Abstract
The leukaemia inhibitory factor (LIF) belongs to the interleukin (IL)-6 cytokine superfamily and is constitutively expressed in skeletal muscle. We tested the hypothesis that LIF expression in human skeletal muscle is regulated by exercise. Fifteen healthy young male volunteers performed either 3 h of cycle ergometer exercise at ∼60% of 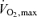 (n = 8) or rested (n = 7). Muscle biopsies were obtained from the vastus lateralis prior to exercise, immediately after exercise, and at 1.5, 3, 6 and 24 h post exercise. Control subjects had biopsy samples taken at the same time points as during the exercise trial. Skeletal muscle LIF mRNA increased immediately after the exercise and declined gradually during recovery. However, LIF protein was unchanged at the investigated time points. Moreover, we tested the hypothesis that LIF mRNA and protein expressions are modulated by calcium (Ca2+) in primary human skeletal myocytes. Treatment of myocytes with the Ca2+ ionophore, ionomycin, for 6 h resulted in an increase in both LIF mRNA and LIF protein levels. This finding suggests that Ca2+ may be involved in the regulation of LIF in endurance-exercised skeletal muscle. In conclusion, primary human skeletal myocytes have the capability to produce LIF in response to ionomycin stimulation and LIF mRNA levels increase in skeletal muscle following concentric exercise. The finding that the increase in LIF mRNA levels is not followed by a similar increase in skeletal muscle LIF protein suggests that other exercise stimuli or repetitive stimuli are necessary in order to induce a detectable accumulation of LIF protein.
(n = 8) or rested (n = 7). Muscle biopsies were obtained from the vastus lateralis prior to exercise, immediately after exercise, and at 1.5, 3, 6 and 24 h post exercise. Control subjects had biopsy samples taken at the same time points as during the exercise trial. Skeletal muscle LIF mRNA increased immediately after the exercise and declined gradually during recovery. However, LIF protein was unchanged at the investigated time points. Moreover, we tested the hypothesis that LIF mRNA and protein expressions are modulated by calcium (Ca2+) in primary human skeletal myocytes. Treatment of myocytes with the Ca2+ ionophore, ionomycin, for 6 h resulted in an increase in both LIF mRNA and LIF protein levels. This finding suggests that Ca2+ may be involved in the regulation of LIF in endurance-exercised skeletal muscle. In conclusion, primary human skeletal myocytes have the capability to produce LIF in response to ionomycin stimulation and LIF mRNA levels increase in skeletal muscle following concentric exercise. The finding that the increase in LIF mRNA levels is not followed by a similar increase in skeletal muscle LIF protein suggests that other exercise stimuli or repetitive stimuli are necessary in order to induce a detectable accumulation of LIF protein.
During the past few years, skeletal muscle has been acknowledged as a cytokine-producing organ. It has been demonstrated that skeletal muscles produce and express cytokines belonging to distinctly different families. Thus, skeletal muscles have the capacity to express, for example, tumour necrosis factor (TNF)-α, interleukin (IL)-6, IL-8, IL-15 and IL-18 (Nieman et al. 2003; Chan et al. 2004; Nielsen et al. 2007; Petersen et al. 2007). Among these cytokines evidence exists that IL-6 (Febbraio & Pedersen, 2002; Pedersen et al. 2003) and IL-8 (Chan et al. 2004; Akerstrom et al. 2005) are regulated by muscle contractions, both at the mRNA and at the protein levels. IL-6 and IL-8 are both released from working skeletal muscle. IL-6 release contributes to the systemic circulation (Steensberg et al. 2000), whereas only a small and transient net release of IL-8 is found (Akerstrom et al. 2005)
The IL-6 cytokine superfamily consists of structurally and functionally related proteins named neuropoietins (or gp130 cytokines), which include IL-6, leukaemia inhibitory factor (LIF), oncostatin M (OSM), ciliary neurotrophic factor (CNTF), IL-11, and cardiotrophin-1 (CT-1). The neuropoietins exhibit pleiotropy and redundancy in biological activities and they all share the gp130 receptor component, a common transducer of their receptor complexes (Heinrich et al. 1998).
With regard to skeletal muscle the cytokine LIF is of major interest. LIF is constitutively expressed at a low level primarily in type 1 muscle fibres (Schoser et al. 1998; Sakuma et al. 2000) and has been demonstrated to be implicated in conditions affecting skeletal muscle growth and regeneration (Kami & Senba, 1998; Sakuma et al. 1998, 2000; Austin et al. 2000; Reardon et al. 2000; Spangenburg & Booth, 2006). LIF protein expression is augmented, for example, in mechanically overloaded rat plantaris muscle (Sakuma et al. 1998) and in denervated fast-type, but not slow-type rat muscle (Sakuma et al. 2000). Furthermore, LIF restores the hypertrophic response to increased loading in the LIF (–/–) mouse and in that respect LIF has been denoted as an important factor in skeletal muscle hypertrophy (Spangenburg & Booth, 2006). In addition, LIF mRNA is increased in human skeletal muscle following muscle injury (Kami & Senba, 1998; Reardon et al. 2000) and ameliorates muscle fibre degeneration in the dystrophin-deficient mdx mice (Austin et al. 2000).
Austin & Burgess, (1991) demonstrated that recombinant LIF has the potency to induce myoblast proliferation in culture. Furthermore, it has been found that LIF can induce muscle satellite cell proliferation, an event relying on activation of the JAK2–STAT3 signalling pathway (Spangenburg & Booth, 2002). Studies in cultured cardiac myocytes demonstrate an anti-apoptotic effect of LIF (Negoro et al. 2001) and also reveal that cultured primary cardiac myocytes have the capacity to synthesize and secrete LIF (Ancey et al. 2002).
Given that LIF is expressed by human skeletal muscle and knowing that IL-6 and LIF belong to the same cytokine family we hypothesized that LIF would be regulated by muscle contractions. In the present study, we tried to elucidate whether LIF expression is regulated by exercise and calcium (Ca2+) in skeletal muscle and primary human skeletal myocytes, respectively.
Methods
Subjects
Fifteen healthy male subjects with a mean (±s.d.) age, height, weight and body mass index (BMI) of 24.9 ± 4 years, 180.9 ± 1 cm, 82.0 ± 8 kg and 24.9 ± 2 kg m−2, respectively, participated in this study. All subjects had a normal medical history and physical examination revealed no abnormalities. Eight subjects exercised and seven individuals rested to serve as controls for an effect of repeated muscle biopsies. There was no difference between the two groups with regard to age, weight, height, BMI, or maximal oxygen uptake 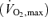 .
.
Ethics
Before the experimental procedures, the subjects received both oral and written information about the experimental procedures before providing their written informed consent. All studies were approved by the Copenhagen and Frederiksberg Ethical Committee, Denmark, and were performed in accordance with the Declaration of Helsinki.
Experimental procedures
In the present study cycle ergometer exercise was chosen as intervention because this type of exercise is mainly concentric and induces minimal muscle damage and subsequent inflammation (Sorichter et al. 1997; Brenner et al. 1999). The subjects performed two incremental maximal exercise tests to determine 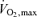 on a cycle ergometer (Monark 839E, Monark Ltd, Varberg, Sweden). The first test, a familiarization trial, was performed 5 days before the first experimental day. The second test was performed 2 days before the experimental day. The tests started with a 5 min warm-up at ∼40% of
on a cycle ergometer (Monark 839E, Monark Ltd, Varberg, Sweden). The first test, a familiarization trial, was performed 5 days before the first experimental day. The second test was performed 2 days before the experimental day. The tests started with a 5 min warm-up at ∼40% of 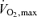 and continued with equal and even increases in load until volitional fatigue. The tests lasted 8–12 min (excluding the warm-up) as recommended by Buchfuhrer et al. (1983). On the experimental day, subjects arrived at 07.00 h after an overnight fast. The subjects rested for approximately 10 min in the supine position after which a venous catheter was placed in an antecubital vein. Subsequently, the subjects performed 3 h of cycling at ∼60% of
and continued with equal and even increases in load until volitional fatigue. The tests lasted 8–12 min (excluding the warm-up) as recommended by Buchfuhrer et al. (1983). On the experimental day, subjects arrived at 07.00 h after an overnight fast. The subjects rested for approximately 10 min in the supine position after which a venous catheter was placed in an antecubital vein. Subsequently, the subjects performed 3 h of cycling at ∼60% of 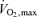 , followed by 6 h of recovery. On the experimental day water was allowed ad libitum. Muscle biopsies were obtained from the vastus lateralis prior to exercise, immediately after exercise, and 1.5 h, 3 h, 6 h and 24 h post exercise, using the percutaneous needle biopsy technique with suction as explained in detail previously (Frydelund-Larsen et al. 2007). To acquire the 24 h samples the subjects reported to the laboratory the following day after an overnight fast. Control subjects rested in the laboratory for 9 h, reported to the laboratory the day after in a fasted state, and had biopsy samples taken at the same time points as during the exercise trial. Biopsy samples were immediately placed on an ice-cold glass plate, cleaned of connective tissue and blood, frozen in liquid nitrogen, and stored at –80°C until further analysis.
, followed by 6 h of recovery. On the experimental day water was allowed ad libitum. Muscle biopsies were obtained from the vastus lateralis prior to exercise, immediately after exercise, and 1.5 h, 3 h, 6 h and 24 h post exercise, using the percutaneous needle biopsy technique with suction as explained in detail previously (Frydelund-Larsen et al. 2007). To acquire the 24 h samples the subjects reported to the laboratory the following day after an overnight fast. Control subjects rested in the laboratory for 9 h, reported to the laboratory the day after in a fasted state, and had biopsy samples taken at the same time points as during the exercise trial. Biopsy samples were immediately placed on an ice-cold glass plate, cleaned of connective tissue and blood, frozen in liquid nitrogen, and stored at –80°C until further analysis.
Primary human skeletal muscle satellite cell isolation
Primary human skeletal muscle satellite cells were isolated using a modification of a previously published method (Roques & Vidal, 1999). During operating procedures, a small (less than 1 g) portion of the human lumbar erector spinae muscle was removed and placed in ice-cold HAM-F10 medium (Invitrogen, Taastrup, Denmark) and stored at 4°C overnight. The next morning, the muscle piece was placed in 10 ml ice-cold Dulbecco's phosphate-buffered saline (PBS) and all connective and fat tissue was removed. The muscle sample was then cut into very small pieces using a pair of caraveijo scissors and transferred to a 50 ml tube containing 20 ml ice-cold PBS and centrifuged at 550 g for 10 min at 4°C. The PBS was aspirated and 10 ml of a digestion cocktail (0.1% collagenase IV (Sigma-Aldrich, USA), 0.1% bovine serum albumin (BSA; Sigma-Aldrich)), 0.01% DNase I (Sigma-Aldrich), 0.05% trypsin-EDTA (Invitrogen) in PBS) was added followed by incubation at 37°C with gentle shaking for 5 min. Following digestion the muscle pieces were allowed to settle on ice and the supernatant was transferred to another 50 ml tube. Another round of digestion was carried out, this time for 10 min, and the supernatants were pooled before being centrifuged at 550 g for 10 min at 4°C. The supernatant was aspirated and the cell pellet resuspended in HAM-F10 containing 20% fetal calf serum supplemented with penicillin (100 μg ml−1), streptomycin (100 μg ml−1) and amphotericin B (1.25 μg ml−1) (growth medium). The cells were then filtered through a 70 μm nylon mesh (Becton Dickinson, Franklin Lakes, NJ, USA) and pre-plated on a tissue culture-coated 150 mm dish (Nunc, Roskilde, Denmark) for 1 h at 37°C in the incubator. Following pre-plating, the supernatant was transferred to a T-75 flask (Nunc). The medium was changed 4 days after cell isolation and subsequently every second day. When the cells were 50–70% confluent they were either frozen and stored or used for experimental procedures.
Ionomycin stimulation of primary human skeletal muscle cells
Isolated satellite cells were propagated in growth medium and plated in either 6-well or 12-well plates and grown in growth medium until confluence. On the day following cell confluence the medium was changed to HAM-F10 containing 2% horse serum (Invitrogen, Taastrup, Denmark) supplemented by penicillin (100 μg ml−1) and streptomycin (100 μg ml−1) (differentiation medium). The medium was changed every 2 days. Cultures were fully differentiated by day 8, as determined by visual confirmation of myotube formation (80–90% of the single cells fused into multi-nuclear myotubes), and stimulation with 1 μm ionomycin was then carried out in differentiation medium for 6 h. RNA isolation (12-well plates) was carried out using RNeasy (Qiagen, Valencia, CA, USA) following the manufacturer's instructions. Cell lysates for SDS-PAGE and Western blotting were prepared from 6-well plates by removing the medium, washing twice with ice-cold PBS, and scraping cells in a lysis buffer (50 mm Tris-HCl, 150 mm NaCl, 1 mm EGTA, 1 mm EDTA, 0.25% sodium deoxycholate and 1% Triton X-100, pH 7.4) containing a complete protease inhibitor cocktail (Roche, Basel, Switzerland) on ice. The homogenate was transferred to an Eppendorf tube, pulled through a small-calibre syringe 3–4 times and centrifuged at 13 000 g for 15 min at 4°C. The supernatant was transferred to a new tube and stored at –80°C until further analysis. All experiments were carried out either in duplicate (protein) or triplicate (RNA).
Real time RT-PCR
Total RNA was extracted from the muscle tissue using TRIzol (Invitrogen, Grand Island, NY, USA) or from human primary skeletal muscle cell cultures using RNeasy according to the manufacturer's instructions. Reverse transcription (RT) reactions were performed using random hexamers on 2 microg RNA employing an RT kit (Applied Biosystems, Foster City, CA, USA) in a reaction volume of 100 ml. The resulting cDNA product was stored at –20°C until further analysis. The primers and probes for LIF and 18S rRNA were pre-developed TaqMan probes and primer sets from Applied Biosystems, Hs00171455_m1 (LIF). All assay reagents were obtained from Applied Biosystems. The mRNA levels of LIF and the endogenous control, 18S rRNA were determined in triplicate by real time RT-PCR using an ABI PRISM 7900 sequence detector (Applied Biosystems). The amplification mixtures were amplified according to standard conditions using 50 cycles. The relative expression of LIF was determined after normalization to the endogenous control 18S. Exercise did not alter 18S mRNA quantities compared to pre-exercise values.
Western blot analysis
Muscle tissue was freeze-dried and dissected free of visual blood, fat and connective tissues. Depending on weight, muscle lysate was then prepared by the addition of 0.4–1.0 ml homogenization buffer (50 mm Hepes, 12 mm sodium pyrophosphate, 100 mm sodium fluoride, 10 mm EDTA, 10% Triton X, pH 7.4) containing sodium orthovanadate, phosphatase inhibitor cocktails 1 and 2 (Sigma-Aldrich) and a complete protease inhibitor cocktail (Roche) to the freeze-dried muscle tissue. The muscle tissue was then homogenized using cooled racks in a TissueLyser (Qiagen) for 1 min at 30 Hz followed by 15 min incubation on ice. The homogenization and incubation on ice were repeated twice or three times depending on the degree of homogenization of the tissue. Homogenates were then rotated end over end for 1 h at 4°C and centrifuged at 16 000 g at 4°C for 25 min. The supernatant protein concentrations were determined with a Bio-Rad DC kit (Bio-Rad, Hercules, CA, USA) using BSA as standard. All determinations were done in triplicate.
Twenty-five milligrams of protein was mixed with 5 × sample buffer (167 mm, 0.5 m dithiothreitol, 30% glycerol, 10% SDS (w/v), 0.05%), boiled at 100°C for 10 min and separated using a NuPage 4–12% Bis-Tris gel (Invitrogen, Taastrup, Denmark), followed by immunoblotting to PVDF membranes (hybond-P, GE Healthcare, Little Chalfont, UK). Membranes were then blocked for 1 h at room temperature in blocking buffer (Tris-buffered saline with 0.1% Tween-20 and 5% BSA (Sigma-Aldrich) and washed 3 times for 5 min in wash buffer (Tris-buffered saline with 0.1% Tween-20). Subsequently the membranes were incubated overnight at 4°C in blocking buffer containing a primary antibody against LIF (AF-250-NA, R&D Systems, Minneapolis, MN, USA) at a final concentration of 0.2 μg ml−1. The membranes were then washed 3 times in wash buffer and incubated for 1 h at room temperature with rabbit anti-goat horseradish peroxidase (Dako, Glostrup, Denmark) secondary antibody at 1 : 15 000 dilution in blocking buffer followed by 3 times 5 min washing in wash buffer. Bands were detected using Supersignal West Femto (Pierce, Rockford, IL, USA) and quantified using a CCD image sensor (ChemiDocXRS, Bio-Rad) and software (Quantity One, Bio-Rad). Probing against LIF revealed a protein band at ∼45 kDa, which is consistent with the finding in other studies (Cullinan et al. 1996; Sakuma et al. 2000).
Statistical analysis
All mRNA data were log transformed in order to obtain normal distribution. The mRNA values are presented as geometric mean ± geometric s.e.m. All other values are presented as means ±s.e.m. The area under the curve was used to evaluate the effect of exercise over time and between groups. Post hoc analysis (Bonferroni-adjusted paired t tests) was performed to identify specific differences across time. Student's paired t test was performed to identify differences between treatments in the cell experiments. Differences were considered significant at P < 0.05.
Results
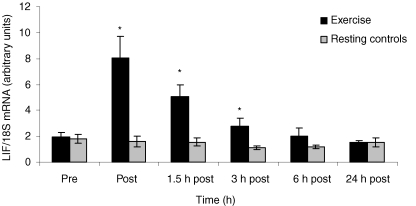
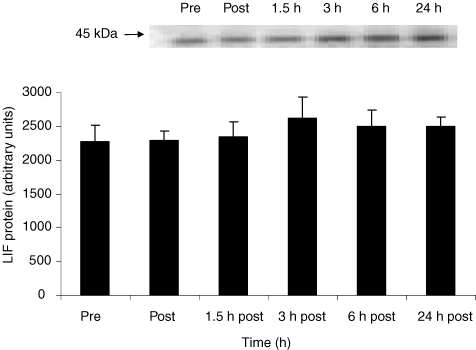
The level of LIF mRNA expression in skeletal muscle biopsies increased 4.5-fold (95% confidence interval; 1.9- to 7.1-fold) with exercise. Compared to the control group the exercising group had significantly elevated LIF mRNA levels immediately after exercise, 1.5 h and 3 h post exercise (P < 0.05) (Fig. 1). LIF protein expression in skeletal muscle was unchanged at the investigated time points (Fig. 2). Plasma LIF was measured in blood samples taken before exercise, post exercise, 1.5 h, 3 h and 6 h post exercise using an ELISA kit for LIF (Quantine, R&D systems, UK). Plasma LIF was undetectable at all time points (data not shown).
Figure 1. LIF mRNA levels in skeletal muscle in response to exercise or rest.
LIF mRNA levels were examined in biopsies taken from m. vastus lateralis before (Pre) 3 h of ergometer bicycle exercise, immediately after (Post) and at time points 1.5 h, 3 h, 6 h and 24 h after exercise. Data are expressed as geometric means ±s.e.m. (n = 8, exercise; and n = 7, control). Effect of time area under the curve, P = 0.002; *post hoc analysis (Bonferroni-adjusted paired t tests), P < 0.05.
Figure 2. LIF protein expression in skeletal muscle in response to exercise.
LIF protein expression was examined in biopsies taken from m. vastus lateralis before (Pre) 3 h ergometer bicycle exercise, immediately after (Post) and at time points 1.5 h, 3 h, 6 h and 24 h after exercise. Data are expressed as means ±s.e.m. (n = 8). A representative immunoblot is shown.
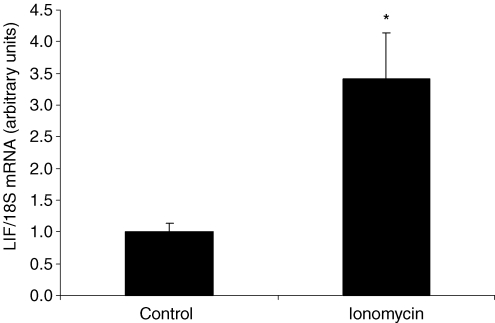
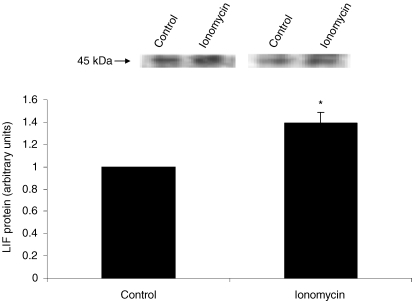
Treatment of primary human skeletal myocytes with ionomycin resulted in a 3.4-fold induction in LIF mRNA (P < 0.01) compared to control conditions (Fig. 3). Furthermore, a significant increase in LIF protein expression was observed in ionomycin-treated cells compared to control cells (P < 0.05) (Fig. 4).
Figure 3. LIF mRNA levels in primary human skeletal myocytes in response to ionomycin stimulation.
Primary human skeletal myocytes were incubated with the Ca2+ ionophore ionomycin for 6 h. Data are expressed as geometric means ±s.e.m. (n = 4). *P < 0.01.
Figure 4. LIF protein expression in primary human skeletal myocytes in response to ionomycin stimulation.
Primary human skeletal myocytes were incubated with the Ca2+ ionophore ionomycin for 6 h. Representative immunoblots are shown. Data are expressed as means ±s.e.m. (n = 3). *P < 0.05.
Discussion
In the present study we demonstrate that acute exercise induces an expression of LIF mRNA in human skeletal muscle. However, quantification of LIF protein by Western blotting revealed no significant change in LIF protein following acute exercise. Moreover, we demonstrate for the first time that primary human skeletal myocytes express LIF mRNA and protein and that Ca2+ modulates the level of both.
The finding that human skeletal muscles have the capacity to express LIF mRNA and LIF protein is in accordance with earlier studies (Schoser et al. 1998; Reardon et al. 2000) confirming that skeletal muscle is a source of endogenous LIF. However, very little is known about the physiological regulation of LIF in healthy skeletal muscle. The observation that LIF mRNA was significantly increased following exercise indicates a regulatory effect of muscle contractions upon LIF mRNA level. However, the finding that the LIF protein level did not change demonstrates that even acute exercise of a relatively long duration does not mediate a detectable increase in LIF at the translational level. In support of this finding, Sakuma et al. (2000) found that chronic mechanical overloading of mouse plantaris muscle induced by ablation of synergists was followed by an increase in LIF protein from 2 to 14 days of overload. However, no LIF protein expression was detected on the first day (Sakuma et al. 2000). This suggests that repetitive bouts of exercise might be necessary to induce accumulation of LIF protein in skeletal muscle.
With regard to skeletal muscle cells, the main effect of LIF is its potency to induce human myoblast and satellite cell proliferation (Austin & Burgess, 1991; Spangenburg & Booth, 2002). Skeletal muscle satellite cell proliferation is essential in two processes known to be of importance following exercise, namely skeletal muscle regeneration and skeletal muscle hypertrophy (Rosenblatt et al. 1994; Hawke & Garry, 2001; Mitchell & Pavlath, 2001). LIF is highly involved in both processes (Kami & Senba, 1998; Reardon et al. 2000; Sakuma et al. 2000) acting to enhance muscle regeneration (Barnard et al. 1994) and functioning as an indispensable factor during skeletal muscle hypertrophy (Spangenburg & Booth, 2006). The extent to which exercise elicits damage to muscle fibres or stimulates hypertrophy depends on the mode, duration and intensity of exercise (Sorichter et al. 1997; Brenner et al. 1999; Wernbom et al. 2007). In the present study, subjects performed pure concentric exercise which induces none or minimal muscle damage (Sorichter et al. 1997; Brenner et al. 1999) and stimulates a smaller hypertrophic response (Colliander & Tesch, 1990). Thus, a reasonable explanation for the lack of LIF protein expression could be that LIF translation is not stimulated by the minor regenerative and hypertrophic response elicited by the concentric muscle work performed in this study. Concentric contractions may therefore regulate LIF at the transcriptional level but not at the protein level suggesting that the LIF transcripts are not translated into functional LIF proteins. Alternatively, LIF mRNA may exist in a translationally inactive pool, which is stored in the cell and ready for translation or LIF may be secreted immediately into the circulation in amounts which do not influence systemic LIF concentrations.
To delineate regulatory events modulating LIF at the transcriptional level during contractions we examined the effect of stimulating primary human skeletal myocytes with the Ca2+ ionophore, ionomycin. We observed an increase in LIF mRNA and protein confirming that Ca2+ is involved in the regulation of LIF in skeletal myocytes. Since a minor proportion of the primary human skeletal myocyte culture consisted of unfused single cells (10–20%), presumably either fibroblasts or unfused satellite cells, we must state the limitation that the possibility exists that ionomycin also increased LIF in either fibroblasts or satellite cells. However, 80–90% of the cell culture consisted of differentiated myotubes and we therefore believe that skeletal myocytes are the dominant source of the increase in LIF mRNA and protein following ionomycin stimulation. During exercise the intra-myocellular concentration of Ca2+ rises temporarily in association with skeletal muscle contractions. These oscillations in Ca2+ concentration act as signals conveying neuromuscular activity into changes in the transcription of specific genes (Dolmetsch et al. 1998; Li et al. 1998). Three main Ca2+-triggered regulatory pathways acting through calcineurin, Ca2+–calmodulin-dependent kinases (CaMKs) and Ca2+-dependent protein kinase C (PKC) transduce alterations in cytosolic Ca2+ concentration to target genes (Koulmann & Bigard, 2006). Due to the downstream signalling events initiated by calcineurin this molecule may constitute a possible link between fluxes in Ca2+ concentration and regulation of LIF in skeletal muscle. Once activated by Ca2+–calmodulin, calcineurin dephosphorylates the nuclear factor of activated T-cells (NFAT), which is translocated from the cytoplasm to the nucleus where it activates transcription of target genes containing the NFAT binding site (Schiaffino & Serrano, 2002). Seeing that the human LIF promoter contains two NFAT binding sites (Bamberger et al. 2004) we hypothesize that this pathway could represent a possible mechanism of LIF gene activation by Ca2+ in myocytes.
In summary, concentric contractions regulate LIF at the mRNA level. However, the increase in LIF mRNA level is not paralleled by a similar change in muscular LIF protein expression. This finding does not exclude the possibility that different training stimuli or repetitive bouts of exercise may induce an increase in skeletal muscle LIF protein content. LIF mRNA and protein levels increase in ionomycin-stimulated primary human skeletal myocytes, suggesting that Ca2+ may be involved in the regulation of LIF expression in skeletal muscle.
Acknowledgments
Ruth Rousing and Hanne Villumsen are acknowledged for their technical assistance. The Centre of Inflammation and Metabolism is supported by a grant from the Danish National Research Foundation (no. 02-512-55). The study was further supported by the Danish Medical Research Council and the Commission of the European Communities (contract no. LSHM-CT-2004-005272). The Copenhagen Muscle Research Centre is supported by grants from the Capital Region of Denmark and the University of Copenhagen.
References
- Akerstrom T, Steensberg A, Keller P, Keller C, Penkowa M, Pedersen BK. Exercise induces interleukin-8 expression in human skeletal muscle. J Physiol. 2005;563:507–516. doi: 10.1113/jphysiol.2004.077610. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ancey C, Corbi P, Froger J, Delwail A, Wijdenes J, Gascan H, Potreau D, Lecron JC. Secretion of IL-6, IL-11 and LIF by human cardiomyocytes in primary culture. Cytokine. 2002;18:199–205. doi: 10.1006/cyto.2002.1033. [DOI] [PubMed] [Google Scholar]
- Austin L, Bower JJ, Bennett TM, Lynch GS, Kapsa R, White JD, Barnard W, Gregorevic P, Byrne E. Leukemia inhibitory factor ameliorates muscle fiber degeneration in the mdx mouse. Muscle Nerve. 2000;23:1700–1705. doi: 10.1002/1097-4598(200011)23:11<1700::aid-mus5>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Austin L, Burgess AW. Stimulation of myoblast proliferation in culture by leukaemia inhibitory factor and other cytokines. J Neurol Sci. 1991;101:193–197. doi: 10.1016/0022-510x(91)90045-9. [DOI] [PubMed] [Google Scholar]
- Bamberger AM, Jenatschke S, Schulte HM, Ellebrecht I, Beil FU, Bamberger CM. Regulation of the human leukemia inhibitory factor gene by ETS transcription factors. Neuroimmunomodulation. 2004;11:10–19. doi: 10.1159/000072964. [DOI] [PubMed] [Google Scholar]
- Barnard W, Bower J, Brown MA, Murphy M, Austin L. Leukemia inhibitory factor (LIF) infusion stimulates skeletal muscle regeneration after injury: injured muscle expresses lif mRNA. J Neurol Sci. 1994;123:108–113. doi: 10.1016/0022-510x(94)90211-9. [DOI] [PubMed] [Google Scholar]
- Brenner IK, Natale VM, Vasiliou P, Moldoveanu AI, Shek PN, Shephard RJ. Impact of three different types of exercise on components of the inflammatory response. Eur J Appl Physiol Occup Physiol. 1999;80:452–460. doi: 10.1007/s004210050617. [DOI] [PubMed] [Google Scholar]
- Buchfuhrer MJ, Hansen JE, Robinson TE, Sue DY, Wasserman K, Whipp BJ. Optimizing the exercise protocol for cardiopulmonary assessment. J Appl Physiol. 1983;55:1558–1564. doi: 10.1152/jappl.1983.55.5.1558. [DOI] [PubMed] [Google Scholar]
- Chan MH, Carey AL, Watt MJ, Febbraio MA. Cytokine gene expression in human skeletal muscle during concentric contraction: evidence that IL-8, like IL-6, is influenced by glycogen availability. Am J Physiol Regul Integr Comp Physiol. 2004;287:R322–R327. doi: 10.1152/ajpregu.00030.2004. [DOI] [PubMed] [Google Scholar]
- Colliander EB, Tesch PA. Effects of eccentric and concentric muscle actions in resistance training. Acta Physiol Scand. 1990;140:31–39. doi: 10.1111/j.1748-1716.1990.tb08973.x. [DOI] [PubMed] [Google Scholar]
- Cullinan EB, Abbondanzo SJ, Anderson PS, Pollard JW, Lessey BA, Stewart CL. Leukemia inhibitory factor (LIF) and LIF receptor expression in human endometrium suggests a potential autocrine/paracrine function in regulating embryo implantation. Proc Natl Acad Sci U S A. 1996;93:3115–3120. doi: 10.1073/pnas.93.7.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolmetsch RE, Xu K, Lewis RS. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 1998;392:933–936. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- Febbraio MA, Pedersen BK. Muscle-derived interleukin-6: mechanisms for activation and possible biological roles. FASEB J. 2002;16:1335–1347. doi: 10.1096/fj.01-0876rev. [DOI] [PubMed] [Google Scholar]
- Frydelund-Larsen L, Penkowa M, Akerstrom T, Zankari A, Nielsen S, Pedersen BK. Exercise induces interleukin-8 receptor (CXCR2) expression in human skeletal muscle. Exp Physiol. 2007;92:233–240. doi: 10.1113/expphysiol.2006.034769. [DOI] [PubMed] [Google Scholar]
- Hawke TJ, Garry DJ. Myogenic satellite cells: physiology to molecular biology. J Appl Physiol. 2001;91:534–551. doi: 10.1152/jappl.2001.91.2.534. [DOI] [PubMed] [Google Scholar]
- Heinrich PC, Behrmann I, Muller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998;334:297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kami K, Senba E. Localization of leukemia inhibitory factor and interleukin-6 messenger ribonucleic acids in regenerating rat skeletal muscle. Muscle Nerve. 1998;21:819–822. doi: 10.1002/(sici)1097-4598(199806)21:6<819::aid-mus20>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Koulmann N, Bigard AX. Interaction between signalling pathways involved in skeletal muscle responses to endurance exercise. Pflugers Arch. 2006;452:125–139. doi: 10.1007/s00424-005-0030-9. [DOI] [PubMed] [Google Scholar]
- Li W, Llopis J, Whitney M, Zlokarnik G, Tsien RY. Cell-permeant caged InsP3 ester shows that Ca2+ spike frequency can optimize gene expression. Nature. 1998;392:936–941. doi: 10.1038/31965. [DOI] [PubMed] [Google Scholar]
- Mitchell PO, Pavlath GK. A muscle precursor cell-dependent pathway contributes to muscle growth after atrophy. Am J Physiol Cell Physiol. 2001;281:C1706–C1715. doi: 10.1152/ajpcell.2001.281.5.C1706. [DOI] [PubMed] [Google Scholar]
- Negoro S, Oh H, Tone E, Kunisada K, Fujio Y, Walsh K, Kishimoto T, Yamauchi-Takihara K. Glycoprotein 130 regulates cardiac myocyte survival in doxorubicin-induced apoptosis through phosphatidylinositol 3-kinase/Akt phosphorylation and Bcl-xL/caspase-3 interaction. Circulation. 2001;103:555–561. doi: 10.1161/01.cir.103.4.555. [DOI] [PubMed] [Google Scholar]
- Nielsen AR, Mounier R, Plomgaard P, Mortensen OH, Penkowa M, Speerschneider T, Pilegaard H, Pedersen BK. Expression of interleukin-15 in human skeletal muscle effect of exercise and muscle fibre type composition. J Physiol. 2007;584:305–312. doi: 10.1113/jphysiol.2007.139618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieman DC, Davis JM, Henson DA, Walberg-Rankin J, Shute M, Dumke CL, Utter AC, Vinci DM, Carson JA, Brown A, Lee WJ, McAnulty SR, McAnulty LS. Carbohydrate ingestion influences skeletal muscle cytokine mRNA and plasma cytokine levels after a 3-h run. J Appl Physiol. 2003;94:1917–1925. doi: 10.1152/japplphysiol.01130.2002. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Steensberg A, Fischer C, Keller C, Keller P, Plomgaard P, Febbraio M, Saltin B. Searching for the exercise factor: is IL-6 a candidate? J Muscle Res Cell Motil. 2003;24:113–119. doi: 10.1023/a:1026070911202. [DOI] [PubMed] [Google Scholar]
- Petersen AM, Penkowa M, Iversen M, Frydelund-Larsen L, Andersen JL, Mortensen J, Lange P, Pedersen BK. Elevated levels of IL-18 in plasma and skeletal muscle in chronic obstructive pulmonary disease. Lung. 2007;185:161–171. doi: 10.1007/s00408-007-9000-7. [DOI] [PubMed] [Google Scholar]
- Reardon KA, Kapsa RM, Davis J, Kornberg AJ, Austin L, Choong P, Byrne E. Increased levels of leukemia inhibitory factor mRNA in muscular dystrophy and human muscle trauma. Muscle Nerve. 2000;23:962–966. doi: 10.1002/(sici)1097-4598(200006)23:6<962::aid-mus18>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Roques M, Vidal H. A phosphatidylinositol 3-kinase/p70 ribosomal S6 protein kinase pathway is required for the regulation by insulin of the p85a regulatory subunit of phosphatidylinositol 3-kinase gene expression in human muscle cells. J Biol Chem. 1999;274:34005–34010. doi: 10.1074/jbc.274.48.34005. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JD, Yong D, Parry DJ. Satellite cell activity is required for hypertrophy of overloaded adult rat muscle. Muscle Nerve. 1994;17:608–613. doi: 10.1002/mus.880170607. [DOI] [PubMed] [Google Scholar]
- Sakuma K, Watanabe K, Sano M, Uramoto I, Totsuka T. Differential adaptation of growth and differentiation factor 8/myostatin, fibroblast growth factor 6 and leukemia inhibitory factor in overloaded, regenerating and denervated rat muscles. Biochim Biophys Acta. 2000;1497:77–88. doi: 10.1016/s0167-4889(00)00044-6. [DOI] [PubMed] [Google Scholar]
- Sakuma K, Watanabe K, Totsuka T, Uramoto I, Sano M, Sakamoto K. Differential adaptations of insulin-like growth factor-I, basic fibroblast growth factor, and leukemia inhibitory factor in the plantaris muscle of rats by mechanical overloading: an immunohistochemical study. Acta Neuropathol (Berl) 1998;95:123–130. doi: 10.1007/s004010050775. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Serrano A. Calcineurin signaling and neural control of skeletal muscle fiber type and size. Trends Pharmacol Sci. 2002;23:569–575. doi: 10.1016/s0165-6147(02)02111-9. [DOI] [PubMed] [Google Scholar]
- Schoser BG, Storjohann S, Kunze K. Immunolocalization of leukemia inhibitory factor in normal and denervated human muscle. Neuroreport. 1998;9:2843–2846. doi: 10.1097/00001756-199808240-00029. [DOI] [PubMed] [Google Scholar]
- Sorichter S, Mair J, Koller A, Gebert W, Rama D, Calzolari C, Artner-Dworzak E, Puschendorf B. Skeletal troponin I as a marker of exercise-induced muscle damage. J Appl Physiol. 1997;83:1076–1082. doi: 10.1152/jappl.1997.83.4.1076. [DOI] [PubMed] [Google Scholar]
- Spangenburg EE, Booth FW. Multiple signaling pathways mediate LIF-induced skeletal muscle satellite cell proliferation. Am J Physiol Cell Physiol. 2002;283:C204–C211. doi: 10.1152/ajpcell.00574.2001. [DOI] [PubMed] [Google Scholar]
- Spangenburg EE, Booth FW. Leukemia inhibitory factor restores the hypertrophic response to increased loading in the LIF (–/–) mouse. Cytokine. 2006;34:125–130. doi: 10.1016/j.cyto.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Steensberg A, van Hall G, Osada T, Sacchetti M, Saltin B, Pedersen BK. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J Physiol. 2000;529:237–242. doi: 10.1111/j.1469-7793.2000.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernbom M, Augustsson J, Thomee R. The influence of frequency, intensity, volume and mode of strength training on whole muscle cross-sectional area in humans. Sports Med. 2007;37:225–264. doi: 10.2165/00007256-200737030-00004. [DOI] [PubMed] [Google Scholar]