ channel. Proc Natl Acad Sci U S A. 2002;99:4325–4330. doi: 10.1073/pnas.072073399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardestuncer T, Wu HG, Lim AL, Neer EJ. Cardiac myocytes express mRNA for ten RGS proteins: changes in RGS mRNA expression in ventricular myocytes and cultured atria. FEBS Lett. 1998;438:285–288. doi: 10.1016/s0014-5793(98)01319-2. [DOI] [PubMed] [Google Scholar]
- Keren-Raifman T, Bera AK, Zveig D, Peleg S, Witherow DS, Slepak VZ, Dascal N. Expression levels of RGS7 and RGS4 proteins determine the mode of regulation of the G protein-activated K+ channel and control regulation of RGS7 by Gβ5. FEBS Lett. 2001;492:20–28. doi: 10.1016/s0014-5793(01)02220-7. [DOI] [PubMed] [Google Scholar]
- Kim D. β-Adrenergic regulation of the muscarinic-gated K+ channel via cyclic AMP-dependent protein kinase in atrial cells. Circ Res. 1990;67:1292–1298. doi: 10.1161/01.res.67.5.1292. [DOI] [PubMed] [Google Scholar]
- Koyrakh L, Lujan R, Colon J, Karschin C, Kurachi Y, Karschin A, Wickman K. Molecular and cellular diversity of neuronal G-protein-gated potassium channels. J Neurosci. 2005;25:11468–11478. doi: 10.1523/JNEUROSCI.3484-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krispel CM, Chen D, Melling N, Chen YJ, Martemyanov KA, Quillinan N, Arshavsky VY, Wensel TG, Chen CK, Burns ME. RGS expression rate-limits recovery of rod photoresponses. Neuron. 2006;51:409–416. doi: 10.1016/j.neuron.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Mintert E, Bösche LI, Rinne A, Timpert M, Kienitz MC, Pott L, Bender K. Generation of a constitutive Na+-dependent inward-rectifier current in rat adult atrial myocytes by overexpression of Kir3.4. J Physiol. 2007;585:3–13. doi: 10.1113/jphysiol.2007.140772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittmann C, Chung CH, Hoppner G, Michalek C, Nose M, Schuler C, Schuh A, Eschenhagen T, Weil J, Pieske B, Hirt S, Wieland T. Expression of ten RGS proteins in human myocardium: functional characterization of an upregulation of RGS4 in heart failure. Cardiovasc Res. 2002;55:778–786. doi: 10.1016/s0008-6363(02)00459-5. [DOI] [PubMed] [Google Scholar]
- Müllner C, Vorobiov D, Bera AK, Uezono Y, Yakubovich D, Frohnwieser-Steinecker B, Dascal N, Schreibmayer W. Heterologous facilitation of G protein-activated K+ channels by β-adrenergic stimulation via cAMP-dependent protein kinase. J Gen Physiol. 2000;115:547–558. doi: 10.1085/jgp.115.5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müllner C, Yakubovich D, Dessauer CW, Platzer D, Schreibmayer W. Single channel analysis of the regulation of GIRK1/GIRK4 channels by protein phosphorylation. Biophys J. 2003;84:1399–1409. doi: 10.1016/S0006-3495(03)74954-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinne A, Littwitz C, Kienitz M-C, Gmerek A, Bösche LI, Pott L, Bender K. Gene silencing in adult rat cardiac myocytes in vitro by adenovirus-mediated RNA interference. J Muscle Res Cell Motil. 2006;27:413–421. doi: 10.1007/s10974-006-9087-0. [DOI] [PubMed] [Google Scholar]
- Sadja R, Alagem N, Reuveny E. Gating of GIRK channels: details of an intricate, membrane-delimited signalling complex. Neuron. 2003;39:9–12. doi: 10.1016/s0896-6273(03)00402-1. [DOI] [PubMed] [Google Scholar]
- Saitoh O, Kubo Y, Miyatani Y, Asano T, Nakata H. RGS8 accelerates G-protein-mediated modulation of K+ currents. Nature. 1997;390:525–529. doi: 10.1038/37385. [DOI] [PubMed] [Google Scholar]
- Saitoh O, Kubo Y, Odagiri M, Ichikawa M, Yamagata K, Sekine T. RGS7 and RGS8 differentially accelerate G protein-mediated modulation of K+ currents. J Biol Chem. 1999;274:9899–9904. doi: 10.1074/jbc.274.14.9899. [DOI] [PubMed] [Google Scholar]
- Sato M, Blumer JB, Simon V, Lanier SM. Accessory proteins for G proteins: partners in signalling. Annu Rev Pharmacol Toxicol. 2006;46:151–187. doi: 10.1146/annurev.pharmtox.46.120604.141115. [DOI] [PubMed] [Google Scholar]
- Siderovski DP, Willard FS. The GAPs, GEFs, and GDIs of heterotrimeric G-protein alpha subunits. Int J Biol Sci. 2005;1:51–66. doi: 10.7150/ijbs.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield PR, Nakajima S, Nakajima Y. Constitutively active and G-protein coupled inward rectifier K+ channels: Kir2.0 and Kir3.0. Rev Physiol Biochem Pharmacol. 2002;145:47–179. doi: 10.1007/BFb0116431. [DOI] [PubMed] [Google Scholar]
- Tu Y, Popov S, Slaughter C, Ross EM. Palmitoylation of a conserved cysteine in the regulator of G protein signalling (RGS) domain modulates the GTPase-activating activity of RGS4 and RGS10. J Biol Chem. 1999;274:38260–38267. doi: 10.1074/jbc.274.53.38260. [DOI] [PubMed] [Google Scholar]
- Wellner-Kienitz M-C, Bender K, Meyer T, Bünemann M, Pott L. Overexpressed A1 adenosine receptors reduce activation of IK(ACh) by native muscarinic M2 receptors in rat atrial myocytes. Circ Res. 2000;86:643–648. doi: 10.1161/01.res.86.6.643. [DOI] [PubMed] [Google Scholar]
- Wellner-Kienitz M-C, Bender K, Meyer T, Pott L. Coupling to Gs and Gq/11 of histamine H2 receptors heterologously expressed in adult rat atrial myocytes. Biochim Biophys Acta. 2002;1642:67–77. doi: 10.1016/s0167-4889(03)00101-0. [DOI] [PubMed] [Google Scholar]
- Wellner-Kienitz MC, Bender K, Pott L. Overexpression of β1 and β2 adrenergic receptors in rat atrial myocytes. Differential coupling to G protein-gated inward rectifier K+ channels via Gs and Gi/o. J Biol Chem. 2001;276:37347–37354. doi: 10.1074/jbc.M106234200. [DOI] [PubMed] [Google Scholar]
- Wesley RC, Jr, Boykin MT, Belardinelli L. Role of adenosine as mediator of bradyarrhythmias during hypoxia in isolated guinea pig hearts. Cardiovasc Res. 1986;20:752–759. doi: 10.1093/cvr/20.10.752. [DOI] [PubMed] [Google Scholar]
- Wieland T, Mittmann C. Regulators of G-protein signalling: multifunctional proteins with impact on signalling in the cardiovascular system. Pharmacol Ther. 2003;97:95–115. doi: 10.1016/s0163-7258(02)00326-1. [DOI] [PubMed] [Google Scholar]
- Yamada M. The role of muscarinic K+ channels in the negative chronotropic effect of a muscarinic agonist. J Pharmacol Exp Ther. 2002;300:681–687. doi: 10.1124/jpet.300.2.681. [DOI] [PubMed] [Google Scholar]
- Zhang W, Anger T, Su J, Hao J, Xu X, Zhu M, Gach A, Cui L, Liao R, Mende U. Selective loss of fine-tuning of Gq/11 signalling by RGS2 protein exacerbates cardiomyocyte hypertrophy. J Biol Chem. 2005;281:5811–5820. doi: 10.1074/jbc.M507871200. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Pacheco MA, Doupnik CA. Gating properties of GIRK channels activated by Gαo- and Gαi-coupled muscarinic m2 receptors in Xenopus oocytes: the role of receptor precoupling in RGS modulation. J Physiol. 2002;545:355–373. doi: 10.1113/jphysiol.2002.032151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Watson N, Zahner J, Rottmann JN, Blumer KJ, Muslin AJ. RGS3 and RGS4 are GTPase activating proteins in the heart. J Mol Cell Cardiol. 1998;30:269–276. doi: 10.1006/jmcc.1997.0591. [DOI] [PubMed] [Google Scholar]