Abstract
The perinatal environment is a powerful determinant of risk for developing disease in later life. Here, we have shown that maternal undernutrition causes dramatic changes in heart structure and hypothalamo-pituitary-adrenal (HPA) function across two generations. Pregnant guinea pigs were fed 70% of normal intake from gestational days 1–35 (early restriction; ER), or 36–70 (late restriction; LR). Female offspring (F1) were mated and fed ad libitum to create second generation (F2) offspring. Heart morphology, blood pressure, baroreceptor and HPA function were assessed in male F1 and F2 offspring. ERF1 males exhibited elevated blood pressure, increased left ventricular (LV) wall thickness and LV mass. These LV effects were maintained in the ERF2 offspring. Maternal undernutrition increased basal cortisol and altered HPA responsiveness to challenge in both generations; effects were greatest in LR groups. In conclusion, moderate maternal undernutrition profoundly modifies heart structure and HPA function in adult male offspring for two generations.
There is now considerable evidence that cardiovascular disease has its origins in prenatal life (Hanson & Gluckman, 2005). Epidemiological studies have demonstrated that size at birth is related to subsequent risk of Type 2 diabetes, insulin resistance, hypertension, cardiovascular disease and stroke (Barker et al. 1993). These relationships are strong, supported both by clinical investigation and experimental research (Hanson & Gluckman, 2005). Studies of human populations following famine have suggested that pathologies in later life are dependent on the timing of nutritional insult during pregnancy. Follow-up of the Dutch Hunger Winter cohort showed that cardiovascular disease was more prevalent in offspring of mothers who were severely undernourished during the first trimester of their pregnancies in 1944/5 compared to those born to mothers whose pregnancies were more advanced at the time of nutritional insult (Roseboom et al. 2001a).
A number of mechanisms by which the early environment can influence cardiovascular function have been proposed. These include changes in the renin–angiotensin system (Dodic et al. 1998), expression of glucocorticoid-inducible genes (Gardner et al. 1998; Bertram et al. 2001), vascular smooth muscle properties (Bendeck et al. 1994) and endothelial function (Torrens et al. 2003). The origins of these relationships remain largely unknown, but maternal exposure to stress or elevated glucocorticoids have been suggested as important causative factors. Recent studies in guinea pigs, rats and sheep suggest that exposure to suboptimal uterine environments caused by physiological insults such as restraint, poor nutrition, hypoxia or elevated gluco-corticoid exposure can induce changes in hypothalamic-pituitary-adrenal (HPA) axis function which persist throughout postnatal life (McCormick et al. 1995; Levitt et al. 1996; Liu et al. 2001; Lingas & Matthews, 2001; Sloboda et al. 2002; Lesage et al. 2002). Long-term alterations in HPA function have been linked to premature development of adult onset pathologies including insulin resistance and hypertension (Nyirenda et al. 1998; Reynolds et al. 2001; Welberg & Seckl, 2001; Matthews, 2002).
The guinea pig represents a good model for investigation of susceptibility to programming stimuli at specific time windows since, like humans, this species gives birth to relatively mature offspring (Dobbing & Sands, 1970, 1979; Owen & Matthews, 2003; Owen et al. 2005). While gestation length in the guinea pig is long for a small mammal (70 days), it is considerably shorter than that of larger mammals (e.g. primates and sheep), making transgenerational studies feasible.
There is emerging evidence that developmental effects induced by an environmental stimulus are not limited to the first generation (Drake & Walker, 2004). However, in epidemiological studies it is difficult to determine the relative importance of genomic and non-genomic effects of the pre- and postnatal environment, against heterogeneous backgrounds of genetic susceptibility. The impact of any effects in different populations will vary, making it difficult to assign cause to any one environmental factor. In contrast, animal studies provide strong support for the hypothesis that non-genomic intergenerational effects operate across a number of generations. Such effects have been demonstrated for insulin resistance and hepatic glucose regulation in the rat model (Drake et al. 2005; Zambrano et al. 2005, 2006). However, no studies have reported transgenerational effects on cardiovascular or HPA function. In the present study, we assessed the effect of timed-restricted nutrition during pregnancy on the cardiovascular system and HPA axis of guinea pig offspring, and ascertained whether effects were transmitted to the second generation in the absence of an additional nutritional challenge.
Methods
Animals and experimental treatments
All studies described were carried out in accordance with the UK Animals (Scientific Procedures) Act 1986. The protocols were also approved by the Ethics Committee of the University of Southampton. Nulliparous 3- to 4-month-old female Dunkin Hartley guinea pigs (n = 46; Halls Farm, Shropshire, UK) were mated as previously described (Banjanin et al. 2004). Briefly, onset of oestrus was defined as the time of vaginal membrane rupture. At this time, females were placed with one of four unrelated breeding males. After mating, guinea pigs were assigned to one of three groups, early restriction (ER, n = 15), late restriction (LR, n = 14) and control (C, n = 17), and housed in individual cages under 12 : 12 h light–dark conditions in visual, auditory and olfactory contact with other pregnant guinea pigs. Standard guinea pig/rabbit chow (Special Diet Services, Witham, UK: 3.4% fat, 18.5% protein, 10.2% cellulose, 9.4% ash, 0.33% methionine) was fed. In addition, all animals had free access to tap water supplemented with vitamin C (400 mg l−1, guinea pigs, like humans, are unable to produce vitamin C). Food-restricted animals were fed between 08.30 and 09.30 h daily. Animals on a restricted diet were given 70% of the dietary provision of the control group for either days 1–35 (ER) or days 36–70 (LR) of pregnancy. Daily intake was calculated using a small pilot study of pregnant guinea pigs under control conditions. During gestation, maternal weight was recorded twice weekly and food intake measured daily. At birth, litter size, weight, nose–rump and abdominal circumference of all pups were recorded. First generation CF1 (n = 10 female, n = 9 male), ERF1 (n = 15 female, n = 18 male) and LRF1 (n = 22 female, n = 18 male) offspring were weaned at 21 days of age, at which point males were housed individually. Non-sibling F1 females were used to generate the F2 generation. Where there was more than one female in a litter, the female that had a birth weight closest to the mean weight of the litter was used (CF1, n = 7; ERF1, n = 7; LRF1, n = 11). Animals were group housed until their second oestrus and mated with control males. F1 females were not subjected to any food restriction. Their offspring are referred to as CF2 (n = 10 female, n = 9 male), ERF2 (n = 7 female, n = 10 male) and LRF2 (n = 18 female, n = 22 male). Animals were left undisturbed apart from daily animal care and bi-weekly weight measurement.
At 90 days of age, polyvinyl catheters (PE90, Royem Scientific, Luton, UK) were surgically implanted into a carotid artery and a jugular vein of non-sibling male offspring (CF1n = 8, ERF1n = 7, LRF1n = 8, CF2n = 6, ERF2n = 6, LRF2n = 6), as previously described (Liu et al. 2001). Briefly, anaesthesia was induced by halothane inhalation (4% in O2), and maintained by isofluorane (1.5–2.0% in O2) and surgery was carried out under standard aseptic conditions. Local anaesthetic (lidocaine (lignocaine) hydrochloride, Astra Zeneca, UK) was administered prior to closure of the skin to minimize post-operative discomfort. A small guinea pig jacket with an integral spring was fitted, through which the arterial catheter was passed to attach to a Teflon swivel (Lomir Biomedicals, Montreal, Canada). This allowed full rotation of the catheter and unrestricted movement of the guinea pig. Analgesic (buprenorphine 0.1 mg kg−1i.m., Schering-Plough Ltd, UK) was given before the anaesthetic was discontinued and 24 h following surgery. Catheters were filled with heparinized saline and flushed daily. Animals were allowed to recover for a minimum of 5 days following surgery. We have shown that repeated sampling of animals catheterized in this way does not result in activation of the HPA axis (Liu & Matthews, 1999; Liu et al. 2001).
Cardiovascular analysis
Prior to surgery, cardiac function and morphology was assessed in conscious adult F1 and F2 males using echocardiography (by an operator blinded to treatment group) to measure the left ventricular wall (LVW) thickness, left ventricular mass (LVM) and fractional shortening (FS; a measure of cardiac function) using previously validated techniques. LVW thickness was measured using a Sonos 2500 scanner with 5 or 7.5 MHz linear transducers, and images taken from the left or right parasternal windows in either prone or right lateral decubitus position. Short axis views at the level of the tip of the mitral valve were used to obtain M mode targeted recordings. Left ventricular diastolic and systolic dimensions were measured using the leading edge technique. FS and LVM were calculated using the equations:
and
where LVD is left ventricular wall diameter in diastole, LVS is left ventricular wall diameter in systole and IVS is interventricular septal wall thickness. Baseline blood pressure and heart rate were measured 8 days post-surgery via the carotid artery catheter, using a small displacement pressure transducer connected to a MacLab/4e (AD Instruments, Chalgrove, UK) data acquisition system and using MacLab Chart 4.5.6 software. Blood pressure was measured continuously between 09.00 and 12.00 h. Chart analysis was performed using the same software to calculate systolic, diastolic and mean arterial pressure (MAP) and heart rate. Following this baseline recording, a bolus of phenylephrine (1.0 mg kg−1) was administered i.v. to measure both the sensitivity of the arterial vasculature to α-adrenergic stimulation, and also the set-point and gain of the baroreflex. The gain of the baroreflex was derived from the plot of blood pressure versus heart rate, as the slope of the linear portion of the plot; set-point of the reflex was taken as the blood pressure at which the mid-point between baseline heart rate and that following the phenylephrine bolus occurred.
Hypothalamo-pituitary-adrenal analysis
At 95 days of age, animals were challenged with dexamethasone (DEX) and corticotrophin-releasing hormone (CRH) to assess glucocorticoid feedback sensitivity and pituitary–adrenal responsiveness to activation by CRH, respectively. DEX (1 mg kg−1, i.v.; Sigma, Poole, UK) was administered at 09.00 h. Blood samples (250 μl per time point) were taken just prior to DEX administration (−30 and 0 min) and at +30, 60, 120 and 240 min following injection. This was followed by an injection of human CRH (0.5 μg kg−1, i.v.; Sigma, Poole, UK) at 13.00 h (240 min) and continued blood sample collection at 245, 255, 270, 300 and 360 min. The doses of DEX and CRH used in the current study were derived from our previous studies (Liu & Matthews, 1999; Liu et al. 2001; Banjanin et al. 2004). All sampling was done out of the line of sight of the animals, to reduce the possibility of stress to the animal at the time of drug administration/blood removal.
Double-antibody and coated tube radioimmunoassay (RIA) kits (ICN Biomedical Inc., Belgium) were used to determine plasma concentrations of adrenocorticotrophin (ACTH) and cortisol, respectively. These assays have been previously used in the guinea pig (Liu et al. 2001; Owen & Matthews, 2003). All samples from within each test were run in the same assay to eliminate interassay variability. Intra-assay coefficients of variance for ACTH and cortisol were 4.9% and 5.2%, respectively.
At the end of the sampling protocol, animals were killed with an overdose of pentobarbital (i.v.), immediately decapitated and trunk blood taken. The brains, pituitary, adrenals, livers and kidneys were removed and weighed. For each animal, the right hippocampus was dissected and weighed.
Statistical analysis
All data were expressed as mean ±s.e.m. For all tests, significance was set at P < 0.05. Statistical analysis was performed using multivariate analysis of variance (ANOVA), followed by Duncan's method of post hoc comparison or a Bonferroni post hoc test to compare replicate means. Gestation length, litter size, birth weight, blood pressure, heart rate, organ weights, body weights, organ-to-body/brain weight ratios, were analysed by one-way (prenatal treatment) ANOVA with two-sample t tests to test specific hypotheses. The growth data were analysed by repeated-measures ANOVA.
For analysis of treatment differences, ACTH and cortisol data were divided into DEX suppression (0–240 min) and CRH activation (240–360 min) periods and analysed using one-way (treatment) ANOVA on the integrated net area above (DEX suppression, AAC) or under (CRH activation, AUC) the curve. AAC was calculated using net change in cortisol or ACTH concentration between basal values at 0 (prior to DEX administration) and values at 240 min using the trapezoidal rule. Similarly, AUC was calculated using net change from values at 240–360 min.
Results
Physiological measurements
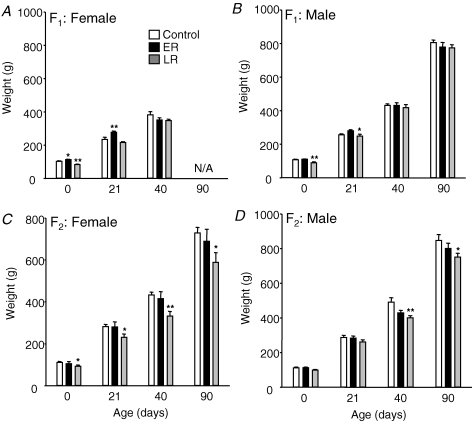
In the first generation, prenatal nutritional restriction had no effect on gestation length (CF1 69.1 ± 0.3 days, ERF1 69.2 ± 0.3 days, LRF1 69.1 ± 0.4 days). ERF1 females, but not males, were significantly heavier than controls at birth (Fig. 1). Longitudinal analysis across all time points in Fig. 1 showed increased absolute growth in ERF1 females (P < 0.04) but not males. In contrast, for LRF1, birth weight and growth were reduced in both females (P < 0.003) and males (P < 0.05). In the second generation, birth weight and growth in ERF2 did not differ from that of the controls. However, birth weight in the LRF2 females was significantly reduced. Longitudinal analysis across all time points showed reduced absolute growth in both LRF2 females (P < 0.02) and LRF2 males (P < 0.05). LRF1 females took significantly (P < 0.01) longer to conceive than CF1 or ERF1 females (age at pregnancy: C: 85 ± 13.7 days, ER 92.7 ± 13.6 days, LR 164.8 ± 11.8 days), although this delay was not associated with either changes in age or in weight at 1st oestrous (Table 1). Maternal body weight during pregnancy is shown in Table 2. There was considerable variability within groups, probably a result of the fact that guinea pigs carry 2–4 fetuses per litter and these each weigh approximately 100 g at birth.
Figure 1. Birth weight and growth.
Animal weights at birth and at 21 and 40 days (mean ±s.e.m.) in F1 females (A) and at birth and 21, 40 and 90 days of age in F1 male (B), F2 female (C) and F2 male (D) offspring born to control mothers (male F1n = 9, F2n = 9; female F1n = 10, F2n = 10) or mothers that had been nutritionally restricted (30%) during early (ER; gestational days 1–35; male F1n = 18, F2n = 10; female F1n = 15, F2n = 7) or late (LR; gestational days 36–70; male F1n = 18, F2n = 22; female F1n = 22, F2n = 18) pregnancy. *P < 0.05, **P < 0.01 denote significant differences compared with controls. N/A, data not available as F1 females were mated after day 40.
Table 1.
Reproductive function
| Age (days) | Body weight (g) | Successful mating (oestrous) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| First oestrous | First mating | Pregnancy | First oestrous | First mating | Pregnancy | 2nd | 3rd | 4th | 5th | |
| CF1 | 41.7 (1.7) | 56.7 (1.9) | 85.0 (13.7) | 389 (27.5) | 479 (28.4) | 692 (55.5) | 3 | 2 | 1 | 1 |
| ERF1 | 43.3 (2.6) | 54.0 (4.5) | 92.7 (13.6) | 376 (18.1) | 494 (21.7) | 565 (55.2) | 2 | 4 | 1 | 0 |
| LRF1 | 42.0 (1.8) | 54.7 (1.5) | 164.8 (11.8)** | 372 (14.5) | 452 (31.4) | 794 (26.4)** | 0 | 4 | 4 | 3 |
Age and weight at first oestrous and mating, together with body weight and mating outcome, in F1 generation female offspring whose mothers were nutritionally restricted during early (ER; n = 7) or late (LR; n = 11) pregnancy or fed ad libitum (control, C; n = 7). Group data (mean ±s.e.m.) were analysed using one-way analysis of variance (ANOVA) and Bonferroni post hoc comparison.
P < 0.01 denotes significant difference compared with controls.
Table 2.
Weight (g) during pregnancy of F0 and F1 females at different stages of gestation (days)
| 10 | 20 | 30 | 40 | 50 | 60 | 70 | |
|---|---|---|---|---|---|---|---|
| CF0 (n = 17) | 714.3 (32.2) | 747.0 (33.3) | 782.6 (30.4) | 856.6 (31.4) | 940.9 (32.6) | 1054.9 (36.4) | 1136.9 (37.5) |
| ERF0 (n = 15) | 645.1 (44.0) | 678.5 (45.4) | 713.8 (46.1) | 804.9 (48.9) | 933.8 (53.9) | 1051.9 (58.7) | 1133.8 (60.3) |
| LRF0 (n = 14) | 703.1 (38.9) | 738.3 (40.2) | 789.3 (34.7) | 864.0 (34.4) | 964.5 (36.5) | 1058.2 (40.9) | 1140.2 (42.1) |
| CF1 (n = 7) | 750.8 (71.8) | 787.9 (63.9) | 834.0 (67.4) | 898.1 (49.5) | 1002.8 (45.2) | 1098.9 (57.8) | 1138.9 (53.7) |
| ERF1 (n = 7) | 634.6 (44.0) | 687.8 (38.1) | 728.7 (38.1) | 816.0 (32.5) | 927.7 (38.3) | 1023.2 (45.8) | 1068.6 (42.6) |
| LRF2 (n = 11) | 840.6 (27.2) | 864.6 (23.1) | 888.7 (23.3) | 955.1 (27.9) | 1054.8 (30.3) | 1134.0 (34.6) | 1171.5 (32.1) |
Comparison of weight gain of F0 and F1 females through pregnancy. F0 females were nutritionally restricted during early (ERF0) or late (LRF0) pregnancy or fed ad libitum (CF0). These F0 females were the mothers of the F1 females (F1 females were fed ad libitum throughout pregnancy). Group data (mean ±s.e.m.) were analysed using one-way analysis of variance (ANOVA) and Bonferroni post hoc comparison.
Organ to body weight ratios for male offspring from the different prenatal treatment groups (both generations) are presented in Table 3. In the F1 generation, there was a significant decrease in pituitary-to-brain and adrenal-to-body weight ratios in both the ERF1 and LRF1 animals (P < 0.01). These corresponded to significant decreases in both pituitary and adrenal weights. Kidney- and brain-to-body weight ratios were significantly increased in the ERF2 and LRF2 groups. Liver to body weight ratio was higher in the LRF2 group compared to the controls (CF2); however, this appears to be related to a reduction in the CF2 liver to body weight ratio rather than an absolute increase in the LRF2. In contrast, there were significant reductions in relative adrenal weight in the LRF2 (but not ERF2) group, and although there were no differences in absolute brain weight, there were reductions in the hippocampal and pituitary weights (expressed as a proportion of brain weight) in both the ERF2 and LRF2 groups.
Table 3.
Organ weights in male offspring
| Percentage of body weight | Percentage of brain weight | ||||||
|---|---|---|---|---|---|---|---|
| Liver | Kidney | Adrenal | Brain | Brain weight (g) | Hippocampus | Pituitary | |
| CF1 | 3.6 (0.3) | 0.42 (0.03) | 0.035 (0.001) | 0.58 (0.01) | 4.2 (0.07) | 4.3 (0.33) | 0.47 (0.03) |
| ERF1 | 3.3 (0.1) | 0.44 (0.01) | 0.031 (0.001)** | 0.60 (0.01) | 4.3 (0.03) | 4.1 (0.33) | 0.41 (0.04)** |
| LRF1 | 3.2 (0.1) | 0.38 (0.01) | 0.026 (0.001)** | 0.58 (0.01) | 4.3 (0.06) | 4.2 (0.35) | 0.43 (0.03)** |
| CF2 | 2.9 (0.2) | 0.33 (0.01) | 0.039 (0.001) | 0.57 (0.01) | 4.7 (0.10) | 4.3 (0.23) | 0.43 (0.02) |
| ERF2 | 3.1 (0.1) | 0.41 (0.01)** | 0.041 (0.001) | 0.63 (0.02)** | 4.7 (0.08) | 3.8 (0.41)** | 0.39 (0.08) |
| LRF2 | 3.4 (0.1)** | 0.43 (0.01)** | 0.033 (0.001)** | 0.62 (0.01)** | 4.7 (0.06) | 4.0 (0.30)* | 0.40 (0.02)** |
Organ weights as percentage of body weight (brain, adrenal, liver and kidney) and hippocampal or pituitary weights as a percentage of brain weights in adult offspring whose mothers or grandmothers had been subjected to nutritional restriction during either days 1–35 of gestation (ERF1 (n = 7) and ERF2 (n = 6)) or days 36–70 (LRF1 (n = 8) and LRF2 (n = 6)) compared to control animals (CF1 (n = 8) and CF2 (n = 6)). Results are expressed as mean ±s.e.m.
P < 0.05,
P < 0.01 denote significant difference compared with controls of the same generation.
Cardiovascular function
Heart rate and blood pressure
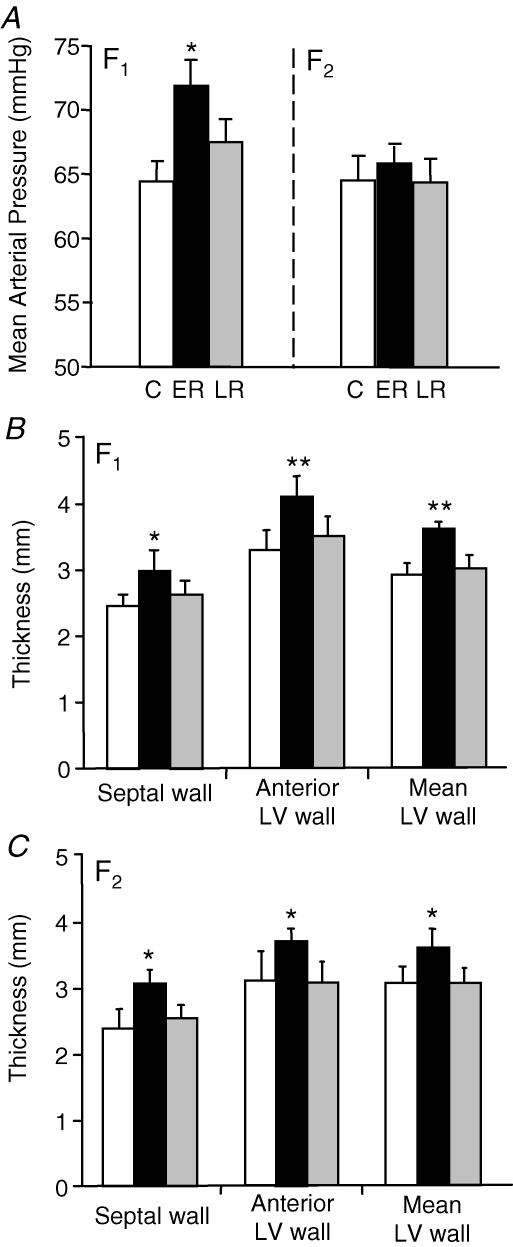
In the F1 generation males, MAP was significantly (P < 0.05) elevated in the ERF1 compared to control and LRF1 groups (Fig. 2A). There were no significant differences in heart rate between prenatal treatment groups (CF1 325 ± 6, ERF1 324 ± 9, LRF1 322 ± 10 beats min−1). In the F2 generation males, neither MAP (Fig. 2A) nor heart rate (data not shown) were different between groups.
Figure 2. Cardiovascular function.
A, mean arterial pressure (mean ±s.e.m.) in adult F1 and F2 male offspring whose mothers (F1) or grandmothers (F2) had been subjected to 30% reduction in total food intake during early (ER; black bars; F1n = 7, F2n = 6) or late (LR; grey bars; F1n = 8, F2n = 6) pregnancy or in offspring from control mothers (C; white bars; F1n = 8, F2n = 6). Echocardiography: interventricular septal wall thickness, anterior left ventricular wall thickness and mean left ventricular (LV) wall thickness (mean ±s.e.m.) in adult F1 (B) and F2 (C) male offspring in the same groups of animals. *P < 0.05, **P < 0.01 denote significant differences compared with controls of same generation.
Echocardiography
In the F1 generation males, IVS (P < 0.05), anterior left ventricle wall (LVW; P < 0.01) and mean LVW (P < 0.01) thicknesses were significantly greater in ERF1 than in CF1 or LRF1 groups (Fig. 2B). There was no difference in fractional shortening (FS; a measure of cardiac function) between the groups (Table 4), but the corrected left ventricular (LV) mass of ERF1 animals was significantly (P < 0.05) increased compared with the CF1 and LRF1 groups. In the F2 generation males, septal wall (P < 0.05), anterior LVW (P < 0.05) and mean LVW thicknesses (P < 0.05) were also significantly greater in ERF2 animals compared to CF2 and LRF2 groups (Fig. 2C). As with the F1 generation, there was no significant difference in FS between groups, but the corrected LV mass was significantly (P < 0.05) higher in the ERF2 group (Table 4).
Table 4.
Echocardiography
| Control | ER | LR | ||||
|---|---|---|---|---|---|---|
| F1 (n = 8) | F2 (n = 6) | F1 (n = 7) | F2 (n = 6) | F1 (n = 8) | F2 (n = 6) | |
| LV diameter systole (mm) | 8.75 (0.3) | 8.28 (0.5) | 8.80 (0.3) | 7.90 (0.2) | 8.62 (0.2) | 7.81 (0.2) |
| LV diameter diastole (mm) | 11.57 (0.3) | 11.47 (0.4) | 11.95 (0.3) | 10.80 (0.2) | 11.42 (0.1) | 10.44 (0.1) |
| Fractional shortening | 24.61 (1.1) | 25.12 (1.4) | 25.51 (1.3) | 26.67 (1.9) | 24.07 (1.7) | 25.49 (0.9) |
| Corrected LV mass (mg g−1) | 4.27 (0.2) | 4.22 (0.3) | 5.93 (0.3)* | 5.30 (0.1)* | 4.15 (0.3) | 3.82 (0.2) |
Biometric and cardiovascular data at 90 days of age in male offspring whose mothers (F1) or grandmothers (F2) had been subjected to 30% nutritional restriction during either days 1–35 of gestation (ER) or days 36–70 (LR) compared to control animals. Results are expressed as mean ±s.e.m.
P < 0.05 denotes significant difference compared to controls of same generation. LV, left ventricle.
Baroreflex responses
The baroreflex response sensitivity, in terms of the prolongation of R–R interval induced by elevation of blood pressure following a phenylephrine bolus, was significantly greater (P < 0.05) in the LRF1 males compared to the control (CF1 0.021 ± 0.002, ERF1 0.025 ± 0.004; LRF1 0.036 ± 0.007 ms mmHg−1). In addition, the set-point on the blood pressure axis of the baroreceptor response curve in the ERF1 males was significantly reduced (P < 0.05) compared to controls (CF1 95.0 ± 1.9, ERF1 86.4 ± 2.7; LRF1 91.8 ± 2.6 mmHg). There were no significant differences in baroreflex responses between groups in the F2 generation males.
Hypothalamo-pituitary-adrenal function
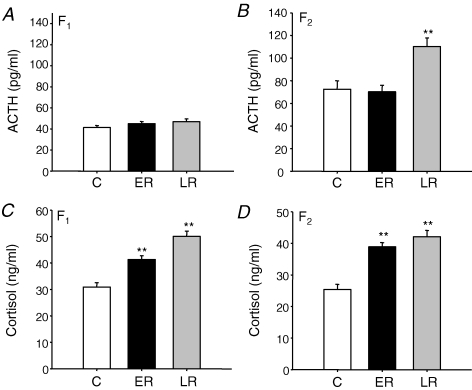
Basal plasma ACTH concentrations were not significantly different between prenatal treatment groups in the F1 generation males but were significantly increased in LRF2 males compared to the ERF2 and CF2 groups (Fig. 3A and B). However, in the F1 and F2 generations, basal plasma cortisol concentrations were significantly elevated in the ER and LR males compared to controls (Fig. 3C and D).
Figure 3. Basal HPA function.
ACTH (A and B) and cortisol (C and D) concentrations (mean ±s.e.m.) in adult F1 and F2 male offspring whose mothers or grandmothers had been subjected to 30% reduction in total food intake during early (ER; gestational days 1–35; F1n = 7, F2n = 6) or late (LR; gestational days 36–70; F1n = 8, F2n = 6) pregnancy or in controls (C; F1n = 8, F2n = 6). **P < 0.01 denotes significant differences compared with controls of same generation.
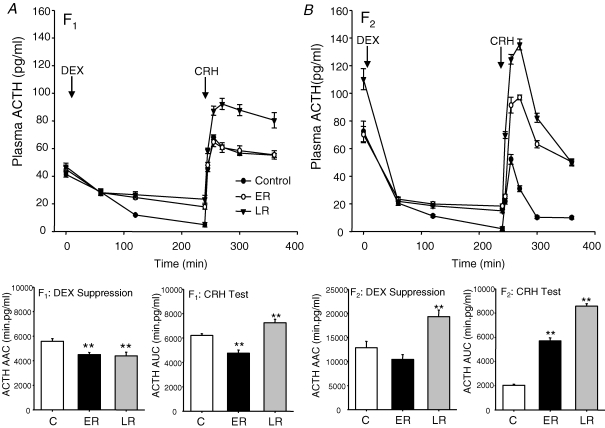
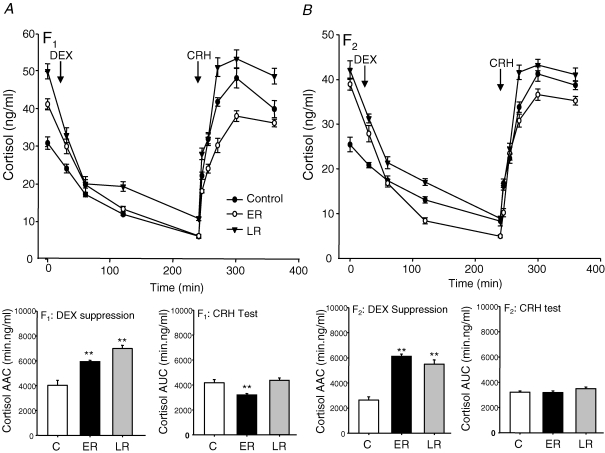
In the F1 generation, administration of DEX caused a significant suppression of plasma ACTH concentrations (CF1P < 0.001; ERF1P < 0.005; LRF1P < 0.005; Fig. 4A). Analysis of the net area above the curve (AAC) following suppression by DEX treatment revealed that both ERF1 and LRF1 animals exhibited a significantly reduced overall suppression of plasma ACTH concentrations compared to control animals. Following CRH injection, ERF1 animals exhibited a similar peak response to the controls, but because of the higher baseline following DEX suppression, the incremental change in ACTH concentrations was lower when analysis of the net AUC was undertaken (Fig. 4A). However, the LRF1 group exhibited a substantially increased plasma ACTH response whether assessed incrementally or absolutely (Fig. 4A). Administration of DEX caused a significant suppression of plasma cortisol concentrations (CF1P < 0.001; ERF1P < 0.005; LRF1P < 0.005; Fig. 5A). Net AAC analysis of the cortisol response following DEX administration showed significantly greater cortisol suppression in ERF1 and LRF1 animals compared to CF1 (Fig. 5A), although the post-DEX level of cortisol suppression measured at 120 and 240 min remained significantly higher in the LRF1 animals than the controls. Following CRH stimulation, AUC analysis revealed that the ERF1 group exhibited a significantly reduced plasma cortisol response compared with the other groups (Fig. 5A).
Figure 4. Dexamethasone suppression and corticotrophin-releasing hormone challenge (ACTH).
Plasma ACTH concentrations (mean ±s.e.m.) following dexamethasone (DEX, 1 mg kg−1) and corticotrophin-releasing hormone (CRH, 0.5 μg kg−1) challenge in adult male offspring whose mothers (A, F1) or grandmothers (B, F2) had been subjected to 30% reduction in total food intake during early (ER; gestational days 1–35; F1n = 7, F2n = 6) or late (LR; gestational days 36–70; F1n = 8, F2n = 6) pregnancy or in controls (C; F1n = 8, F2n = 6). **P < 0.01 denotes significant differences compared with controls of the same generation. Histograms represent net area above the curve (AAC) of plasma ACTH concentrations after suppression by DEX and incremental area under the curve (AUC) plasma ACTH after activation with CRH.
Figure 5. Dexamethasone suppression and corticotrophin-releasing hormone challenge (cortisol).
Plasma cortisol concentrations (mean ±s.e.m.) following dexamethasone (DEX, 1 mg kg−1) and corticotrophin-releasing hormone (CRH, 0.5 μg kg−1) challenge in adult male offspring whose mothers (A, F1) or grandmothers (B, F2) had been subjected to 30% reduction in total food intake during early (ER; gestational days 1–35; F1n = 7, F2n = 6) or late (LR; gestational days 36–70; F1n = 8, F2n = 6) pregnancy or in controls (C; F1n = 8, F2n = 6). **P < 0.01 denotes significant differences compared with controls of the same generation. Histograms represent net area above the curve (AAC) of plasma cortisol concentrations after suppression by DEX and incremental area under the curve (AUC) plasma cortisol after activation with CRH.
In the F2 generation, DEX administration led to significant suppression of plasma ACTH levels (CF2P < 0.001; ERF2P < 0.005; LRF2P < 0.005; Fig. 4B). Analysis of the plasma ACTH suppression (AAC) by DEX revealed a substantially increased net suppression in the LRF2 group of animals. Analysis of the net ACTH response (AUC) following CRH injection revealed very dramatic increases in the ACTH responses of both the ERF2 and LRF2 animals compared to controls (Fig. 4B). Administration of DEX led to significant suppression of cortisol (CF2P < 0.001; ERF2P < 0.005; LRF2P < 0.005; Fig. 5B). Plasma cortisol concentrations at 09.00 h were significantly elevated (P < 0.05) in LRF2 and ERF2 animals compared to CF2, and AAC analysis of the cortisol response to suppression by DEX administration showed a significant increase in overall cortisol suppression in ERF2 and LRF2 animals compared to CF2 (Fig. 5B). AUC analysis, however, revealed no overall differences in the level of plasma cortisol following activation by CRH in either ERF2 or LRF2 when compared to CF2 (Fig. 5B).
Discussion
We have demonstrated that reduced nutrition during pregnancy has profound long-term effects on specific aspects of cardiovascular and HPA function, and that these effects are dependent on the timing of insult. Importantly, this study also shows that effects remain in the second generation offspring, despite no manipulation of the F1 pregnancy.
Females exposed to early restriction had higher birth weights and postnatal growth, while both males and females exposed to late undernutrition had reduced birth and postnatal weights. These findings are intriguing as they accord with data from a human famine study. In the Dutch Hunger Winter Study, females who as fetuses were exposed to famine during the first trimester of pregnancy tended to have higher body weights, while those exposed in the last trimester had lower body weights; there was little effect in males (Ravelli et al. 1999). Strikingly, in the present study, an even greater effect of nutrient restriction in late gestation was identified in the F2 generation, despite no manipulation during their development. The late restricted F2 generation are also characterized by hypercortisolaemia and it is likely that their growth restriction is, at least in part, a consequence of glucocorticoid-mediated inhibition of growth, although other mechanisms may be involved, for example epigenetic modification of genes that regulate growth.
LRF1 females had a marked delay in conception which was not a result of altered body weight. This resulted in pregnant LRF1 females being twice the age of the CF1 and ERF1 females at conception. This novel observation may have parallels with the increasing body of human evidence that prenatal growth restriction results in reduced ovarian and uterine size, reduced fraction of primordial follicles and anovulation in late adolescence (Ibáñez et al. 2000). Another apparent difference between the pregnant LRF1 females and the CF1 and ERF1 females was the reduced body weight gain during pregnancy in the pregnant LRF1 females. This is most probably related to age at pregnancy, as these animals were approximately 70 days older at the time of pregnancy than the CF1 and ERF1 groups. While guinea pigs are sexually mature at approximately 8 weeks of age they are still growing at this time. Therefore, part of the increase in weight during pregnancy in the CF1 and ERF1 groups is due to normal growth. In contrast, the older LRF1 animals are likely to have achieved adult body weight prior to pregnancy. We had not anticipated the reduced conception rate in the LRF1 and so were not able to factor differences in body weight into the experimental design. However, it is possible that maternal metabolic differences between the CF1 and ERF1 groups during pregnancy compared to the LRF1 group may impact on fetal development. Notwithstanding, our data illustrate more profound influences of nutritional restriction on cardiovascular and endocrine phenotype in ERF2 offspring compared to LRF2 offspring. Measurement of endocrine and nutrient levels in the blood of the F1 dams would have given us additional mechanistic insight. However, catheterization or venepuncture are stressful, and we have previously shown that stress during pregnancy has profound effects on the offspring (Kapoor & Matthews, 2005). We did not make such measurements because the aim of this study was to examine the effect of suboptimal maternal nutrition on the offspring, and the results would have been confounded by additional stress.
The increase in MAP that we identified in the ERF1 offspring was associated with increased IVS and anterior LVW thickness. There was no growth restriction at any time in these offspring. In contrast, the LRF1 offspring were growth restricted but did not display alterations in blood pressure or LV structure. Clearly there is no direct relation between reduction in fetal growth and these cardiovascular effects. This accords with the idea that reduced growth is not on the causal pathway to later cardiovascular dysfunction, although it may be part of an adaptive strategy adopted by the developing organism (Hanson & Gluckman, 2005). Likewise, effects on cardiac development, or endothelial function leading to alterations in blood pressure, may represent distinct strategies which are induced to give later adaptive advantage (Hanson & Gluckman, 2005). The induction of these effects by an early gestation challenge has important implications for humans, since retrospective studies of the Dutch Winter Famine have indicated that adults whose mothers were exposed to famine in the first trimester of pregnancy had increased risk of cardiovascular disease (Roseboom et al. 2001b). In sheep, global undernutrition during the first half of pregnancy leads to increased fetal LVM by mid-gestation (Vonnahme et al. 2003).
In the F2 generation, male offspring of ERF1 mothers demonstrated a strikingly similar LV enlargement to that in the ERF1 males, although this was not accompanied by elevated blood pressure. Therefore, the increased LV thickness in ERF2 animals is not a response to increased blood pressure. These findings clearly indicate that prenatal undernutrition can induce changes in cardiovascular structure and function which are passed across generations. Such non-genomic transmission clearly merits further mechanistic investigation. In addition, it was only feasible in this study to examine transmission via the female F1 lineage. Future studies are needed to determine if similar effects can be passed via the male F1 lineage, and indeed whether they are greater in F2 offspring from female F1 and male F1 crosses.
The mechanisms by which the early environment influences the developing fetal heart are not known. It is possible that maternal undernutrition causes increased growth of the fetal heart, either directly, or in response to changes in the fetal peripheral circulation. This may involve an increase in the number of cardiomyocytes (Lijnen & Petrov, 1999; Pham et al. 2003; Han et al. 2004). Alternatively, maternal undernutrition during early gestation may induce premature maturation of the myocardium, reducing the number of cardiomyocytes at birth and thus exposing the heart to greater stress and hence hypertrophic increase in cardiomyocyte size postnatally (Yang et al. 2001). Finally, it is possible that early nutrient restriction causes epigenetic modification of genes that mediate cardiac hypertrophy in response to load; this may not affect fetal cardiomyocyte size or number but would predispose to the development of hypertrophy postnatally (Kuznetsova et al. 2003).
In the present study, baroreflex sensitivity to phenylephrine was increased in LRF1 offspring, while baroreflex set-point was significantly reduced in ERF1 offspring. Prenatal nutritional challenge has been shown to modify baroreflex function in rats and sheep (Gardner et al. 2004; Pladys et al. 2004). Interestingly, Gardner also found a left-shift in baroreflex function in lambs whose mothers had been nutritionally challenged during the periconceptual period (Gardner et al. 2004). Baroreceptors are critical for adapting to immediate changes in blood pressure. The lower set-point in the ERF1 group may be viewed as a compensatory mechanism to reduce the elevated blood pressure in this group. Similarly, it is possible that the greater gain of the reflex in the LRF1 animals had successfully brought MAP back to control levels.
Previous studies have shown that manipulation of the prenatal environment can alter HPA function in young and adult offspring (Welberg & Seckl, 2001; Owen et al. 2005). However, this is the first to demonstrate that the effects of undernutrition on HPA function are also present in the F2 generation. Prenatal nutrient restriction had no effect on basal plasma ACTH concentrations in the F1 generation. However, in F2, basal ACTH levels were very substantially increased in the LR but not the ER group. In contrast to ACTH, plasma cortisol concentrations were significantly elevated in the ER and LR offspring in both F1 and F2; the effect was greatest in the LR group. This would suggest a substantial increase in adrenal sensitivity to circulating ACTH; except for in the LRF2 offspring where both basal plasma ACTH and cortisol were elevated. Alternatively, there may be a reduction in glucocorticoid metabolism, possibly resulting from an increase in corticosteroid-binding globulin. In a previous study, an acute period (48 h) of nutrient withdrawal, at the time of maximal fetal brain growth, resulted in adult F1 male guinea pig offspring that exhibited a reduction in basal plasma ACTH and cortisol concentrations (Lingas & Matthews, 2001). In contrast, chronic severe nutrient restriction (50%) during the perinatal period in the rat had no effect on basal ACTH or corticosterone concentrations in adult male offspring (Lesage et al. 2002). Together these studies suggest that the effects of nutrient restriction on HPA function in adult F1 offspring are dependent on the severity and timing of the nutrient deprivation. In the present study, there was a reduction in adrenal to body weight ratio in the ERF1, LRF1 and the LRF2 offspring indicating that hypercortisolaemia was not associated with adrenal hypertrophy or hyperplasia. Chronic elevation of glucocorticoids can result in a reduction in hippocampal volume in the rat and human (Sapolsky, 1999; Lupien & Lepage, 2001; Roy & Sapolsky, 2003). In the present study, there was a significant reduction in hippocampal weight relative to brain weight in the ERF2 and LRF2 offspring compared to controls. Further analysis of neurological function in these animals is clearly warranted.
DEX suppression followed by CRH challenge is used for clinical assessment in humans (Newport et al. 2004). Reduced suppression of plasma cortisol following DEX exposure represents a sensitive test for depression. This approach provides a reproducible analysis of HPA function across many species (Banjanin et al. 2004). In the present study, there was a reduction in suppression of ACTH following DEX treatment in both the ERF1 and LRF1 offspring. The anterior pituitary represents the major feedback site for DEX (Newport et al. 2004), and as such the reduced ACTH suppression may result from a reduction in pituitary glucocorticoid receptors (GR). Surprisingly, the reduced ACTH suppression was associated with a greater suppression of plasma cortisol in ERF1 and LRF1 offspring. However, the latter probably resulted from the fact that basal plasma cortisol levels were elevated in both groups. CRH challenge following DEX suppression activated pituitary–adrenal function. Interestingly, there was a reduced net response of ACTH and cortisol in the ERF1 offspring compared to controls. This would suggest a reduced pituitary sensitivity to CRH and a resultant reduction in pituitary–adrenocortical response.
There were very significant effects of maternal nutrient restriction on HPA reactivity to the DEX–CRH challenge in F2 offspring. The increased suppression of plasma ACTH by DEX in the LRF2 offspring was probably a result of the significant elevation in basal ACTH. Interestingly, DEX treatment in control F1 and F2 offspring resulted in an almost total loss of ACTH in the circulation, whereas this was not the case in ER and LR offspring of both generations, further indicating reduced GR sensitivity at the anterior pituitary. The net suppression of cortisol following DEX treatment was > 2-fold elevated in the ERF2 and LRF2 offspring compared to controls. In humans, similar super-suppression of cortisol by DEX has been identified in post-traumatic stress disorder (Fries et al. 2005). In this regard, future studies of stress-related behaviour in animal models following nutrient restriction are required. In the present study, CRH challenge resulted in 2- and 3-fold greater ACTH response in the ERF2 and LRF2 offspring compared to controls. This would suggest a dramatic increase in pituitary sensitivity to CRH. However, these elevations in the ACTH response to CRH were not reflected by increased cortisol secretion from the adrenal cortex. This may result from a reduction in adrenocortical sensitivity or a decrease in the bioactivity of ACTH isoforms being secreted from the pituitary. Alternatively, it is possible that plasma ACTH (following CRH treatment) is maximally driving the adrenal cortex, such that further elevation of ACTH, as in ERF2 and LRF2 offspring, can cause no further elevation of cortisol secretion (i.e. a ‘ceiling’ effect). The latter may be resolved by decreasing the concentration of exogenously administered CRH, and clearly further studies are required.
The mechanisms that underlie transgenerational programming of cardiovascular and HPA function remain to be determined. Two major possibilities exist. Altered endocrine function in the ERF1 and LRF1 females results in altered metabolic, cardiovascular or endocrine adaptations which are known to be critical for pregnancy; this in turn influences development of the fetus. Alternatively, transgenerational transmission may occur via persistent epigenetic modification of DNA. Recent studies have shown that the early environment can have dramatic influences on methylation and histone acetylation in specific gene promoters including the GR, in F1 offspring (Weaver et al. 2004; Lillycrop et al. 2005). Furthermore, a recent study has indicated that these epigenetic effects can be transgenerationally transmitted (Burdge et al. 2007), in which it is shown for the first time that altered methylation of gene promoters for peroxisome proliferator-activated receptor-alpha (PPARalpha) and GR induced in the F1 generation by protein restriction during pregnancy is transmitted to the F2 generation. There is also emerging evidence for transgenerational transmission down the paternal as well as the maternal line (Drake & Walker, 2004; Gluckman et al. 2007). Future studies will be designed to investigate this possibility in our undernutrition model.
In conclusion, moderate global nutrient restriction during pregnancy has profound effects on growth, reproductive efficiency, cardiovascular system, baro-receptor function and the HPA axis, and the timing of insult profoundly influences outcome. We have also demonstrated, for the first time, that it is possible for the effects which maternal nutritional restriction have on the cardiovascular system and the HPA axis, manifested in the first generation, to be passed to the second generation in the absence of any further manipulation. This has clear implications for our understanding of the development of human disease in later life as well as its non-genomic transgenerational transmission.
Acknowledgments
The authors would like to thank Claire Loades for her assistance in these studies. This work was supported by the British Heart Foundation (M.A.H. and S.G.M. PG/02/139/14633), the HOPE charity (M.A.H.) and the Canadian Institutes of Health Research (S.G.M. MOP-49511).
References
- Banjanin S, Kapoor A, Matthews S. Prenatal glucocorticoid exposure alters hypothalamic-pituitary-adrenal function and blood pressure in mature male guinea pigs. J Physiol. 2004;558:305–318. doi: 10.1113/jphysiol.2004.063669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia. 1993;36:62–67. doi: 10.1007/BF00399095. [DOI] [PubMed] [Google Scholar]
- Bendeck MP, Keeley FW, Langille BL. Perinatal accumulation of arterial wall constituents: relation to hemodynamic changes at birth. Am J Physiol Heart Circ Physiol. 1994;267:H2268–H2279. doi: 10.1152/ajpheart.1994.267.6.H2268. [DOI] [PubMed] [Google Scholar]
- Bertram C, Trowern AR, Copin N, Jackson AA, Whorwood CB. The maternal diet during pregnancy programs altered expression of the glucocorticoid receptor and type 2, 11β-hydroxysteroid dehydrogenase: potential molecular mechanisms underlying the programming of hypertension in utero. Endocrinology. 2001;142:2841–2853. doi: 10.1210/endo.142.7.8238. [DOI] [PubMed] [Google Scholar]
- Burdge GC, Slater-Jefferies J, Torrens C, Phillips ES, Hanson MA, Lillycrop KA. Dietary protein restriction of pregnant rats in the F0 generation induces altered methylation of hepatic gene promoters in the adult male offspring in the F1 and F2 generations. Br J Nutr. 2007;97:435–439. doi: 10.1017/S0007114507352392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Growth and development of the brain and spinal cord of the guinea pig. Brain Res. 1970;17:115–123. doi: 10.1016/0006-8993(70)90311-2. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Dodic M, May CN, Wintour EM, Coghlan JP. An early prenatal exposure to excess glucocorticoid leads to hypertensive offspring in sheep. Clin Sci. 1998;94:149–155. doi: 10.1042/cs0940149. [DOI] [PubMed] [Google Scholar]
- Drake AJ, Walker BR. The intergenerational effects of fetal programming: non-genomic mechanisms for the inheritance of low birth weight and cardiovascular risk. J Endocrinol. 2004;180:1–16. doi: 10.1677/joe.0.1800001. [DOI] [PubMed] [Google Scholar]
- Drake AJ, Walker BR, Seckl JR. Intergenerational consequences of fetal programming by in utero exposure to glucocorticoids in rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R34–R38. doi: 10.1152/ajpregu.00106.2004. [DOI] [PubMed] [Google Scholar]
- Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30:1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Gardner DS, Jackson AA, Langley-Evans SC. The effect of prenatal diet and glucocorticoids on growth and systolic blood pressure in the rat. Proc Nutr Soc. 1998;57:235–240. doi: 10.1079/pns19980037. [DOI] [PubMed] [Google Scholar]
- Gardner DS, Pearce S, Dandrea J, Walker R, Ramsay MM, Stephenson T, Symonds ME. Peri-implantation undernutrition programs blunted angiotensin II evoked baroreflex responses in young adult sheep. Hypertension. 2004;43:1290–1296. doi: 10.1161/01.HYP.0000126991.67203.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Beedle AS. Early life events and their consequences for later disease: a life history and evolutionary perspective. Am J Hum Biol. 2007;19:1–19. doi: 10.1002/ajhb.20590. [DOI] [PubMed] [Google Scholar]
- Han HC, Austin KJ, Nathanielsz PW, Ford SP, Nijland MJ, Hansen TR. Maternal nutrient restriction alters gene expression in the ovine fetal heart. J Physiol. 2004;558:111–121. doi: 10.1113/jphysiol.2004.061697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MA, Gluckman PD. Developmental processes and the induction of cardiovascular function: conceptual aspects. J Physiol. 2005;565:27–34. doi: 10.1113/jphysiol.2004.082339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibáñez L, Potau N, Enriquez G, de Zegher F. Reduced uterine and ovarian size in adolescent girls born small for gestational age. Pediatr Res. 2000;47:575–577. doi: 10.1203/00006450-200005000-00003. [DOI] [PubMed] [Google Scholar]
- Kapoor A, Matthews SG. Short periods of prenatal stress affect growth, behaviour and hypothalamo-pituitary-adrenal axis activity in male guinea pig offspring. J Physiol. 2005;566:967–977. doi: 10.1113/jphysiol.2005.090191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova T, Staessen JA, Olszanecka A, Ryabikov A, Stolarz K, Malyutina S, Fagard R, Kawecka-Jaszcz K, Nikitin Y. Maternal and paternal influences on left ventricular mass of offspring. Hypertension. 2003;41:69–74. doi: 10.1161/01.hyp.0000042429.62541.a9. [DOI] [PubMed] [Google Scholar]
- Lesage J, Dufourny L, Laborie C, Bernet F, Blondeau B, Avril I, Breant B, Dupouy JP. Perinatal malnutrition programs sympathoadrenal and hypothalamic-pituitary-adrenal axis responsiveness to restraint stress in adult male rats. J Neuroendocrinol. 2002;14:135–143. doi: 10.1046/j.0007-1331.2001.00753.x. [DOI] [PubMed] [Google Scholar]
- Levitt NS, Lindsay RS, Holmes MC, Seckl JR. Dexamethasone in the last week of pregnancy attenuates hippocampal glucocorticoid receptor gene expression and elevates blood pressure in the adult offspring in the rat. Neuroendocrinology. 1996;64:412–418. doi: 10.1159/000127146. [DOI] [PubMed] [Google Scholar]
- Lijnen P, Petrov V. Renin-angiotensin system, hypertrophy and gene expression in cardiac myocytes. J Mol Cell Cardiol. 1999;31:949–970. doi: 10.1006/jmcc.1999.0934. [DOI] [PubMed] [Google Scholar]
- Lillycrop KA, Phillips ES, Jackson AA, Hanson MA, Burdge GC. Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. J Nutr. 2005;135:1382–1386. doi: 10.1093/jn/135.6.1382. [DOI] [PubMed] [Google Scholar]
- Lingas R, Matthews SG. A short period of maternal nutrient restriction in late gestation modifies pituitary-adrenal function in adult guinea pig offspring. Neuroendocrinology. 2001;73:302–311. doi: 10.1159/000054647. [DOI] [PubMed] [Google Scholar]
- Liu L, Li A, Matthews SG. Maternal glucocorticoid treatment programs HPA regulation in adult offspring: sex-specific effects. Am J Physiol Endocrinol Metab. 2001;280:E729–E739. doi: 10.1152/ajpendo.2001.280.5.E729. [DOI] [PubMed] [Google Scholar]
- Liu L, Matthews SG. Adrenocortical response profiles to corticotrophin-releasing hormone and adrenocorticotrophin challenge in the chronically catheterized adult guinea-pig. Exp Physiol. 1999;84:971–977. [PubMed] [Google Scholar]
- Lupien SJ, Lepage M. Stress, memory, and the hippocampus: can't live with it, can't live without it. Behav Brain Res. 2001;127:137–158. doi: 10.1016/s0166-4328(01)00361-8. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Smythe JW, Sharma S, Meaney MJ. Sex-specific effects of prenatal stress on hypothalamic-pituitary-adrenal responses to stress and brain glucocorticoid receptor density in adult rats. Dev Brain Res. 1995;84:55–61. doi: 10.1016/0165-3806(94)00153-q. [DOI] [PubMed] [Google Scholar]
- Matthews SG. Early programming of the hypothalamo-pituitary-adrenal axis. Trends Endocrinol Metab. 2002;13:373–380. doi: 10.1016/s1043-2760(02)00690-2. [DOI] [PubMed] [Google Scholar]
- Newport DJ, Heim C, Bonsall R, Miller AH, Nemeroff CB. Pituitary-adrenal responses to standard and low-dose dexamethasone suppression tests in adult survivors of child abuse. Biol Psychiatry. 2004;55:10–20. doi: 10.1016/s0006-3223(03)00692-9. [DOI] [PubMed] [Google Scholar]
- Nyirenda MJ, Lindsay RS, Kenyon CJ, Burchell A, Seckl JR. Glucocorticoid exposure in late gestation permanently programs rat hepatic phosphoenolpyruvate carboxykinase and glucocorticoid receptor expression and causes glucose intolerance in adult offspring. J Clin Invest. 1998;101:2174–2181. doi: 10.1172/JCI1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen D, Andrews MH, Matthews SG. Maternal adversity, glucocorticoids and programming of neuroendocrine function and behaviour. Neurosci Biobehav Rev. 2005;29:209–226. doi: 10.1016/j.neubiorev.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Owen D, Matthews SG. Glucocorticoids and sex-dependent development of brain glucocorticoid and mineralocorticoid receptors. Endocrinology. 2003;144:2775–2784. doi: 10.1210/en.2002-0145. [DOI] [PubMed] [Google Scholar]
- Pham TD, MacLennan NK, Chiu CT, Laksana GS, Hsu JL, Lane RH. Uteroplacental insufficiency increases apoptosis and alters p53 gene methylation in the full-term IUGR rat kidney. Am J Physiol Regul Integr Comp Physiol. 2003;285:R962–R970. doi: 10.1152/ajpregu.00201.2003. [DOI] [PubMed] [Google Scholar]
- Pladys P, Lahaie I, Cambonie G, Thibault G, Le NL, Abran D, Nuyt AM. Role of brain and peripheral angiotensin II in hypertension and altered arterial baroreflex programmed during fetal life in rat. Pediatr Res. 2004;55:1042–1049. doi: 10.1203/01.PDR.0000127012.37315.36. [DOI] [PubMed] [Google Scholar]
- Ravelli AC, Der Meulen JH, Osmond C, Barker DJ, Bleker OP. Obesity at the age of 50 y in men and women exposed to famine prenatally. Am J Clin Nutr. 1999;70:811–816. doi: 10.1093/ajcn/70.5.811. [DOI] [PubMed] [Google Scholar]
- Reynolds RM, Walker BR, Syddall HE, Andrew R, Wood PJ, Whorwood CB, Phillips DI. Altered control of cortisol secretion in adult men with low birth weight and cardiovascular risk factors. J Clin Endocrinol Metabolism. 2001;86:245–250. doi: 10.1210/jcem.86.1.7145. [DOI] [PubMed] [Google Scholar]
- Roseboom TJ, Van Der Meulen JH, Osmond C, Barker DJ, Ravelli AC, Bleker OP. Adult survival after prenatal exposure to the Dutch famine 1944–45. Paediatr Perinat Epidemiol. 2001a;15:220–225. doi: 10.1046/j.1365-3016.2001.00336.x. [DOI] [PubMed] [Google Scholar]
- Roseboom TJ, Van Der Meulen JH, Van Montfrans GA, Ravelli AC, Osmond C, Barker DJ, Bleker OP. Maternal nutrition during gestation and blood pressure in later life. J Hypertens. 2001b;19:29–34. doi: 10.1097/00004872-200101000-00004. [DOI] [PubMed] [Google Scholar]
- Roy M, Sapolsky RM. The exacerbation of hippocampal excitotoxicity by glucocorticoids is not mediated by apoptosis. Neuroendocrinology. 2003;77:24–31. doi: 10.1159/000068337. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Why stress is bad for your brain. Science. 1999;273:749–750. doi: 10.1126/science.273.5276.749. [DOI] [PubMed] [Google Scholar]
- Sloboda DM, Moss TJ, Gurrin LC, Newnham JP, Challis JR. The effect of prenatal betamethasone administration on postnatal ovine hypothalamic-pituitary-adrenal function. J Endocrinol. 2002;172:71–81. doi: 10.1677/joe.0.1720071. [DOI] [PubMed] [Google Scholar]
- Torrens C, Brawley L, Barker AC, Itoh S, Poston L, Hanson MA. Maternal protein restriction in the rat impairs resistance artery but not conduit artery function in pregnant offspring. J Physiol. 2003;547:77–84. doi: 10.1113/jphysiol.2002.026120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonnahme KA, Hess BW, Hansen TR, McCormick RJ, Rule DC, Moss GE, Murdoch WJ, Nijland MJ, Skinner DC, Nathanielsz PW, Ford SP. Maternal undernutrition from early- to mid-gestation leads to growth retardation, cardiac ventricular hypertrophy, and increased liver weight in the fetal sheep. Biol Reprod. 2003;69:133–140. doi: 10.1095/biolreprod.102.012120. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Welberg LA, Seckl JR. Prenatal stress, glucocorticoids and the programming of the brain. J Neuroendocrinol. 2001;13:113–128. doi: 10.1046/j.1365-2826.2001.00601.x. [DOI] [PubMed] [Google Scholar]
- Yang G, Meguro T, Hong C, Asai K, Takagi G, Karoor VL, Sadoshima J, Vatner DE, Bishop SP, Vatner SF. Cyclosporine reduces left ventricular mass with chronic aortic banding in mice, which could be due to apoptosis and fibrosis. J Mol Cell Cardiol. 2001;33:1505–1514. doi: 10.1006/jmcc.2001.1413. [DOI] [PubMed] [Google Scholar]
- Zambrano E, Bautista CJ, Deas M, Martinez-Samayoa PM, Gonzalez-Zamorano M, Ledesma H, Morales J, Larrea F, Nathanielsz PW. A low maternal protein diet during pregnancy and lactation has sex- and window of exposure-specific effects on offspring growth and food intake, glucose metabolism and serum leptin in the rat. J Physiol. 2006;571:221–230. doi: 10.1113/jphysiol.2005.100313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrano E, Martinez-Samayoa PM, Bautista CJ, Deas M, Guillen L, Rodriguez-Gonzalez GL, Guzman C, Larrea F, Nathanielsz PW. Sex differences in transgenerational alterations of growth and metabolism in progeny (F2) of female offspring (F1) of rats fed a low protein diet during pregnancy and lactation. J Physiol. 2005;566:225–236. doi: 10.1113/jphysiol.2005.086462. [DOI] [PMC free article] [PubMed] [Google Scholar]