Abstract
Studies on intact muscle fibres indicate that reactive oxygen species (ROS) produced during muscle activity, or applied exogenously, can cause decreased force responses primarily by reducing the Ca2+ sensitivity of the contractile apparatus. Identification of the molecular basis of this effect is complicated by the fact that studies on skinned muscle fibres in general have not observed reduced contractile Ca2+ sensitivity when applying ROS, predominantly H2O2. Here, using skinned fibres from rat extensor digitorum longus (EDL) and soleus muscle, it is shown that although H2O2 (≥ 100 μm) has little effect by itself, when added in the presence of myoglobin it causes marked reduction in the Ca2+ sensitivity of the contractile apparatus, probably due to production of hydroxyl radicals (OH•). Maximum force production is also reduced, but only with larger or more prolonged treatments. The effects are not prevented by tempol, a potent superoxide scavenger. Dithiotreitol (DTT) produces little reversal of the sensitivity change if applied afterwards, but it does substantially reverse all the changes if applied before the fibre undergoes an activation sequence. When glutathione (GSH, 5 mm) is present, exposure of EDL fibres to H2O2 and myoglobin causes an increase in Ca2+ sensitivity, with longer treatments causing a subsequent decrease, whereas in soleus fibres it causes only decreases in sensitivity and maximum force. The increased Ca2+ sensitivity in EDL fibres is evidently due to the summed actions of (i) a potentiating effect of glutathionylation, which can be reversed by DTT and only occurs in fast-twitch fibres, and (ii) a less reversible reduction in sensitivity. Western blotting showed that reductions in Ca2+ sensitivity were not due to loss of troponin-C. The present findings help provide a mechanistic basis for diverse findings on the effects of ROS in muscle fibres and implicate OH• radicals and glutathione as likely mediators of the effects.
There is substantial evidence that reactive oxygen species (ROS) are generated in skeletal muscle fibres during exercise and that they may contribute to the development of muscle fatigue in some situations (Clanton et al. 1999; Reid, 2001; Sostaric et al. 2006; Bruton et al. 2007; Allen et al. 2008). Some of the principal ROS that may be involved are superoxide, hydrogen peroxide (H2O2) and hydroxyl radicals (OH•). Although ROS could adversely affect many cellular processes, including the release and re-uptake of intracellular Ca2+, it appears that their inhibitory effect on tetanic force arises primarily from their actions on the contractile apparatus, particularly in reducing Ca2+ sensitivity (Moopanar & Allen, 2005, 2006; Bruton et al. 2007). Similar conclusions have also been reached from experiments applying exogenous H2O2 to isolated skeletal muscle fibres (Andrade et al. 1998).
Determining the specific molecular events involved in these ROS effects, however, is made difficult by the fact that experiments on isolated contractile apparatus using skinned muscle fibres for the most part have not found any comparable reduction in Ca2+ sensitivity. For example, skinned fibre experiments with rat soleus and extensor digitorum longus (EDL) muscle found no effect of 30 min exposure to H2O2, even at concentrations up to 1 mm, on either the pCa50 (i.e. −log10[Ca2+] producing 50% of maximum force) or the maximum Ca2+-activated force, with the only significant change being ∼30% decrease in the Hill coefficient (h), indicating a reduction in the steepness of the force–pCa relationship (Plant et al. 2000; Lamb & Posterino, 2003). Similarly, in skinned fibres from rat diaphragm there was no significant effect on any of the contractile parameters of 5 min exposure to either 10 μm or 1 mm H2O2 (Callahan et al. 2001). The latter study further found that treatments with either superoxide or hydroxyl radicals also had no significant effect on pCa50 or h, though they did cause substantial reduction in maximum Ca2+-activated force. The conclusions concerning the effects of the hydroxyl radicals, however, are complicated by the fact that they were generated by exposing the skinned fibres to a mixture of H2O2, ascorbate and Fe2+ (each at 1 mm), and the latter two treatments alone also caused significant reductions in maximum force, albeit less than the combination of all three reagents which was meant to generate the hydroxyl radicals (Callahan et al. 2001).
The only instances where H2O2 exposure caused significant reductions in maximum Ca2+-activated force and pCa50 were with prolonged exposure (≥ 20 min) to extremely high concentrations (≥ 5 mm) (Plant et al. 2000; Lamb & Posterino, 2003; Prochniewicz et al. 2008), there being reductions of ∼15–20% and ∼0.08–0.4 pCa units, respectively, in fast-twitch fibres with 5–10 mm H2O2. With 50 mm H2O2 the reduction in maximum force was > 80% and there was > 95% decrease in unloaded velocity of shortening, the effects possibly being due to oxidation of multiple methionine residues in the essential light chains and in the heavy chain myosin S1 (Prochniewicz et al. 2008). Given the extremely high concentrations of H2O2 required for such effects, it seems possible that they were mediated not by the H2O2 itself but instead by hydroxyl radicals formed by homolytic fission of the H2O2 (Halliwell & Gutteridge, 2007), as such radicals are known to readily attack methionine residues.
Such production of hydroxyl radicals can occur in muscle fibres via the Fenton reaction when H2O2 reacts with Fe2+ in myoglobin (Halliwell & Gutteridge, 2007). In view of the possible important physiological relevance of this to ROS effects in muscle, the present study used skinned muscle fibres to examine the effects on the contractile apparatus of applying H2O2 in the presence of myoglobin (0.5 mm; Wittenberg, 1970). This revealed potent inhibitory actions of ROS on the contractile apparatus in such circumstances. The study further examined how these effects were modified by the presence of glutathione, a key intracellular reducing agent present in muscle and other tissue (Halliwell & Gutteridge, 2007). This also gave insight into the possible role in ROS effects played by glutathionylation of the contractile apparatus, which in previous experiments has been shown to cause very marked potentiation of Ca2+ sensitivity in fast-twitch (EDL) fibres but have no effect at all in slow-twitch soleus fibres (Lamb & Posterino, 2003), in a process probably analogous to myosin light chain phosphorylation. These observations help relate disparate findings of many different studies and provide new physiological insight into the effects of ROS in muscle function and fatigue.
Methods
Preparations
With approval of the La Trobe University Animal Ethics Committee, male Long–Evans hooded rats (∼6–8 months old) were killed by overdose with fluothane (2% vol : vol) in a restricted air space. Both extensor digitorum longus (EDL) and soleus muscles were rapidly excised and pinned at resting length under paraffin oil and kept cool (∼10°C) on an ice pack. Single muscle fibres were mechanically skinned with fine jeweller's forceps as previously described (Lamb & Stephenson, 1994). Briefly, a skinned fibre segment was attached to a force transducer (AME801, resonance frequency > 2 kHz; SensoNor, Horten, Norway), set at 120% of resting length and pre-equilibrated for 2 min in ‘intracellular’ solution (either K-HDTA based or K-EGTA based, see below). Force responses were recorded using a Bioamp pod and Powerlab 4/20 series hardware (ADInstruments, Sydney, Australia). All experiments were conducted at ∼23 ± 2°C.
Skinned fibre solutions
The standard K-HDTA intracellular solution used for examining twitch and tetanic force properties contained (mm): HDTA2− (Fluka, Buchs, Switzerland), 50; total ATP, 8; creatine phosphate (CrP), 10; Na+, 36; K+, 126; total Mg2+, 8.5; total EGTA, 0.075; Hepes, 90; pH 7.10 and pCa (=−log10[Ca2+]) 6.9. For examination of contractile apparatus properties, solutions similar to the K-HDTA solution were made with all HDTA replaced by 50 mm EGTA (pCa > 9, ‘relaxing’ solution) or 50 mm Ca-EGTA (pCa ∼4.7, ‘Max’ solution), with total Mg2+ adjusted to maintain 1 mm free. These two solutions were mixed in appropriate ratio to produce solutions with pCa in the range 6.7–4.7. The fibre was then activated by exposure to sequences of solutions with progressively higher free [Ca2+] (pCa 9 to pCa 4.7). These solutions had an osmolality of 295 ± 5 mosmol l−1 and a calculated free [Mg2+] of 1 mm (Lamb & Stephenson, 1994). The pCa of solutions (for pCa < 7.2) was measured with a Ca2+-sensitive electrode (Orion Research, Cambridge, MA, USA). All chemicals were purchased from Sigma-Aldrich (St Louis, MO, USA) unless specified otherwise.
Additional solutions
Myoglobin was directly added as a solid to the appropriate bathing solution at the final concentration. Hydrogen peroxide was added from a 30% aqueous stock solution to the experimental solutions at a final concentration of 1 or 10 mm or diluted to give 100, 200 or 300 μm. The 100 mm stock of reduced glutathione (GSH) was made in K-HDTA solution with the pH re-adjusted to 7.10 with KOH. Importantly, when combinations of myoglobin, H2O2 and GSH solutions were mixed together, this was done immediately (< 3 min) prior to the treatment exposure and then discarded after only one use. Dithiothreitol (DTT) was made as a 1 m stock in double distilled water and diluted 100-fold into final solution. A 100 mm stock solution of 2,2′-dithiodipyridine (DTDP) was made in absolute ethanol and was diluted 1000-fold in the final solutions to 100 μm; matching control solutions with the same amount of ethanol (0.1%) had no noticeably different effect than controls without ethanol.
Contractile apparatus properties
The force–[Ca2+] relationship in each fibre was determined by exposing the skinned fibre to a sequence of solutions at progressively higher free [Ca2+], as previously described (Lamb & Posterino, 2003). The last in each sequence was the solution at pCa ∼4.7, and the force reached in that solution was defined as the maximum Ca2+-activated force (‘Max’). The fibre was then fully relaxed in the pCa > 9 solution for at least 1 min before repeating the activation sequence. Successive control sequences were normally highly reproducible, apart from a small progressive reduction in maximum force (see below and Results). The same fibre was then subjected to one or more experimental protocols (e.g. 10 mm H2O2 with myoglobin) in the relaxing solution (pCa > 9) for a set time, washed for 1 min in the relaxing solution and then tested again (usually 3 times in succession) with the control activating solution sequence (e.g. Fig. 1). Where appropriate, the fibre was then subjected to another reagent (e.g. 10 mm DTT) as above and tested again.
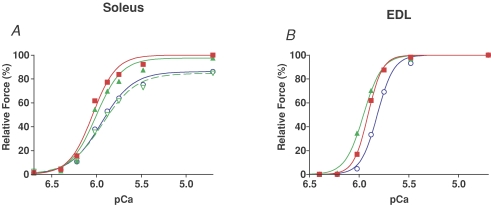
Figure 1. H2O2 in the presence of myoglobin reduces Ca2+ sensitivity and maximum force.
A, isometric force production in a skinned EDL fibre exposed to solutions with successively higher free [Ca2+] (small ticks, solution order: pCa > 9, 6.40, 6.22, 6.02, 5.88, 5.75, 5.48, 4.7, > 9). The entire sequence was repeated several times both before and after a 20 min exposure to 300 μm H2O2 in presence of 0.5 mm myoglobin (in solution at pCa > 9) and again after 10 min exposure to 10 mm DTT. The peak of each force staircase, reached at pCa 4.7, indicates maximum Ca2+-activated force. Horizontal arrows mark the force level achieved at pCa 5.75. B, Hill fits to the force–pCa data in A, for second control sequence shown (▪), first and third sequence after H2O2–myoglobin (• and ○, respectively), and first after DTT (▵, dashed line); force responses in each ‘staircase’ normalized to their own maximum.
Each time a skinned EDL fibre was subjected to a force–pCa staircase, which involved continuous activation for ∼1 min, there was a small reduction in the absolute maximum force reached, typically ∼3–5% with each staircase for the first 4 or 5 times and then progressively less on later staircases. The change in soleus fibres was typically only ∼1% per staircase. These reductions occurred when repeating control staircase sequences without any treatment and depended only on the overall duration of each force staircase and not on the length of time between staircases. Consequently, the effect of a given treatment on maximum Ca2+-activated force was quantified after taking into account the decline in force occurring on each staircase, based on measurements in the same fibre for the three force staircases immediately preceding the treatment. pCa50 also became very slightly lower after each staircase; this was a change of only ∼0.005–0.010 pCa units per staircase in EDL fibres and considerably less in soleus fibres, and was similarly taken into account when determining the effect of a treatment on pCa50. The Hill coefficient typically did not show any appreciable change upon repeated force staircases unless the fibre was subjected to certain specific treatments.
Electrical stimulation experiments
The segment of skinned fibre was positioned parallel to, and midway between, two platinum electrodes in a stimulating bath containing 130 μl of K-HDTA ‘control’ solution, as previously described (Dutka et al. 2005). After a 2 min equilibration period, the skinned fibre segment was then electrically stimulated (strength 75 V cm−1, 1 ms pulse duration) eliciting twitch or tetanic (50 Hz, 20 pulses) force responses, after which the fibre was exposed to 0.5 mm myoglobin and/or 10 mm H2O2 in K-HDTA for 2 min (without stimulation). The fibre was then washed in K-HDTA solution (30 s) to remove all myoglobin and/or H2O2 before being stimulated again back in K-HDTA solution. Twitch and tetanic force responses were highly reproducible. At the end of each experiment maximum Ca2+-activated force was determined in the ‘Max’ solution (pCa 4.7).
Western blotting
Individual fibre segments were analysed for troponin-C (TnC) protein content by Western blotting. Following the physiological experiments, skinned fibre segments that had been exposed to either myoglobin alone (n = 4) or myoglobin with 10 mm H2O2 (n = 4) were collected into 5 μl solubilizing buffer (0.125 m Tris-Cl, pH 6.8, 4% SDS, 10% glycerol, 4 m urea, 10% mercaptoethanol, 0.001% bromophenol blue) and heated to ∼95°C for 4 min and placed at −20°C until analysed by Western blotting. An intact EDL muscle fibre was treated the same and run alongside the treated fibres. Proteins from fibre segments were separated on 15% SDS-PAGE gels (Bio-Rad, Hercules, CA, USA) for 15 min at 100 V and 60 min at 160 V. Proteins were semi-dry transferred to nitrocellulose (15 V for 60 min). Following transfer the SDS-PAGE gel was stained with BioSafe Coommassie Stain (Bio-Rad) for detection of myosin heavy chain which was used as an indicator of the relative amount of protein between the lanes as previously described (Murphy et al. 2006). After a series of washes, transferred membranes were exposed overnight to polyclonal rabbit anti-troponin-C (Santa Cruz Biotechnologies, Santa Cruz, CA, USA) diluted 1 in 200 in 1% BSA in phosphate-buffered saline with 0.025% Tween. Following a number of washes in blocking buffer (5% skimmed milk powder in tris-buffered saline with 0.025% Tween (TBST)), the membrane was exposed to secondary antibody (goat anti-rabbit HRP; Pierce, Rockford, IL, USA), diluted 1 in 20 000 in blocking buffer for 60 min. Following a further series of washes in TBST, bands were visualized using chemiluminescent detection (Pierce). A single band at ∼18 kDa was detected on the membrane and images were collected using a CCD camera attached to a ChemiDoc XRS (Bio-Rad, USA) and using Quantity One software (Bio-Rad). Densitometry was performed with the Quantity One software.
Statistics
Data are expressed as mean ± standard error of the mean (s.e.m.), with the number of fibres studied denoted as ‘n’. Student's t test (paired or unpaired as appropriate) was used to determine statistical significance (probability value, P < 0.05).
Results
Effect of H2O2 in the presence of myoglobin
Throughout the text we refer to a segment of a skinned muscle fibre simply as a ‘skinned fibre’ or a ‘fibre’. When myoglobin (0.5 mm) was present, exposing a skinned EDL fibre to H2O2 caused a marked reduction in Ca2+ sensitivity of the contractile apparatus. In the example in Fig. 1 with a 20 min exposure to 300 μm H2O2, there was a decrease in pCa50 by ∼0.15 pCa units (from 5.87 to 5.72) and a decrease in Hill coefficient (h) by ∼40% (from 6.2 to 3.6). The treatment also caused a small reduction in maximum Ca2+-activated force (∼6% in this case); this value takes into account the reduction in maximum force that occurs irrespective of treatment when subjecting a fibre to each ‘force staircase’ (see Methods). Similar effects of the H2O2–myoglobin treatment were observed in every EDL fibre examined; the mean results are shown in Fig. 2. After an EDL fibre had undergone one force staircase following the H2O2–myoglobin exposure, the Ca2+ sensitivity was slightly increased (see horizontal arrows in Fig. 1A), possibly due to cross-linking of oxidized elements of the contractile apparatus during the first post-treatment staircase; this would be in accord with an activation-dependent effect of oxidation reported previously (see Lamb & Posterino, 2003). Treating the fibre with the reducing agent DTT (10 mm) for 10 min reversed only this small activation-dependent increase in Ca2+ sensitivity, but did not appreciably reverse the main deleterious effects of the H2O2–myoglobin exposure.
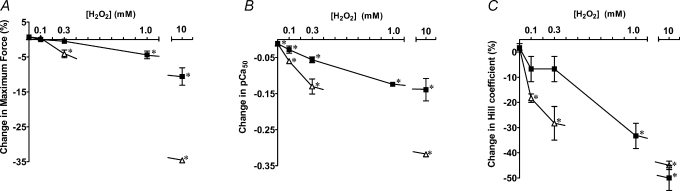
Figure 2. Concentration-dependent effects of H2O2 with myoglobin on maximum force and Ca2+ sensitivity in EDL fibres.
A, change in maximum force (as percentage of initial) following 5 min (▪) or 20 min (▵) exposure to a solution (pCa > 9) with indicated concentration of H2O2 and 0.5 mm myoglobin, as in Fig. 1A. Values are mean change (±s.e.m.) in following number of fibres: 5 min exposure to 0 mm H2O2 (7), 0.1 mm (6), 0.3 mm (4), 1 mm (4), 10 mm (17); 20 min exposure to 0.1 mm H2O2 (3), 0.3 mm (6), 10 mm (3). B, corresponding change in pCa50. C, corresponding change in Hill coefficient. *Significantly different from zero.
Figure 2 shows the mean changes in maximum Ca2+-activated force, pCa50 and h in EDL fibres plotted against the concentration of H2O2 for both a 5 min and a 20 min exposure; in all cases myoglobin (0.5 mm) was present during the treatment period. Fibres were exposed to H2O2–myoglobin whilst relaxed in solution at pCa > 9 and indistinguishable results were obtained when the treatment was conducted with solutions at pCa 7 (not shown). Exposure to myoglobin alone (i.e. with zero H2O2) for the same periods had little or no effect (Fig. 2). Consistent with previous results (Lamb & Posterino, 2003), exposure to H2O2 alone had comparatively little effect; a 5 min exposure to 10 mm H2O2 caused no significant change in maximum force in the three EDL fibres examined (−2.1 ± 1.3%) and caused only a relatively small reduction in pCa50 (−0.030 ± 0.007), with the only substantial effect being a reduction in h (by ∼25%). This contrasts with the effects of a 5 min exposure to the same [H2O2] with myoglobin present: 10.6 ± 2.5% reduction in maximum force, −0.139 ± 0.031 change in pCa50 and ∼50 ± 5% reduction in h (n = 17 fibres; Fig. 2). DTT treatment (10 mm, 10 min) caused only slight or no reversal of these latter changes (+0.8 ± 0.6%, +0.014 ± 0.004 and +0.14 ± 0.03, n = 9). However, if fibres exposed to 10 mm H2O2–myoglobin (5 min) were kept relaxed until after they had been treated with DTT (10 mm, 10 min), the reduction in maximum force (−4.9 ± 1.3%, n = 7) was significantly smaller than for similarly treated fibres activated before the DTT treatment (−10.0 ± 2.1% net reduction, n = 9, P < 0.05, one-tailed unpaired t test), and it was also much less than when a subset of the same seven fibres was subsequently re-exposed to H2O2–myoglobin without DTT treatment (further reduction: −14.3 ± 0.4%, n = 4, P < 0.05, paired t test). This indicates that DTT is indeed able to partially reverse the decline in maximum force but that this ability is largely lost if the skinned fibre is subjected to a force–pCa staircase whilst still ‘oxidised’ (see also van der Poel & Stephenson, 2002). Similarly, in the fibres where complete force–pCa data were obtained in the above cases, it was found that the reductions in pCa50 and h were also significantly smaller if DTT treatment was applied before rather than after activation (pCa50: −0.086 ± 0.023, n = 5, and −0.151 ± 0.010, n = 9; h: −1.2 ± 0.4, n = 5, and −2.3 ± 0.2, n = 9, respectively).
As can be seen in Fig. 2, the effects of the H2O2–myoglobin treatment increased broadly in proportion with the product of the H2O2 concentration and treatment time (i.e. [H2O2]× time), with the effect of a 20 min treatment with 300 μm H2O2 being similar to that of a 5 min exposure with 1 mm H2O2 for each of the three parameters examined (maximum force, pCa50 and h). This did not hold true, however, if the [H2O2] was increased above 1 mm, where the effects showed evidence of saturation. It is also important to note that the above sub-maximal treatment regimes (i.e. 20 min with 300 μm H2O2 and myoglobin or 5 min with 1 mm H2O2 and myoglobin) caused relatively little reduction in maximum force (i.e. only ∼4 or 5%) but caused substantial reduction in Ca2+ sensitivity, reducing pCa50 by ∼0.14 units and h by ∼30% (see Fig. 2). The major effect that such a change in Ca2+ sensitivity would have on sub-maximal tetanic force development is evident from the force–pCa curves in Fig. 1B.
In soleus muscle fibres the effects of H2O2 exposure with myoglobin present (Table 1) were similar to those in EDL fibres: a 5 min exposure to 300 μm H2O2 with myoglobin caused a significant decrease in pCa50 with no change in maximum Ca2+-activated force, and higher [H2O2] and/or longer exposure resulted in a greater decrease in pCa50 and decreases in maximum force and h. It appeared that the change in pCa50 with the milder treatments was somewhat smaller in soleus fibres than in EDL fibres (e.g. 5 min with 300 μm H2O2 and myoglobin resulted in decrease of 0.032 ± 0.005 in soleus fibres and 0.056 ± 0.008 in EDL fibres), but extremely stringent treatment nevertheless caused a similar decline in both muscle types (e.g. 20 min with 10 mm H2O2 and myoglobin caused ∼35–40% decline in maximum force, ∼0.3 unit decline pCa50, and ∼40–50% decline in h) (Table 1 and Fig. 2). In these soleus fibres myoglobin exposure alone had no significant effect (Table 1), and it has been previously shown in the same preparation that 5 min treatment with 10 mm H2O2 alone also has no significant effect (Lamb & Posterino, 2003). After the soleus fibres had been exposed to H2O2–myoglobin and activated, DTT treatment (10 mm, 10 min) produced only a small recovery in pCa50 with no significant change in maximum force or h (Table 1). Longer exposure to DTT had no further effects in either soleus or EDL fibres (not shown). The ability of DTT to reverse the changes if applied before activation was not examined in the soleus fibres.
Table 1.
Effect of myoglobin and H2O2 exposure on contractile apparatus properties in soleus fibres
| Treatment | (n) | ΔMax | ΔpCa50 | Δh | |
|---|---|---|---|---|---|
| 1 | Myoglobin, 5 min | 3 | +1.7 ± 1.9% | +0.012 ± 0.008 | +0.1 ± 0.1 |
| 2 | Myo + 300 μm H2O2, 5 min | 4 | −1.1 ± 0.6% | −0.032 ± 0.005* | −0.1 ± 0.1 |
| 3 | Myo + 10 mm H2O2, 5 min | 6 | −13.7 ± 1.6%* | −0.086 ± 0.009* | −1.0 ± 0.2* |
| - subsequent DTT | 6 | +1.0 ± 0.6% | +0.015 ± 0.004* | +0.1 ± 0.04 | |
| 4 | Myoglobin, 20 min | 3 | −1.1 ± 2.5% | −0.011 ± 0.009 | −0.1 ± 0.1 |
| 5 | Myo + 100 μm H2O2, 20 min | 3 | −3.1 ± 0.3%* | −0.024 ± 0.004* | −0.5 ± 0.2 |
| 6 | Myo + 10 mm H2O2, 20 min | 3 | −39.1 ± 2.7%* | −0.272 ± 0.041* | −1.3 ± 0.2* |
| - subsequent DTT | 3 | +1.7 ± 0.6% | +0.025 ± 0.003* | 0.0 ± 0.1 | |
Mean ±s.e.m. change in maximum Ca2+-activated force (Max), pCa50 and h following the indicated treatment in each of ‘n’ fibres. Values reflect the difference in paired measurements of the given variable after treatment compared to that before treatment in each fibre.
Significantly different from zero (i.e. the treatment produced a significant change). The pCa50 in control conditions before any treatment was typically ∼6.02 and h was ∼3.1 in the soleus fibres here.
The greatly increased efficacy of H2O2 when added in the presence of myoglobin is probably due to the production of hydroxyl radicals (OH•) via the Fenton reaction (see introduction). Although substantial production of superoxide would not be expected in this experimental situation, this was explicitly considered by examining the effect of tempol, a superoxide dismutase mimetic (Moopanar & Allen, 2005; Edwards et al. 2007). The presence of tempol (0.5 mm) did not prevent the reductions in maximum force and Ca2+ sensitivity occurring during a 5 min exposure to 10 mm H2O2 with myoglobin (mean change in maximum: −13.0 ± 1.4%; pCa50: −0.239 ± 0.008 pCa units; h: −1.7 ± 0.2 (or ∼18%), n = 5 EDL fibres), with the reduction in pCa50 actually being larger than that found without tempol present. When tempol was present in the same conditions without H2O2, it had no effect on any of the contractile parameters (mean changes, −0.8 ± 1.3%; −0.007 ± 0.003 pCa units; −0.2 ± 0.1, respectively, examined before matching treatment with H2O2 in 3 of the same fibres).
Effect of the presence of glutathione
The presence of glutathione (GSH, 5 mm), a reducing agent present endogenously in muscle fibres, greatly modified the effects of H2O2–myoglobin exposure. In soleus fibres, it attenuated by 5-fold the reduction in maximum force occurring with a 5 min exposure to 10 mm H2O2 with myoglobin (−2.6 ± 0.6% and −13.7 ± 1.6%, respectively, with and without GSH; Tables 1 and 2), and halved the reductions in pCa50 (−0.042 ± 0.008 and −0.086 ± 0.009, respectively) and in h (−0.3 ± 0.1 and −1.0 ± 0.2, respectively) (e.g. Fig. 3A). The protective effects were smaller with longer (20 min) treatment (Tables 1 and 2). In EDL fibres, however, the presence of GSH not only greatly attenuated the decline in maximum force occurring with H2O2–myoglobin exposure (e.g. −1.1 ± 0.6% and −10.6 ± 2.5%, respectively, with and without GSH, for 5 min in 10 mm H2O2–myoglobin; Fig. 2 and Table 2), but also actually caused an increase in pCa50 (+0.032 ± 0.014, Table 2) in all six fibres examined with 5 min exposure (e.g. Fig. 3B). This contrasts greatly with the effect of the H2O2–myoglobin treatment in the absence of GSH, where it was found in other EDL fibres to cause a 0.139 ± 0.031 decrease in pCa50 (middle panel in Fig. 2, n = 17). Moreover, if the GSH was applied after the H2O2–myoglobin treatment, rather than with it, it had no significant effect on pCa50 (treatment 3 in Table 2). When the H2O2–myogloblin–GSH treatment was applied in EDL fibres for a total of 20 min, there was a small reduction in pCa50 (−0.024 ± 0.001) and maximum force did drop but only by ∼30% of that occurring without GSH (−10.2 ± 1.4% and −34.6 ± 0.6%, respectively) (Table 2 and Fig. 2). We have shown previously that addition of GSH alone causes no change in Ca2+ sensitivity if the skinned fibre had been not pre-exposed to specific oxidizing conditions (Lamb & Posterino, 2003), and consistent with this, we found here that exposure to myoglobin and GSH together for 5 min also had no significant effect on the contractile properties (mean changes in maximum force, pCa50 and h: 0.1 ± 0.1%, −0.003 ± 0.003, 0.1 ± 0.03, respectively, n = 3). When H2O2 was then included with the myoglobin and GSH, the effects were indistinguishable from those described above with a 5 min (2 fibres) or 20 min (1 fibre) exposure to the same combination of H2O2, myoglobin and GSH; evidently the fibre remained in a similar non-oxidized state irrespective of whether myoglobin and/or GSH were initially present and the final effects depended on simultaneous exposure to all three agents.
Table 2.
Effect on contractile apparatus properties of myoglobin and H2O2 with glutathione present
| Treatment | (n) | ΔMax | ΔpCa50 | Δh |
|---|---|---|---|---|
| EDL fibres | ||||
| 1 Myo + 300 μm H2O2+ GSH, 5 min | 3 | +1.7 ± 0.7% | −0.010 ± 0.006 | −0.1 ± 0.2 |
| - subsequent DTT | 3 | +0.7 ± 0.7% | −0.010 ± 0.004 | +0.3 ± 0.1 |
| 2 Myo + 10 mm H2O2+ GSH, 5 min | 6 | −1.1 ± 0.6% | +0.032 ± 0.014# | −1.7 ± 0.2* |
| - subsequent DTT | 3 | +0.5 ± 0.4% | −0.114 ± 0.002* | +0.9 ± 0.2* |
| 3 Myo + 10 mm H2O2, 5 min | 3 | −11.7 ± 0.4%* | −0.175 ± 0.008* | −2.1 ± 0.2* |
| - subsequent GSH | 3 | +1.4 ± 0.3%* | +0.017 ± 0.026 | 0.0 ± 0.0 |
| - subsequent DTT | 3 | −0.6 ± 0.3% | −0.021 ± 0.003* | +0.3 ± 0.1 |
| 4 Myo + 10 mm H2O2+ GSH, 20 min | 3 | −10.2 ± 1.4%* | −0.024 ± 0.001* | −1.9 ± 0.1* |
| - subsequent DTT | 3 | +0.0 ± 0.8% | −0.174 ± 0.004* | +0.4 ± 0.03* |
| Soleus fibres | ||||
| 1 Myo + 10 mm H2O2+ GSH, 5 min | 6 | −2.6 ± 0.6%* | −0.042 ± 0.008* | −0.3 ± 0.1 |
| - subsequent DTT (s.d., not s.e.m.) | 2 | +0.3 ± 1.2% | +0.001 ± 0.001 | 0.0 ± 0.1 |
| 2 Myo + 10 mm H2O2+ GSH, 20 min | 3 | −13.3 ± 2.6%* | −0.186 ± 0.016* | −0.8 ± 0.1* |
| - subsequent DTT | 3 | +1.7 ± 0.3%* | +0.012 ± 0.001* | +0.1 ± 0.1 |
Values are mean ±s.e.m. of the parameter changes with the given treatment, as in Table 1 (or ±s.d. in one case indicated).
Significantly different from zero (two-tailed paired t test).
Significantly larger than zero (one-tailed paired t test). Where indicated, the subsequent treatments involved exposure to 10 mm DTT for 10 min, or 5 mm GSH for 2 min, in the same fibres or in a subset.
Figure 3. Glutathione alters the effect of H2O2–myoglobin exposure differentially in EDL and soleus fibres.
A, force–pCa relationships in a soleus fibre before treatment (red squares), after 5 min (green filled triangles) and 20 min (green open triangles, dashed line) exposure to 10 mm H2O2 and myoglobin (0.5 mm) with glutathione (5 mm) present, and following a further 10 min treatment with DTT (10 mm) (blue circles). The presence of glutathione reduced, but did not fully prevent, the deleterious effects on maximum force and Ca2+ sensitivity of exposure to H2O2–myoglobin. B, force–pCa relationships in an EDL fibre before (red squares) and after (green triangles) 5 min exposure to the same H2O2–myoglobin–glutathione solution as in A, and after the DTT treatment (blue circles). With glutathione present the H2O2–myoglobin treatment caused an increase in Ca2+ sensitivity, and subsequent DTT treatment resulted in an overall decrease in sensitivity. Maximum force was not detectably altered (see Table 2, treatment 2) after correcting for small progressive decline occurring on successive staircases (see text).
Importantly too, when EDL fibres that had been exposed to the combination of H2O2–myoglobin–GSH were subsequently treated with DTT (10 mm, 10 min), there was a marked reduction in the pCa50 in every case (e.g. Fig. 3B) with no change in maximum force and a small recovery in h, with the reduction in pCa50 being greater in fibres given the longer initial oxidizing treatment (20 min versus 5 min) (mean data: Table 2, treatments 2 and 4). This was in marked contrast to the effect of such DTT treatment in soleus fibres, where it had either no effect or produced a minor recovery in pCa50 and maximum force (Fig. 3A; Table 2). The DTT exposure in EDL fibres evidently was reversing a substantial increase in pCa50 caused by glutathionylation during the treatment with H2O2–myoglobin and GSH, a process which does not occur in soleus fibres (Lamb & Posterino, 2003) (see introduction). Thus, the overall change in pCa50 in EDL fibres with H2O2–myoglobin–GSH treatment appears to be the sum of two processes: one related to the H2O2–myoglobin, which causes a poorly reversible reduction in pCa50, and another involving glutathionylation, which causes a readily reversible increase in pCa50.
Inducing specific glutathionylation with DTDP and GSH
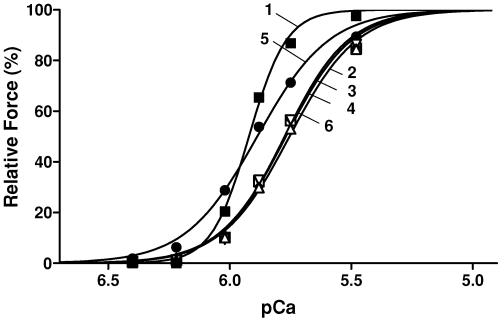
In order to verify that glutathionylation effects did indeed still occur in EDL fibres after oxidation with H2O2–myoglobin, three fibres were treated as in Fig. 4, with virtually identical results in each. The gluthionylation procedure was that used previously (Lamb & Posterino, 2003), which involved applying the highly reactive and specific sulphydryl reagent, DTDP, to oxidize free sulphydryls in the fibres, and then exposing them briefly to GSH; the reagent concentrations and exposure times used were those found previously to produce maximal effect (Lamb & Posterino, 2003). The initial 5 min exposure to 10 mm H2O2 with myoglobin caused a mean reduction in pCa50 by 0.207 ± 0.005 pCa units and a 10 ± 1% reduction in maximum force, comparable with other EDL fibres examined in this study. Each fibre was then given reducing treatment with DTT (10 mm, 10 min), which had no significant effects on the force–pCa responses, and then exposed to DTDP (100 μm, 5 min). The DTDP treatment itself had no significant effect on either maximum force or pCa50 (e.g. Fig. 4) but following subsequent exposure to GSH (5 mm, 2 min), there was a substantial increase in pCa50 (0.111 ± 0.010, n = 3) with no change in maximum force. As seen in Fig. 4, this shift in pCa50 was fully reversed by DTT treatment (10 mm, 10 min) (−0.139 ± 0.003 in same 3 fibres). It could also be reversed instead by much longer exposure to 5 mm GSH, with 20 min reversing approximately half of the increase in sensitivity (not shown), similar to that found previously in control fibres subjected to DTDP–GSH glutathionylation treatment (Lamb & Posterino, 2003). The shift in pCa50 induced by glutathionylation in these fibres pre-treated with H2O2–myoglobin (∼+0.11), was only approximately half of that occurring in EDL fibres that had not undergone such oxidizing pre-treatment (∼+0.22 ± 0.01, Table 2 in Lamb & Posterino, 2003).
Figure 4. H2O2–myoglobin treatment reduces the ability of specific glutathionylation to increase Ca2+ sensitivity.
Force–pCa curves for an EDL fibre before any treatment (1), and then sequentially after the following treatments in pCa > 9 solution: 5 min with 10 mm H2O2–myoglobin (2), 10 min with 10 mm DTT (3), 5 min with 100 μm DTDP (4), 2 min with 5 mm GSH (to produce glutathionylation) (5), and finally after 10 min more DTT treatment (6). Force responses for each case normalized to respective maximum (measured at pCa 4.7); maximum Ca2+-activated force declined ∼10% after H2O2–myoglobin treatment (2) and then was not detectably changed by any subsequent treatment.
Troponin-C levels after H2O2–myoglobin treatment
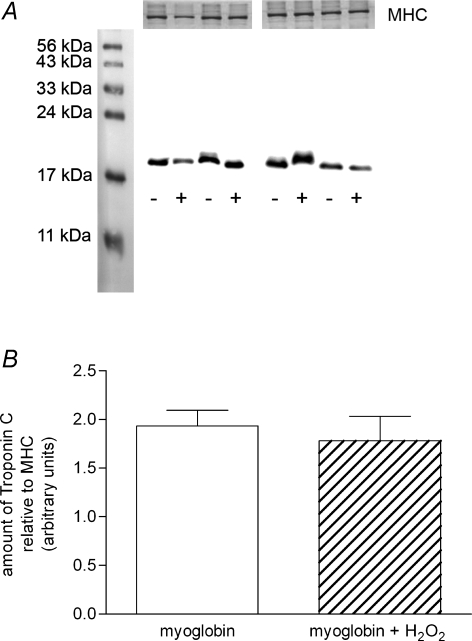
It has been reported previously that a hypoxia-fatigue bout in mouse isolated diaphragm muscle causes a decrease in maximal calcium-activated force and calcium sensitivity and that this is associated with degradation (or rather apparent loss) of troponins TnI and TnC (Brotto et al. 2000; de Paula Brotto et al. 2001). Given the analogous changes occurring in contractile properties here, we investigated whether we could detect any such degradation in TnC in rat EDL fibres following H2O2–myoglobin treatment. The force–pCa characteristics of each skinned EDL fibre was examined both before and after treating it with myoglobin and 10 mm H2O2 for 5 min or with myoglobin alone (treatments applied to successive fibres alternately). The changes in maximum force and Ca2+ sensitivity in these fibres were indistinguishable from other fibres in this study given the same treatments (i.e. from those in Fig. 2). Western blotting of the individual fibre segments for TnC showed no evidence of any loss or degradation of TnC in the fibres treated with H2O2 and myoglobin as compared to those treated with myoglobin alone (Fig. 5). The density of the TnC band for each fibre segment was normalized by the amount of myosin heavy chain (MHC) in each case, so as to control for differences in the amount of fibre present in each case; normalizing TnC density by the amount of actin present gave virtually identical results (not shown). The amount of TnC present in skinned fibres was also not evidently different from that present in an untreated non-skinned fibre segment (not shown).
Figure 5. Troponin-C levels are unchanged after H2O2–myoglobin treatment.
A, Western blots of troponin-C in individual EDL fibres subjected to treatment with myoglobin and 10 mm H2O2 for 5 min (+) or myoglobin alone (−). Force–pCa responses were measured before and after treatment in each case (not shown) and each displayed the typical changes for the respective treatment. Top panel shows corresponding coomassie stain of myosin heavy chain (MHC) for the fibres, which is indicative of the amount of fibre present. B, mean (+s.e.m.) of relative density of troponin-C band normalized to MHC content of the fibre for two treatments (both n = 4 fibres).
Effect on twitches and tetani
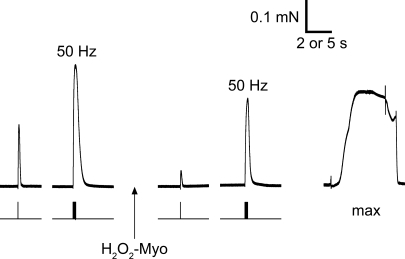
Finally, we examined the effects of H2O2–myoglobin treatment on twitch and tetanic force responses in skinned EDL fibres. In these experiments all solutions were weakly Ca2+-buffered using HDTA rather than EGTA as the primary anion and the pCa was ∼7.0, but otherwise matched the solutions used to examine the properties of the contractile apparatus (see Methods); the change in anion and Ca2+ buffering was necessary to avoid either depleting or over-loading the sarcoplasmic reticulum (SR) with Ca2+. The responses in Fig. 6 are representative of those found in each of the four EDL fibres examined. Before treatment the twitch response to single pulse stimulation was on average 45 ± 6% of the tetanic response to 50 Hz stimulation, the latter being known typically to produce very close to maximum Ca2+-activated force in such fibres (e.g. see Dutka et al. 2008); it was not possible, however, to verify this at that time point because exposure of the fibres to the maximum Ca2+-activating solution would have altered the SR Ca2+ loading and also probably hurt the EC coupling mechanism (Verburg et al. 2006). After exposure to 10 mm H2O2 with myoglobin for 2 min, the twitch and tetanic responses were reduced to 33 ± 5% and 74 ± 2% of their respective values before treatment (n = 4 fibres). The duration of the H2O2–myoglobin treatment in these experiments was kept at 2 min rather than 5 min, because it had been found previously in this skinned EDL fibre preparation that a 5 min treatment to H2O2 alone can have deleterious effects on EC coupling in some cases (Posterino et al. 2003). Here, following the H2O2–myoglobin treatment the tetanic response was found to reach 91 ± 3% of the maximum Ca2+-activated force that could be elicited in the fibres at that time point (Fig. 6), demonstrating that EC coupling was quite functional and that AP stimulation could still evoke substantial Ca2+ release. It was apparent though that the treatment had substantially altered the properties of the contractile apparatus, because the maximum Ca2+-activated force was evidently substantially less than that prevailing before the H2O2–myoglobin treatment, given the comparatively large size of the pre-treatment tetanic response (e.g. Fig. 6). These experiments indicate that the H2O2–myoglobin treatment must have exerted some or all of its deleterious effects on the force responses via actions on the contractile apparatus. In contrast, exposing a fibre for the same duration to 10 mm H2O2 and myoglobin with 5 mm glutathione present had no apparent deleterious effect on the twitch or tetanic responses (not shown).
Figure 6. Effect on twitch and tetanic force responses of treatment with 10 mm H2O2 and myoglobin.
Twitch and tetanic force responses in a skinned EDL fibre elicited by single pulse and 50 Hz (20 pulse) stimulation, both before and after a 2 min exposure to 10 mm H2O2 and 0.5 mm myoglobin in standard K-HDTA solution (‘H202-Myo’). Fibre washed for 30 s (in standard K-HDTA solution) after treatment. Lower trace indicates electric field stimulation. Timescale: 2 s for twitch and tetanic force responses and 5 s for maximum Ca2+-activated force (‘max’, pCa 4.7).
Discussion
This study has demonstrated that exposure to comparatively low concentrations of H2O2 (100 μm) can cause a decrease in Ca2+ sensitivity of the contractile apparatus in skinned fibre preparations if added in the presence of myoglobin, with maximum force also being reduced at higher H2O2 concentrations. The effects occurred in both EDL and soleus fibres, and the extent of the effects broadly depended on the product of the [H2O2] and the exposure time (Table 1 and Fig. 2). The present study did not attempt to mimic closely the levels of H2O2 and other factors prevailing in vivo, because such an endeavour would be fraught with many problems, due to the numerous variables involved, including the existence of specialized microdomains throughout the intracellular space, the many candidate reactants, and the metabolic changes and temperature ranges involved in normal muscle function. Instead, the study aimed to examine the qualitative and quantitative changes in the effects of ROS exposure on the contractile apparatus when two probably important physiological factors were included in the process, namely the presence of myoglobin, which can play a major role in hydroxyl radical production, and glutathione, a key cellular reducing agent. The concentration of myoglobin used (0.5 mm) was only slightly above the range found in predominantly fast-twitch muscles of various terrestrial species (Wittenberg, 1970), and it could be expected that the relevant level for slow-twitch oxidative fibres might be similar to this or somewhat higher.
H2O2 is known to be able to interact with the Fe2+ in myoglobin and generate the hydroxyl radical (OH•) via the Fenton reaction (Halliwell & Gutteridge, 2007). The experiments here utilized this normal in vivo process to generate OH•, both because of its physiological relevance and because it avoided the considerable problems that arise when attempting to produce OH• by other means, such as direct application of millimolar levels of Fe2+ and ascorbic acid to the contractile apparatus (see introduction). In the experiments here, the presence of 50 mm free EGTA ensured that the concentration of free Fe2+ was kept extremely low at all times, so any effects were due to H2O2 attacking the myoglobin and generating free radicals and not to direct effects of Fe2+ on the contractile proteins or other molecules. Hydroxyl radicals are extremely reactive and very short lived (Halliwell & Gutteridge, 2007), so it is very likely that effects found here, as in vivo, are not due solely to direct actions of OH• but also to the actions of other radicals generated secondarily by the OH•. In particular, when GSH (5 mm) was present, it more than likely interacted with OH• to generate the thiyl radical GS• (Halliwell & Gutteridge, 2007), which then may have interacted with the contractile apparatus or other molecules, including the case where two thiyl molecules interact to form reduced glutathione, GSSG, a far less reactive compound. In the experiments here, Cl− was not included in the bathing solutions, and so the effects would not have been due to the OH• reacting with Cl− and forming HOCl (Halliwell & Gutteridge, 2007), another highly reactive molecule.
The findings of this study indicate that OH• is quite possibly responsible, either directly or indirectly, for causing the decrease in Ca2+ sensitivity of the contractile apparatus that underlies the reduced tetanic force (fatigue) occurring in isolated muscle fibres stimulated in vitro at close to physiological temperature (Moopanar & Allen, 2005, 2006; Bruton et al. 2007), and also when applying H2O2 exogenously to isolated fibres (Andrade et al. 1998). It may also explain the similar reduced Ca2+ sensitivity seen when stimulating skeletal muscle fibres from rats with congestive heart failure (Lunde et al. 2006). Clearly, the direct effects of H2O2 itself on the contractile apparatus observed in skinned fibre studies cannot account for the ROS-dependent changes occurring in the intact fibre studies. Furthermore, the experiments of Bruton et al. (2007) indicated that the ROS causing the sensitivity change, in the muscle fibres examined from rat and from mice over-expressing superoxide dismutase, was probably not superoxide itself, but instead followed from the dismutation of the superoxide to H2O2. The results here could readily explain the subsequent events as being mediated by the formation of OH• from the H2O2.
Reversibility with DTT
The study of Bruton et al. (2007) found that the adverse ROS-induced effects on the contractile apparatus could be reversed at least partially by treating the fibres with DTT. This too is broadly consistent with the findings here, where DTT treatment was able to reverse approximately half of the reduction in Ca2+ sensitivity and maximum force occurring after exposure to H2O2 and myoglobin, provided that the skinned fibre had not been subjected to a force–pCa staircase inbetween the exposure and the DTT treatment. If the ‘oxidised’ fibre was instead activated before being treated with DTT, the changes were mostly irreversible. This behaviour is highly analogous to that found previously in skinned fibres from muscle that had been subjected in vitro to high temperatures (> 40°C) (van der Poel & Stephenson, 2002), where there was a ROS-induced reduction in maximum Ca2+-activated force that could not be reversed by DTT once the fibre had been skinned and subjected to a Ca2+-activation sequence, but that could be reversed if it was left quiescent at room temperature for > 60 min before being skinned or was skinned and treated with DTT before being activated. It seems likely that this type of phenomenon is due to the fibre activation allowing one or more ‘oxidised’ sites on the contractile apparatus to come close together and interact to form a product that cannot be reduced by DTT. Such irreversible reactions can involve either protein thiols (Dalle-Donne et al. 2007), or result from oxidative attack on other sites such as protein methionines. Interestingly, although DTT itself cannot reverse oxidative damage to methionine residues, it can be used in in vitro experiments to supply the reducing power to enable the normal endogenous enzyme, methionine sulphoxide reductase, to repair such oxidative damage (Halliwell & Gutteridge, 2007); such an effect might underlie some of the DTT-reversal of ROS-induced changes observed in intact fibres in vitro, and so reversal of oxidative effects by DTT in such experiments cannot be taken as a guarantee that the oxidative changes solely involved formation of disulphide bonds.
Effects of glutathione
The presence of GSH during the H2O2–myoglobin treatment attenuated its deleterious effects, particularly on maximum force, but did not entirely prevent such effects (Fig. 3, Table 2). (Of course the extent of such attenuation probably depends on the relative concentrations of all the various reactants in the given situation.) The GS• radical can not only cause oxidative damage both directly and indirectly, but it can also interact with protein thiols (protein-SH) by a number of different pathways to produce protein glutathionylation (protein-SSG) (Halliwell & Gutteridge, 2007). Such glutathionylation can actually be protective (Klatt & Lamas, 2000; Dalle-Donne et al. 2007; Halliwell & Gutteridge, 2007), by stopping protein thiols from being subject to other more irreversible oxidative changes. Importantly too, glutathionylation of the contractile apparatus has marked functional effects in EDL fast-twitch fibres, greatly increasing the Ca2+ sensitivity (shift in pCa50∼+0.22), whereas in soleus fibres it has no such effect (Lamb & Posterino, 2003). Specific glutathionylation was achieved in the latter study by treating the skinned EDL fibre with the highly reactive and specific sulphydryl reagent, DTDP, and then subsequently exposing the fibre to GSH for only a brief period so the glutathionylation could occur without being subsequently reversed.
A major novel aspect of the experiments here was that the GSH was present during the oxidative treatment itself, as it would be in fibres in vivo, and further that the treatment involved exposure to H2O2 with myoglobin rather than to H2O2 alone. In the EDL fibres here, 5 min exposure to H2O2–myoglobin–GSH caused a small increase in pCa50 and then subsequent DTT treatment produced a large decrease (∼−0.114 pCa units) (e.g. Fig. 3; Table 2), which we suggest was due to the reversal of the gluthathionylation caused by the H2O2–myoglobin–GSH treatment. This is quantitatively consistent with the magnitude of the pCa50 change seen in EDL fibres that were given the same duration exposure to H2O2 and myoglobin without GSH and then subjected to specific glutathionylation treatment with DTDP (e.g. Fig. 4), where there was found to be a virtually identical, DTT-reversible shift in pCa50 (∼0.11 pCa units).
Thus, the net effect on pCa50 of the H2O2–myoglobin–GSH treatment in the EDL fibres was evidently the sum of two independent effects: (i) a reversible effect due to glutathionylation, and (ii) a more poorly reversible effect due to ‘oxidative damage’. Furthermore, it seems that the 5 min exposure to H2O2–myoglobin–GSH caused the maximal shift in pCa50 still possible with glutathionylation in EDL fibres after the oxidation. It does not appear that the glutathionylation at these particular protein thiol sites (see more on sites later) protected the EDL fibres from the overall deleterious effects of the H2O2–myoglobin exposure, because when the glutathionylation effect on Ca2+ sensitivity was reversed with DTT it was apparent that the Ca2+ sensitivity had been adversely affected (e.g. Fig. 3B) (net pCa shift ∼−0.08 pCa units). Although this reduction in pCa50 is less than that in EDL fibres given the same H2O2–myoglobin treatment without GSH present (∼−0.13 pCa unit shift, Fig. 2), it is not appreciably different from the level of protection seen with GSH in soleus fibres, where there is no glutathionylation effect (Tables 1 and 2). It is also interesting to note that the maximal pCa50 shift occurring with glutathionylation in EDL fibres oxidized with H2O2–myoglobin (∼+0.11 pCa units) was approximately only half of that in non-oxidized fibres (∼+0.22 pCa units; Lamb & Posterino, 2003). This may be because the oxidation treatment rendered some of the critical protein thiols dysfunctional before they were glutathionylated, or because the Ca2+-sensitizing effect of the glutathionylation is less marked in EDL fibres that have been deleteriously affected at other sites by the oxidative treatment.
Cause of reduction in Ca2+ sensitivity and possible site of glutathionylation
The reduction in Ca2+ sensitivity found here after oxidative treatment with H2O2 and myoglobin was evidently not due to loss or degradation of TnC (Fig. 5). Instead, the site(s) involved are likely to include or overlap with those recently identified on the myosin heavy chain S1 and the essential light chains, which are altered by exposure to very high concentrations of H2O2 and linked to similar changes in maximum force and Ca2+ sensitivity (Prochniewicz et al. 2008). Previous work had also identified the myosin S1 heads as being involved in such oxidative changes (Wilson et al. 1991). The study of Prochniewicz et al. (2008) suggested that oxidative damage to methionine residues was largely responsible for the effects, but the fact that the effects could be at least partially reversed with DTT in the skinned fibres here (provided the fibres had been not been activated), indicates that the overall oxidative changes also involved cysteine residues (see also Wilson et al. 1991; van der Poel & Stephenson, 2002).
The highly specific features of the glutathionylation effect on the contractile apparatus reported here and previously (Lamb & Posterino, 2003) make it reasonable to speculate about the site(s) involved. The effect closely resembles what happens in skeletal muscle fibres with phosphorylation of the myosin regulatory light chain (RLC). Firstly, it causes a comparable increase in Ca2+ sensitivity without any change in maximum Ca2+-activated force (Persechini et al. 1985; Sweeney & Stull, 1986; Szczesna et al. 2002). Secondly, the increased Ca2+ sensitivity only occurs in fast-twitch fibres and not in slow-twitch fibres (Ryder et al. 2007). Analogous to protein phosphorylation, the glutamate residue of glutathione adds a negative charge to the target protein, and the resulting steric effects could be expected to be similar to or even greater than with phosphorylation (Klatt & Lamas, 2000; Dalle-Donne et al. 2007). It is proposed that phosphorylation of the RLC increases Ca2+ sensitivity by bringing the myosin heads into closer proximity with actin (MacIntosh, 2003), and it seems entirely plausible that glutathionylation of the RLC could have similar effects. Consistent with this, the RLC of fast-twitch muscle has two cysteine residues to act as sites for glutathionylation, one of which (Cys-129) is crucial to regulatory function (Sachdev et al. 2003), whereas the RLC of slow-twitch muscle has no cysteine residues at all (Dalla Libera et al. 1989) and so could not be subject to glutathionylation.
Further comparisons with other studies
The striking difference found here between the effect of the H2O2–myoglobin–GSH treatment in EDL fibres and in soleus fibres (Fig. 3) is very similar to the changes in contractile apparatus Ca2+ sensitivity found in rat EDL and soleus fibres following fatiguing stimulation in vitro (Danieli-Betto et al. 2000). Danieli-Betto et al. (2000) speculated that the overall effect that they observed in EDL fibres may have been the sum of (i) a persistent decrease in pCa50 caused by a fatigue-linked mechanism and (ii) an increase in pCa50 caused by phosphorylation of the RLC. Although this could well be the case, the study found only a relatively minor increase in the level of RLC phosphorylation in the fatigued muscles (61% compared to 44% in rested muscles) and so the effect might be instead explained at least in part by glutathionylation of the RLC. Such an explanation could also apply to the results of Tubman et al. (1996) who found that when muscles were stimulated to fatigue, the level of RLC phosphorylation was relatively small but there was still considerable post-tetanic potentiation suggestive of enhanced Ca2+ sensitivity of the contractile apparatus.
In the experiments here the net effect of the opposing actions of glutathionylation and oxidative damage on Ca2+ sensitivity in EDL fibres varied over time, with the pCa50 first increasing and then decreasing (Table 2). Such effects might thus account for the findings of Andrade et al. (1998), where H2O2 (100–300 μm) applied to intact fast-twitch fibres caused the Ca2+ sensitivity to initially increase and then to subsequently decrease markedly (Andrade et al. 1998). The interplay of these opposing effects and their differential susceptibility to reversal by DTT might also account for other observations in intact fibres. For example, it was found that DTT exposure was able to reverse the decline in Ca2+ sensitivity occurring in fibres fatigued in vitro at 37°C, but that longer exposure to the DTT caused the Ca2+ sensitivity to decrease again (Moopanar & Allen, 2006). Such effects might be the result of the DTT exposure initially restoring intracellular levels of reduced glutathione (GSH), allowing glutathionylation of oxidized sites on the contractile apparatus, but with the continued exposure to the DTT subsequently reversing this glutathionylation and its sensitizing effect. Similar arguments also can explain the findings of Diaz et al. (1998), where intermediate concentrations of DTT enhanced force recovery in fatigued muscle but higher concentrations of DTT had no such beneficial effect.
EC coupling
Finally, we also examined what effects the H2O2–myoglobin treatment had on twitch and tetanic responses in skinned EDL fibres. The treatment caused a substantial reduction in maximum Ca2+-activated force, reducing peak tetanic force in every fibre examined (e.g. Fig. 6). Twitch force was reduced proportionately more, probably owing at least in part to a reduction in Ca2+ sensitivity of the contractile apparatus, as such reductions were found to occur more readily with oxidative treatment than did the reduction in maximum force (e.g. Fig. 2). Although the oxidative treatment quite possibly also adversely affected Ca2+ release or other events preceding it in the EC coupling sequence, there evidently was still sufficient Ca2+ release to activate the contractile apparatus at close to the maximal possible in the circumstances. As the level of maximum Ca2+-activated force measured after the oxidative treatment was less than the peak tetanic force observed before the treatment in every fibre examined, it can be concluded that at least part of the overall reduction in tetanic force must have been due to action of the oxidative treatment on the contractile apparatus. This is in accord with findings on ROS-induced changes occurring in intact fibres with exogenous ROS treatment (Andrade et al. 1998) and during fatigue (Moopanar & Allen, 2005, 2006; Bruton et al. 2007), which were due primarily changes in the contractile apparatus properties rather than to reduced Ca2+ release.
In conclusion, the findings of this study give new insight into the probable mechanistic basis for diverse findings on the effects of ROS on the contractile apparatus in muscle fibres, implicating OH• radicals and glutathione as likely mediators of these effects. The deleterious changes are likely to play a major role in the reduced skeletal muscle performance occurring not only after prolonged exercise but also in many disease states linked to aberrant ROS activity, including reperfusion injury and heart failure. On the other hand, the force potentiating effect of glutathionylation of the contractile proteins reported here is a largely unrecognized but probably important factor that may act to help maintain muscle performance in adverse conditions.
Acknowledgments
We thank Maria Cellini and Aida Yousef for expert technical assistance, and Professor George Stephenson for helpful input. Supported by the National Health & Medical Research Council of Australia (grants 280623, 433034 and 380842).
References
- Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 2008;88:287–332. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- Andrade FH, Reid MB, Allen DG, Westerblad H. Effect of hydrogen peroxide and dithiothreitol on contractile function of single skeletal muscle fibres from the mouse. J Physiol. 1998;509:565–575. doi: 10.1111/j.1469-7793.1998.565bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotto MA, Andreatta-van Leyen S, Nosek CM, Brotto LS, Nosek TM. Hypoxia and fatigue-induced modification of function and proteins in intact and skinned murine diaphragm muscle. Pflugers Arch. 2000;440:727–734. doi: 10.1007/s004240000327. [DOI] [PubMed] [Google Scholar]
- Bruton JD, Place N, Yamada T, Silva JP, Andrade FH, Dahlstedt AJ, Zhang SJ, Katz A, Larsson NG, Westerblad H. Reactive oxygen species and fatigue-induced prolonged low-frequency force depression in skeletal muscle fibres of rats, mice and SOD2 overexpressing mice. J Physiol. 2007;586:175–184. doi: 10.1113/jphysiol.2007.147470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan LA, She ZW, Nosek TM. Superoxide, hydroxyl radical, and hydrogen peroxide effects on single-diaphragm fiber contractile apparatus. J Appl Physiol. 2001;90:45–54. doi: 10.1152/jappl.2001.90.1.45. [DOI] [PubMed] [Google Scholar]
- Clanton TL, Zuo L, Klawitter P. Oxidants and skeletal muscle function: physiologic and pathophysiologic implications. Proc Soc Exp Biol Medical. 1999;222:253–262. doi: 10.1046/j.1525-1373.1999.d01-142.x. [DOI] [PubMed] [Google Scholar]
- Dalla Libera L, Hoffmann E, Floroff M, Jackowski G. Isolation and nucleotide sequence of the cDNA encoding human ventricular myosin light chain 2. Nucl Acids Res. 1989;17:2360. doi: 10.1093/nar/17.6.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalle-Donne I, Rossi R, Giustarini D, Colombo R, Milzani A. S-glutathionylation in protein redox regulation. Free Radic Biol Med. 2007;43:883–898. doi: 10.1016/j.freeradbiomed.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Danieli-Betto D, Germinario E, Esposito A, Biral D, Betto R. Effects of fatigue on sarcoplasmic reticulum and myofibrillar properties of rat single muscle fibers. J Appl Physiol. 2000;89:891–898. doi: 10.1152/jappl.2000.89.3.891. [DOI] [PubMed] [Google Scholar]
- de Paula Brotto M, van Leyen SA, Brotto LS, Jin JP, Nosek CM, Nosek TM. Hypoxia/fatigue-induced degradation of troponin I and troponin C: new insights into physiologic muscle fatigue. Pflugers Arch. 2001;442:738–744. doi: 10.1007/s004240100587. [DOI] [PubMed] [Google Scholar]
- Diaz PT, Costanza MJ, Wright VP, Julian MW, Diaz AJ, Clanton TL. Dithiothreitol improves recovery from in vitro diaphragm fatigue. Med Sci Sports Exerc. 1998;30:421–426. doi: 10.1097/00005768-199803000-00013. [DOI] [PubMed] [Google Scholar]
- Dutka TL, Cole L, Lamb GD. Calcium phosphate precipitation in the sarcoplasmic reticulum reduces action potential-mediated Ca2+ release in mammalian skeletal muscle. Am J Physiol Cell Physiol. 2005;289:C1502–C1512. doi: 10.1152/ajpcell.00273.2005. [DOI] [PubMed] [Google Scholar]
- Dutka TL, Murphy RM, Stephenson DG, Lamb GD. Chloride conductance in the transverse tubular system of rat skeletal muscle fibres: importance in excitation–contraction coupling and fatigue. J Physiol. 2008;586:875–887. doi: 10.1113/jphysiol.2007.144667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JN, Macdonald WA, van der Poel C, Stephenson DG. O2− production at 37°C plays a critical role in depressing tetanic force of isolated rat and mouse skeletal muscle. Am J Physiol Cell Physiol. 2007;293:C650–C660. doi: 10.1152/ajpcell.00037.2007. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. Oxford: Oxford University Press; 2007. [Google Scholar]
- Klatt P, Lamas S. Regulation of protein function by S-glutathiolation in response to oxidative and nitrosative stress. Eur J Biochem. 2000;267:4928–4944. doi: 10.1046/j.1432-1327.2000.01601.x. [DOI] [PubMed] [Google Scholar]
- Lamb GD, Posterino GS. Effects of oxidation and reduction on contractile function in skeletal muscle fibres of the rat. J Physiol. 2003;546:149–163. doi: 10.1113/jphysiol.2002.027896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb GD, Stephenson DG. Effects of intracellular pH and [Mg2+] on excitation–contraction coupling in skeletal muscle fibres of the rat. J Physiol. 1994;478:331–339. doi: 10.1113/jphysiol.1994.sp020253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunde PK, Sejersted OM, Thorud HM, Tonnessen T, Henriksen UL, Christensen G, Westerblad H, Bruton J. Effects of congestive heart failure on Ca2+ handling in skeletal muscle during fatigue. Circ Res. 2006;98:1514–1519. doi: 10.1161/01.RES.0000226529.66545.e5. [DOI] [PubMed] [Google Scholar]
- MacIntosh BR. Role of calcium sensitivity modulation in skeletal muscle performance. News Physiol Sci. 2003;18:222–225. doi: 10.1152/nips.01456.2003. [DOI] [PubMed] [Google Scholar]
- Moopanar TR, Allen DG. Reactive oxygen species reduce myofibrillar Ca2+ sensitivity in fatiguing mouse skeletal muscle at 37°C. J Physiol. 2005;564:189–199. doi: 10.1113/jphysiol.2005.083519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moopanar TR, Allen DG. The activity-induced reduction of myofibrillar Ca2+ sensitivity in mouse skeletal muscle is reversed by dithiothreitol. J Physiol. 2006;571:191–200. doi: 10.1113/jphysiol.2005.101105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy RM, Verburg E, Lamb GD. Ca2+ activation of diffusible and bound pools of μ-calpain in rat skeletal muscle. J Physiol. 2006;576:595–612. doi: 10.1113/jphysiol.2006.114090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persechini A, Stull JT, Cooke R. The effect of myosin phosphorylation on the contractile properties of skinned rabbit skeletal muscle fibers. J Biol Chem. 1985;260:7951–7954. [PubMed] [Google Scholar]
- Plant DR, Lynch GS, Williams DA. Hydrogen peroxide modulates Ca2+-activation of single permeabilized fibres from fast- and slow-twitch skeletal muscles of rats. J Muscle Res Cell Motil. 2000;21:747–752. doi: 10.1023/a:1010344008224. [DOI] [PubMed] [Google Scholar]
- Posterino GS, Cellini MA, Lamb GD. Effects of oxidation and cytosolic redox conditions on excitation–contraction coupling in rat skeletal muscle. J Physiol. 2003;546:149–163. doi: 10.1113/jphysiol.2002.035204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochniewicz E, Lowe DA, Spakowicz D, Higgins L, Oconor K, Thompson LV, Ferrington DA, Thomas DD. Functional, structural and chemical changes in myosin associated with hydrogen peroxide treatment of skeletal muscle fibers. Am J Physiol Cell Physiol. 2008;294:C613–C626. doi: 10.1152/ajpcell.00232.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MB. Nitric oxide, reactive oxygen species, and skeletal muscle contraction. Med Sci Sports Exerc. 2001;33:371–376. doi: 10.1097/00005768-200103000-00006. [DOI] [PubMed] [Google Scholar]
- Ryder JW, Lau KS, Kamm KE, Stull JT. Enhanced skeletal muscle contraction with myosin light chain phosphorylation by a calmodulin-sensing kinase. J Biol Chem. 2007;282:20447–20454. doi: 10.1074/jbc.M702927200. [DOI] [PubMed] [Google Scholar]
- Sachdev S, Raychowdhury MK, Sarkar S. Human fast skeletal myosin light chain 2 cDNA: isolation, tissue specific expression of the single copy gene, comparative sequence analysis of isoforms and evolutionary relationships. DNA Seq. 2003;14:339–350. doi: 10.1080/1042517031000154952. [DOI] [PubMed] [Google Scholar]
- Sostaric SM, Skinner SL, Brown MJ, Sangkabutra T, Medved I, Medley T, Selig SE, Fairweather I, Rutar D, McKenna MJ. Alkalosis increases muscle K+ release, but lowers plasma [K+] and delays fatigue during dynamic forearm exercise. J Physiol. 2006;570:185–205. doi: 10.1113/jphysiol.2005.094615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney HL, Stull JT. Phosphorylation of myosin in permeabilized mammalian cardiac and skeletal muscle cells. Am J Physiol Cell Physiol. 1986;250:C657–C660. doi: 10.1152/ajpcell.1986.250.4.C657. [DOI] [PubMed] [Google Scholar]
- Szczesna D, Zhao J, Jones M, Zhi G, Stull J, Potter JD. Phosphorylation of the regulatory light chains of myosin affects Ca2+ sensitivity of skeletal muscle contraction. J Appl Physiol. 2002;92:1661–1670. doi: 10.1152/japplphysiol.00858.2001. [DOI] [PubMed] [Google Scholar]
- Tubman LA, MacIntosh BR, Maki WA. Myosin light chain phosphorylation and posttetanic potentiation in fatigued skeletal muscle. Pflugers Arch. 1996;431:882–887. doi: 10.1007/s004240050081. [DOI] [PubMed] [Google Scholar]
- van der Poel C, Stephenson DG. Reversible changes in Ca2+-activation properties of rat skeletal muscle exposed to elevated physiological temperatures. J Physiol. 2002;544:765–776. doi: 10.1113/jphysiol.2002.024968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verburg E, Dutka TL, Lamb GD. Long-lasting muscle fatigue: partial disruption of excitation-contraction coupling by elevated cytosolic Ca2+ concentration during contractions. Am J Physiol Cell Physiol. 2006;290:C1199–C1208. doi: 10.1152/ajpcell.00469.2005. [DOI] [PubMed] [Google Scholar]
- Wilson GJ, dos Remedios CG, Stephenson DG, Williams DA. Effects of sulphydryl modification on skinned rat skeletal muscle fibres using 5,5′-dithiobis(2-nitrobenzoic acid) J Physiol. 1991;437:409–430. doi: 10.1113/jphysiol.1991.sp018603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberg JB. Myoglobin-facilitated oxygen diffusion: role of myoglobin in oxygen entry into muscle. Physiol Rev. 1970;50:559–636. doi: 10.1152/physrev.1970.50.4.559. [DOI] [PubMed] [Google Scholar]