Abstract
The ‘distributed chemoreception theory’ attributes the central chemoreflex (the stimulation of breathing by CNS acidification) to the cumulative effects of pH on multiple classes of respiratory neurons as well as on their tonic sources of drive. Opinions differ as to how many classes of pH-sensitive neurons contribute to the central chemoreflex but the number of candidates is high and growing fast. The ‘specialized chemoreceptor theory’, endorsed here, attributes the chemoreflex to a limited number of specialized neurons. These neurons (the central chemoreceptors) would drive a respiratory pattern generator that is not or minimally activated by acidification. In this review we first describe the properties of the retrotrapezoid nucleus (RTN) and argue that this nucleus may contain the most important central chemoreceptors. Next, we subject the assumptions that underlie the distributed chemoreception theory to a critical analysis. We propose several explanations for the apparent contradiction between the two competing theories of central chemoreception. We attribute much of the current controversy to premature extrapolations of the effects of acidification on neurons recorded in vitro (chemosensitivity) and to a semantic confusion between chemosensitivity and chemoreception (the mechanism by which CO2 or pH activates breathing in vivo).
Central chemoreception
Brain 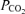 is almost certainly detected via changes in pH (the ‘reaction theory’) (Loeschcke, 1982), but major questions remain as to how pH is detected, where pH is detected and how brain acidification stimulates breathing (central respiratory chemoreception). Most authors currently believe that central respiratory chemoreception relies on many types of pH-sensitive neurons, a view that we call here the ‘distributed chemoreception theory’. Opinions differ as to how many classes of neurons are involved but the number of candidates is at present very high and growing ever larger (Kawai et al. 1996, 2006; Solomon et al. 2000; Bayliss et al. 2001; Richerson, 2004; Putnam et al. 2004; Su et al. 2007; Williams et al. 2007). Proponents of the distributed chemoreception theory also postulate that central chemoreception is due to many types of pH-sensitive channels or receptors (Putnam et al. 2004; Jiang et al. 2005; Duprat et al. 2007; Su et al. 2007). In the final analysis, the distributed chemoreception theory is based on three classes of observations. First, in vitro or in cell culture, a very high proportion of neurons (30–60%) respond to acidification to some degree (Putnam et al. 2004). This statement applies to the entire brain, not just the brainstem. Second, many channels, when studied in vitro, typically in heterologous expression systems but also in their native state in neurons, display some pH sensitivity (Xu et al. 2000; Jiang et al. 2001; O'Connell et al. 2002; Su et al. 2007). Third, if one perfuses brainstem regions known to regulate breathing with CO2-enriched saline (caudal nucleus of the solitary tract (NTS), ventrolateral medulla, raphe, retrotrapezoid nucleus (RTN), fastigial nucleus), some degree of respiratory stimulation is usually produced (Nattie & Li, 2002b, 2006a; Feldman et al. 2003; Nattie, 2006).
is almost certainly detected via changes in pH (the ‘reaction theory’) (Loeschcke, 1982), but major questions remain as to how pH is detected, where pH is detected and how brain acidification stimulates breathing (central respiratory chemoreception). Most authors currently believe that central respiratory chemoreception relies on many types of pH-sensitive neurons, a view that we call here the ‘distributed chemoreception theory’. Opinions differ as to how many classes of neurons are involved but the number of candidates is at present very high and growing ever larger (Kawai et al. 1996, 2006; Solomon et al. 2000; Bayliss et al. 2001; Richerson, 2004; Putnam et al. 2004; Su et al. 2007; Williams et al. 2007). Proponents of the distributed chemoreception theory also postulate that central chemoreception is due to many types of pH-sensitive channels or receptors (Putnam et al. 2004; Jiang et al. 2005; Duprat et al. 2007; Su et al. 2007). In the final analysis, the distributed chemoreception theory is based on three classes of observations. First, in vitro or in cell culture, a very high proportion of neurons (30–60%) respond to acidification to some degree (Putnam et al. 2004). This statement applies to the entire brain, not just the brainstem. Second, many channels, when studied in vitro, typically in heterologous expression systems but also in their native state in neurons, display some pH sensitivity (Xu et al. 2000; Jiang et al. 2001; O'Connell et al. 2002; Su et al. 2007). Third, if one perfuses brainstem regions known to regulate breathing with CO2-enriched saline (caudal nucleus of the solitary tract (NTS), ventrolateral medulla, raphe, retrotrapezoid nucleus (RTN), fastigial nucleus), some degree of respiratory stimulation is usually produced (Nattie & Li, 2002b, 2006a; Feldman et al. 2003; Nattie, 2006).
The second theory, henceforth called the ‘specialized chemoreceptor theory’, is the oldest and the one favoured in this review. According to this theory, in its intact state in vivo, the central respiratory pattern generator (CPG; the network responsible for the rate and relative timing of the contractions of respiratory muscles) is not at all or only weakly pH responsive. The central chemoreflex is due to specialized acid-sensitive neurons (the central chemoreceptors) that are distinct from the CPG and drive this network synaptically (Loeschcke, 1982). From the outset, one should note that this theory does not require that the neurons that make up the CPG be completely insensitive to pH. The theory only implies that the pH sensitivity of these neurons does not translate into a physiologically relevant activation of the breathing network by CO2 and acidification.
The retrotrapezoid nucleus and central chemoreception
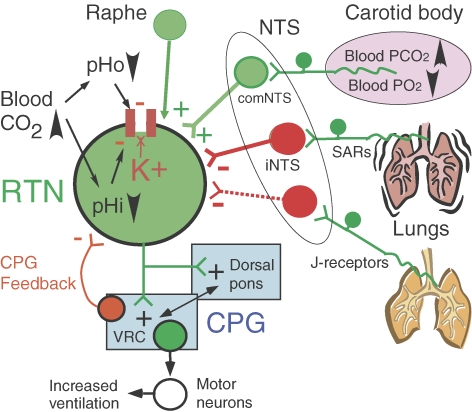
The physiological, cellular and genetic evidence in support of the notion that RTN neurons are important central chemoreceptors is briefly presented below along with the principal remaining uncertainties. Figure 1 is a schematic illustration of the known properties and inputs of these neurons.
Figure 1. RTN and a few of its connections.
CPG, central respiratory pattern generator; NTS, nucleus of the solitary tract; iNTS, interstitial subnucleus; comNTS, commissural subnucleus; SARs, slowly adapting lung stretch receptors; VRC, ventral respiratory column. Acidification activates RTN neurons by reducing an unidentified potassium conductance. There is no evidence that this potassium channel is directly proton sensitive. Whether protons act intra or extracellularly is also unknown, and therefore both possibilities are represented. RTN neurons receive an oligosynaptic excitatory input from the carotid body. It is important to realize that these neurons are not just chemoreceptors. RTN neurons also receive inhibitory inputs from the CPG and from several types of pulmonary receptors. These inhibitory inputs are tentatively interpreted as feedbacks that limit the activity of the CPG under conditions when this network is already vigorously activated by sources of drive other than of chemosensory origin. RTN neurons are activated by brainstem serotonergic neurons that release serotonin, thyrotropin-releasing hormone (TRH) and substance P. These inputs presumably contribute to the ability of serotonergic neurons to stimulate breathing and to facilitate the central chemoreflex. Glutamatergic neurons are in green, inhibitory neurons (GABA or glycine) are in red. The CPG consists of vast numbers of interconnected neurons located primarily within the VRC and the dorsal pons.
RTN neurons are located at the ventral medullary surface in a region suspected since the 1960s to mediate central chemoreception (area M of Loeschcke and Mitchell; Loeschcke, 1982). RTN neurons are only mildly respiratory modulated, their activation by pH is robust and occurs within a physiologically relevant range, they are about equally sensitive to pH in vivo and in vitro and their CO2 sensitivity in vivo persists after pharmacological blockade of the CPG (Mulkey et al. 2004; Guyenet et al. 2005). RTN neurons are glutamatergic and they innervate selectively the pontomedullary regions that contain the central respiratory pattern generator, CPG (Mulkey et al. 2004; Guyenet et al. 2005; Rosin et al. 2006; Takakura et al. 2006; Moreira et al. 2007). Their acid sensitivity in vitro is due to the inhibition of a resting potassium conductance that is not a TASK channel (O'Connell et al. 2002; Mulkey et al. 2004; Mulkey et al. 2007b). Since the responsible channel is unidentified, there is no evidence that RTN neuron chemosensitivity and, by extension, central chemoreception are driven by a channel that is directly sensitive to hydrogen ions. Likewise, local paracrine mechanisms have not yet been ruled out as potential factors contributing to the pH sensitivity of RTN neurons although the theory that ATP or UTP mediates the chemosensitivity of RTN could not be confirmed (Gourine et al. 2005; Mulkey et al. 2006).
Inhibition or lesion of the RTN region suppresses the phrenic outflow (Onimaru & Homma, 2003; Takakura et al. 2006). Acidification of the RTN region stimulates breathing and chronic lesions of RTN reduce the central chemoreflex (Nattie & Li, 2002a, 2006b; Feldman et al. 2003; Nattie, 2003). In intact animals, these manipulations are usually not associated with notable disturbances of the respiratory rhythm, consistent with the interpretation that the RTN drives the CPG but is not part of this network. Other interpretations of the role of the RTN have been proposed, including a rhythmogenic role during the early postnatal period (Feldman & Del Negro, 2006). These theories will not be covered in this short review.
RTN neurons express Phox2b, a transcription factor whose mutation is responsible for the congenital central hypoventilation syndrome, CCHS (Amiel et al. 2003; Stornetta et al. 2006). In mice, the prenatal development of RTN neurons is uniquely sensitive to a mutation that causes CCHS in man, a seven-alanine expansion of the normal twenty-residue poly alanine tract (Dubreuil et al. 2008). These mice (Phox2b27Ala/+) have an 85% reduction of RTN neurons, apparently no anatomical defects elsewhere in the brainstem and, like CCHS patients, they have a virtually complete loss of the central chemoreflex (Spengler et al. 2001; Dubreuil et al. 2008). These mice cannot survive without artificial ventilation suggesting that breathing and chemoreception are both entirely dependent on the integrity of RTN neurons at birth. Chronic brain lesions encompassing parts of the RTN were found to be survivable and to produce relatively limited breathing impairments in unanaesthetized adult rats, but these lesions are likely to have spared a high percentage of the RTN chemoreceptors because the authors did not have at their disposal a marker to identify the targeted neurons (Nattie & Li, 2002a; Nattie & Li, 2006b). Accordingly, the respiratory consequences of a massive (> 90%) impairment of RTN neurons in the conscious adult rat are still unknown.
The only definitive way to demonstrate that RTN neurons are the only central chemoreceptors would be to implement an approach that can selectively disrupt the pH sensing mechanism of these neurons without destroying or inactivating them in the process. In the absence of such evidence, it is premature to assert that RTN neurons are the only cells responsible for the effect of brain  on breathing, but this possibility appears increasingly plausible.
on breathing, but this possibility appears increasingly plausible.
Central CO2 sensitivity: why so many sensing molecules?
This rhetorical question with teleological undertones is borrowed from a recent paper by Jiang et al. (2005). It reflects the very common opinion that central chemoreception derives from the activity of multiple and already identified pH-sensitive molecules that are expressed by vast numbers of neurons. In our view, this interpretation requires at least five assumptions for which supportive evidence is modest at best.
The first (implied) assumption is that the pH sensors are channels (inwardly rectifying potassium channels, TASK channels, etc.) (Jiang et al. 2005). This assumption is eminently plausible but the fact has not been established, not even in the case of RTN neurons as noted above. In theory, acid-sensitive G protein-coupled receptors (Murakami et al. 2004) could also serve as pH sensors.
The second assumption is that central chemoreception in vivo is mediated by a large number of pH-sensitive channels. In the absence of proof that any channel is the physiological pH sensor of RTN neurons, this extrapolation is even less warranted.
The third assumption is that any channel that is pH sensitive in vitro (e.g. in slices, neuronal cell culture, heterologous expression system) is equally pH sensitive in the intact brain in vivo. If this admittedly logical assumption is correct, why is it that TASK channels, which are so commonly expressed by brainstem neurons, especially by motoneurons, make no contribution to central chemoreception (Mulkey et al. 2007b)? One possibility is that there may be some mechanism (e.g. glial cells or extracellular matrix) that protects pH-sensitive ion channels such as TASK channels from being titrated in vivo by blood-derived changes in pH. This possibility could explain why serotonergic neurons, whose pH sensitivity in vitro relies on TASK channels, do not react to CO2 under anaesthesia in vivo, as they should if they were pH sensors (Mulkey et al. 2004, 2007b; Richerson, 2004). Earlier investigators have speculated at length over the possible significance of the superficial location of the chemoreceptors. Some early hypotheses included a required proximity to penetrating arteries or the need for the chemosensitive neurons to be surrounded by a special type of glia (the marginal glia) (for discussion and references see Loeschcke, 1982). As we showed recently, the dendrites of RTN chemoreceptors have a very unusual propensity to invade the marginal layer of the ventral medulla (Mulkey et al. 2004). Unlike CPG neurons, whose dendrites often reach the ventral surface but stop there, more than half of the total dendritic length of RTN neurons may be found in the marginal layer. This RTN region conceivably provides the extracellular environment required for a proton sensor to be freely titrated by changes in blood pH or  . The proton sensor of RTN neurons is not TASK (Mulkey et al. 2007b), but it does not have to be an esoteric molecule. It could be a fairly common pH-sensitive channel that can be titrated only in the RTN in the intact brain in vivo.
. The proton sensor of RTN neurons is not TASK (Mulkey et al. 2007b), but it does not have to be an esoteric molecule. It could be a fairly common pH-sensitive channel that can be titrated only in the RTN in the intact brain in vivo.
The fourth assumption is that, if acidification changes the spontaneous activity of a neuron in vitro, it is capable of doing so in vivo. This assumption has not been verified in the already mentioned specific case of the serotonergic neurons. Although these cells are pH sensitive in culture and, to lesser extent, in slices (Severson et al. 2003; Richerson et al. 2005), they do not respond to CO2 under anaesthesia in vivo (Mulkey et al. 2004). One possible explanation has already been evoked. Another, perhaps more plausible, explanation could be that the relative contribution of pH-sensitive channels to overall whole cell conductance is diminished in vivo, where increased conductance due to synaptic inputs or neurohumoral influences can be expected. Whatever the explanation, the difference between the pH sensitivity of serotonergic neurons in vivo and in vitro shows that neuronal pH sensitivity in vitro does not provide evidence of direct sensitivity to CO2in vivo.
The final assumption is that, if the CPG neurons are individually chemosensitive, the cumulative effects of pH on all these cells should cause a robust activation of the network of which they are a part. The experimental evidence is mixed and difficult to interpret. In cell cultures of brainstem, a network effect causes some amplification of the response of individual neurons to acidification (Su et al. 2007). In the breathing slice, a preparation that does not contain the RTN, acidification increases the rate of the respiratory-like motor output but it does not change its intensity (e.g. Lorier et al. 2004), which is the principal mechanism by which central chemoreceptors control alveolar ventilation. Amplitude modulation is also absent in the neonatal brainstem-spinal cord preparation despite the fact that inspiratory neurons are uniformly depolarized and expiratory neurons are hyperpolarized by acidification (Kawai et al. 1996). The interpretations of the effects of pH in these preparations are subject to assumption number 3, namely that the channels which are titrated by acid in vitro are also titrated in the intact brain. Secondly, some of these preparations contain random networks (Su et al. 2007) or very reduced versions of the respiratory network (breathing slices and Suzue preparation) that may react to pH in unpredictable ways due to the absence of many of the interactions that normally exist between CPG neurons in vivo.
Central CO2 sensitivity: why so many cells?
Many brain regions besides the RTN have been proposed to contain chemosensitive neurons that could regulate breathing (NTS, raphe, ventrolateral medulla, hypothalamic orexinergic neurons) (Feldman et al. 2003; Duprat et al. 2007). As mentioned previously, some authors have even argued that virtually every respiratory neuron is chemosensitive (Kawai et al. 1996) and evidence of widespread neuronal pH sensitivity in vitro is accruing with clockwork regularity (Kawai et al. 2006; Duprat et al. 2007). The evidence supporting these alternative chemoreceptors is much more limited than in the case of the RTN neurons. It is based on two classes of observations: the presence throughout the brainstem of neurons that respond to acid in vitro, and the fact that perfusion of many brainstem areas (NTS, raphe, ventrolateral medulla) with CO2-enriched saline activates respiration to some degree (Li & Nattie, 2002; Nattie & Li, 2002b; Feldman et al. 2003; Hodges et al. 2004; Nattie, 2006).
As discussed above, the first criterion is too broad and this ‘inconvenient truth’ must be confronted. Acid sensitivity in vitro is undoubtedly a necessary attribute of central chemoreceptors but this property is not predictive of acid sensitivity in vivo, which is an important and necessary criterion for a central chemoreceptor. Furthermore, even if chemosensitivity in vitro were predictive of a response to CO2in vivo, the evidence would not mean that the cell in question plays a role in central chemoreception unless its functional connections with the breathing network were demonstrated. This view has been illustrated above by the case of the serotonergic neurons. Our results on TASK KO mice (Mulkey et al. 2007b) and those of Williams et al. (2007) on orexinergic neurons in mice show that the widespread pH sensitivity of neurons in vitro is frequently caused by TASK channels. Yet, despite the fact that TASK channels are abundant in cardiorespiratory neurons throughout the brainstem (Washburn et al. 2003), our results also show that these channels have very little to do with the respiratory consequences of raising CNS  in vivo. The undeniable effects of the raphe on the central chemoreflex (Hodges et al. 2004; Li et al. 2006; Dias et al. 2007) may well be due to the excitatory effects of the serotonergic neurons on RTN cells and the CPG rather than to the ability of these cells to detect changes in
in vivo. The undeniable effects of the raphe on the central chemoreflex (Hodges et al. 2004; Li et al. 2006; Dias et al. 2007) may well be due to the excitatory effects of the serotonergic neurons on RTN cells and the CPG rather than to the ability of these cells to detect changes in 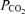 in vivo (Mulkey et al. 2007a). Other opinions have been expressed (Richerson et al. 2005).
in vivo (Mulkey et al. 2007a). Other opinions have been expressed (Richerson et al. 2005).
The second line of evidence is more persuasive but it is likewise not definitive. The reason is that perfusion of brain nuclei with acid solutions requires cannulas that produce local disruption of the neuronal environment, even if microdialysis membranes are used. This technology may therefore suffer from some of the same problems as in vitro experiments, i.e. false positive results caused by disruption of the neuronal environment that may protect channels like TASK from being titrated by small pH changes.
In conclusion, our objections to the existence of central chemoreceptors outside RTN clearly do not rule out such receptors. They should be viewed as a call for more evidence, especially of a cellular nature.
Summary and conclusions
The RTN contains over 2000 glutamatergic neurons that are activated by acidification via reductions in potassium conductance. The anatomical and physiological properties of these neurons are consistent with those expected from specialized central chemoreceptors. The relative importance of RTN neurons versus other central chemoreceptors is not definitively established, but recent results strongly suggest that the RTN could be the main site of central chemoreception. The chemosensitivity of RTN neurons is not explained in molecular terms and this remains one of the most interesting questions to solve. Yet, the principal key to the role of RTN neurons in central chemoreception probably resides in the still unexplored details of their anatomical connections and in the type of synapses that these cells form with the CPG neurons.
Acknowledgments
This work was supported by National Institutes of Health grants HL28785 and HL074011 to P.G.G.
References
- Amiel J, Laudier B, Attie-Bitach T, de Trang HPL, Gener B, Trochet D, Etchevers H, Ray P, Simonneau M, Vekemans M, Munnich A, Gaultier C, Lyonnet S. Polyalanine expansion and frameshift mutations of the paired-like homeobox gene PHOX2B in congenital central hypoventilation syndrome. Nat Genet. 2003;33:459–461. doi: 10.1038/ng1130. [DOI] [PubMed] [Google Scholar]
- Bayliss DA, Talley EM, Sirois JE, Lei QB. TASK-1 is a highly modulated pH-sensitive ‘leak’ K+ channel expressed in brainstem respiratory neurons. Resp Physiol. 2001;129:159–174. doi: 10.1016/s0034-5687(01)00288-2. [DOI] [PubMed] [Google Scholar]
- Dias MB, Nucci TB, Margatho LO, Antunes-Rodrigues J, Gargaglioni LH, Branco LGS. Raphe magnus nucleus is involved in ventilatory but not hypothermic response to CO2. J Appl Physiol. 2007;103:1780–1788. doi: 10.1152/japplphysiol.00424.2007. [DOI] [PubMed] [Google Scholar]
- Dubreuil V, Ramanantsoa N, Trochet D, Vaubourg V, Amiel J, Gallego J, Brunet JF, Goridis C. A human mutation in Phox2b causes lack of CO2 chemosensitivity, fatal central apnoea and specific loss of parafacial neurons. Proc Natl Acad Sci U S A. 2008;105:1067–1072. doi: 10.1073/pnas.0709115105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprat F, Lauritzen I, Patel A, Honore E. The TASK background K2P channels: chemo- and nutrient sensors. Trends Neurosci. 2007;30:573–580. doi: 10.1016/j.tins.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci. 2006;7:232–242. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: Rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Llaudet E, Dale N, Spyer KM. ATP is a mediator of chemosensory transduction in the central nervous system. Nature. 2005;436:108–111. doi: 10.1038/nature03690. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Mulkey DK, Stornetta RL, Bayliss DA. Regulation of ventral surface chemoreceptors by the central respiratory pattern generator. J Neurosci. 2005;25:8938–8947. doi: 10.1523/JNEUROSCI.2415-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Martino P, Davis S, Opansky C, Pan LG, Forster HV. Effects on breathing of focal acidosis at multiple medullary raphe sites in awake goats. J Appl Physiol. 2004;97:2303–2309. doi: 10.1152/japplphysiol.00645.2004. [DOI] [PubMed] [Google Scholar]
- Jiang C, Rojas A, Wang R, Wang X. CO2 central chemosensitivity: why are there so many sensing molecules? Respir Physiol Neurobiol. 2005;145:115–126. doi: 10.1016/j.resp.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Jiang C, Xu HX, Cui NR, Wu JP. An alternative approach to the identification of respiratory central chemoreceptors in the brainstem. Respir Physiol. 2001;129:141–157. doi: 10.1016/s0034-5687(01)00301-2. [DOI] [PubMed] [Google Scholar]
- Kawai A, Ballantyne D, Muckenhoff K, Scheid P. Chemosensitive medullary neurones in the brainstem–spinal cord preparation of the neonatal rat. J Physiol. 1996;492:277–292. doi: 10.1113/jphysiol.1996.sp021308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai A, Onimaru H, Homma I. Mechanisms of CO2/H+ chemoreception by respiratory rhythm generator neurons in the medulla from newborn rats in vitro. J Physiol. 2006;572:525–537. doi: 10.1113/jphysiol.2005.102533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Nattie E. CO2 dialysis in one chemoreceptor site, the RTN: stimulus intensity and sensitivity in the awake rat. Respir Physiol Neurobiol. 2002;133:11–22. doi: 10.1016/s1569-9048(02)00134-9. [DOI] [PubMed] [Google Scholar]
- Li A, Zhou S, Nattie E. Simultaneous inhibition of caudal medullary raphe and retrotrapezoid nucleus decreases breathing and the CO2 response in conscious rats. J Physiol. 2006;577:307–318. doi: 10.1113/jphysiol.2006.114504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeschcke HH. Central chemosensitivity and the reaction theory. J Physiol. 1982;332:1–24. doi: 10.1113/jphysiol.1982.sp014397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorier AR, Peebles K, Brosenitsch T, Robinson DM, Housley GD, Funk GD. P2 receptors modulate respiratory rhythm but do not contribute to central CO2 sensitivity in vitro. Respir Physiol Neurobiol. 2004;142:27–42. doi: 10.1016/j.resp.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Moreira TS, Takakura AC, Colombari E, West GH, Guyenet PG. Inhibitory input from slowly adapting lung stretch receptors to retrotrapezoid nucleus chemoreceptors. J Physiol. 2007;580:285–300. doi: 10.1113/jphysiol.2006.125336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Mistry AM, Guyenet PG, Bayliss DA. Purinergic P2 receptors modulate excitability but do not mediate pH sensitivity of RTN respiratory chemoreceptors. J Neurosci. 2006;26:7230–7233. doi: 10.1523/JNEUROSCI.1696-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Rosin DL, West G, Takakura AC, Moreira TS, Bayliss DA, Guyenet PG. Serotonergic neurons activate chemosensitive retrotrapezoid nucleus neurons by a pH-independent mechanism. J Neurosci. 2007a;27:14128–14138. doi: 10.1523/JNEUROSCI.4167-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci. 2004;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Talley EM, Stornetta RL, Siegel AR, West GH, Chen X, Sen N, Mistry AM, Guyenet PG, Bayliss DA. TASK channels determine pH sensitivity in select respiratory neurons but do not contribute to central respiratory chemosensitivity. J Neurosci. 2007b;27:14049–14058. doi: 10.1523/JNEUROSCI.4254-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami N, Yokomizo T, Okuno T, Shimizu T. G2A is a proton-sensing G-protein-coupled receptor antagonized by lysophosphatidylcholine. J Biol Chem. 2004;279:42484–42491. doi: 10.1074/jbc.M406561200. [DOI] [PubMed] [Google Scholar]
- Nattie E. The cartography of breathing. J Physiol. 2003;548:665. doi: 10.1113/jphysiol.2003.042515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie E. Why do we have both peripheral and central chemoreceptors? J Appl Physiol. 2006;100:9–10. doi: 10.1152/japplphysiol.01097.2005. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A. Substance P–saporin lesion of neurons with NK1 receptors in one chemoreceptor site in rats decreases ventilation and chemosensitivity. J Physiol. 2002a;544:603–616. doi: 10.1113/jphysiol.2002.020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie EE, Li AH. CO2 dialysis in nucleus tractus solitarius region of rat increases ventilation in sleep and wakefulness. J Appl Physiol. 2002b;92:2119–2130. doi: 10.1152/japplphysiol.01128.2001. [DOI] [PubMed] [Google Scholar]
- Nattie E, Li A. Central chemoreception 2005: a brief review. Auton Neurosci. 2006a;126–127:332–338. doi: 10.1016/j.autneu.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Nattie E, Li A. Neurokinin-1 receptor-expressing neurons in the ventral medulla are essential for normal central and peripheral chemoreception in the conscious rat. J Appl Physiol. 2006b;101:1596–1606. doi: 10.1152/japplphysiol.00347.2006. [DOI] [PubMed] [Google Scholar]
- O'Connell AD, Morton MJ, Hunter M. Two-pore domain K+ channels-molecular sensors. Biochim Biophys Acta. 2002;1566:152–161. doi: 10.1016/s0005-2736(02)00597-7. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Homma I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J Neurosci. 2003;23:1478–1486. doi: 10.1523/JNEUROSCI.23-04-01478.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam RW, Filosa JA, Ritucci NA. Cellular mechanisms involved in CO2 and acid signaling in chemosensitive neurons. Am J Physiol Cell Physiol. 2004;287:C1493–C1526. doi: 10.1152/ajpcell.00282.2004. [DOI] [PubMed] [Google Scholar]
- Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci. 2004;5:449–461. doi: 10.1038/nrn1409. [DOI] [PubMed] [Google Scholar]
- Richerson GB, Wang W, Hodges MR, Dohle CI, Diez-Sampedro A. Homing in on the specific phenotype(s) of central respiratory chemoreceptors. Exp Physiol. 2005;90:259–266. doi: 10.1113/expphysiol.2005.029843. [DOI] [PubMed] [Google Scholar]
- Rosin DL, Chang DA, Guyenet PG. Afferent and efferent connections of the rat retrotrapezoid nucleus. J Comp Neurol. 2006;499:64–89. doi: 10.1002/cne.21105. [DOI] [PubMed] [Google Scholar]
- Severson CA, Wang W, Pieribone VA, Dohle CI, Richerson GB. Midbrain serotonergic neurons are central pH chemoreceptors. Nat Neurosci. 2003;6:1139–1140. doi: 10.1038/nn1130. [DOI] [PubMed] [Google Scholar]
- Solomon IC, Edelman NH, O'Neill MH., 3rd CO2/H+ chemoreception in the cat pre-Bötzinger complex in vivo. J Appl Physiol. 2000;88:1996–2007. doi: 10.1152/jappl.2000.88.6.1996. [DOI] [PubMed] [Google Scholar]
- Spengler CM, Gozal D, Shea SA. Chemoreceptive mechanisms elucidated by studies of congenital central hypoventilation syndrome. Resp Physiol. 2001;129:247–255. doi: 10.1016/s0034-5687(01)00294-8. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, West GH, Brunet JF, Mulkey DK, Bayliss DA, Guyenet PG. Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J Neurosci. 2006;26:10305–10314. doi: 10.1523/JNEUROSCI.2917-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J, Yang L, Zhang X, Rojas A, Shi Y, Jiang C. High CO2 chemosensitivity versus wide sensing spectrum: a paradoxical problem and its solutions in cultured brainstem neurons. J Physiol. 2007;578:831–841. doi: 10.1113/jphysiol.2006.115758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, Colombari E, West GH, Stornetta RL, Guyenet PG. Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J Physiol. 2006;572:503–523. doi: 10.1113/jphysiol.2005.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn CP, Bayliss DA, Guyenet PG. Cardiorespiratory neurons of the rat ventrolateral medulla contain TASK-1 and TASK-3 channel mRNA. Respir Physiol Neurobiol. 2003;138:19–35. doi: 10.1016/s1569-9048(03)00185-x. [DOI] [PubMed] [Google Scholar]
- Williams RH, Jensen LT, Verkhratsky A, Fugger L, Burdakov D. Control of hypothalamic orexin neurons by acid and CO2. Proc Natl Acad Sci U S A. 2007;104:10685–10690. doi: 10.1073/pnas.0702676104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Cui N, Yang Z, Qu Z, Jiang C. Modulation of Kir4.1 and Kir5.1 by hypercapnia and intracellular acidosis. J Physiol. 2000;524:725–735. doi: 10.1111/j.1469-7793.2000.00725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]