Abstract
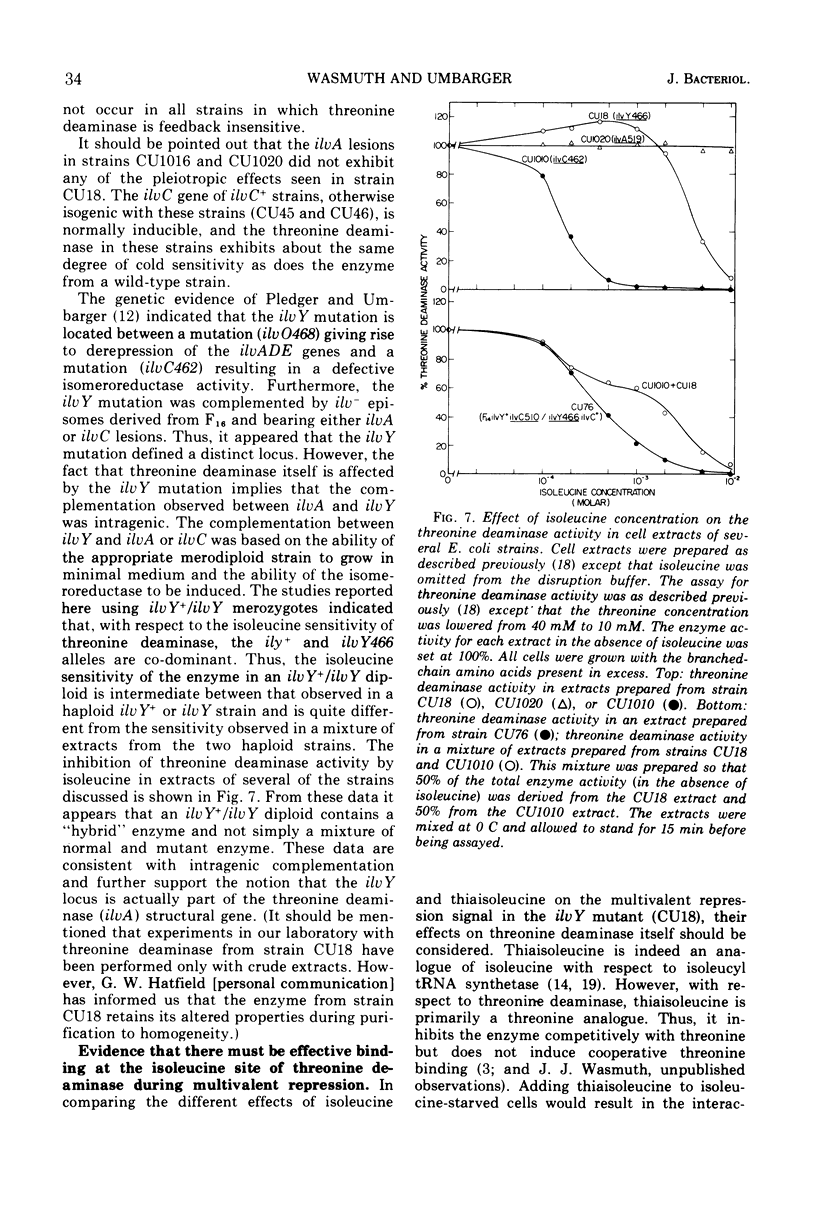
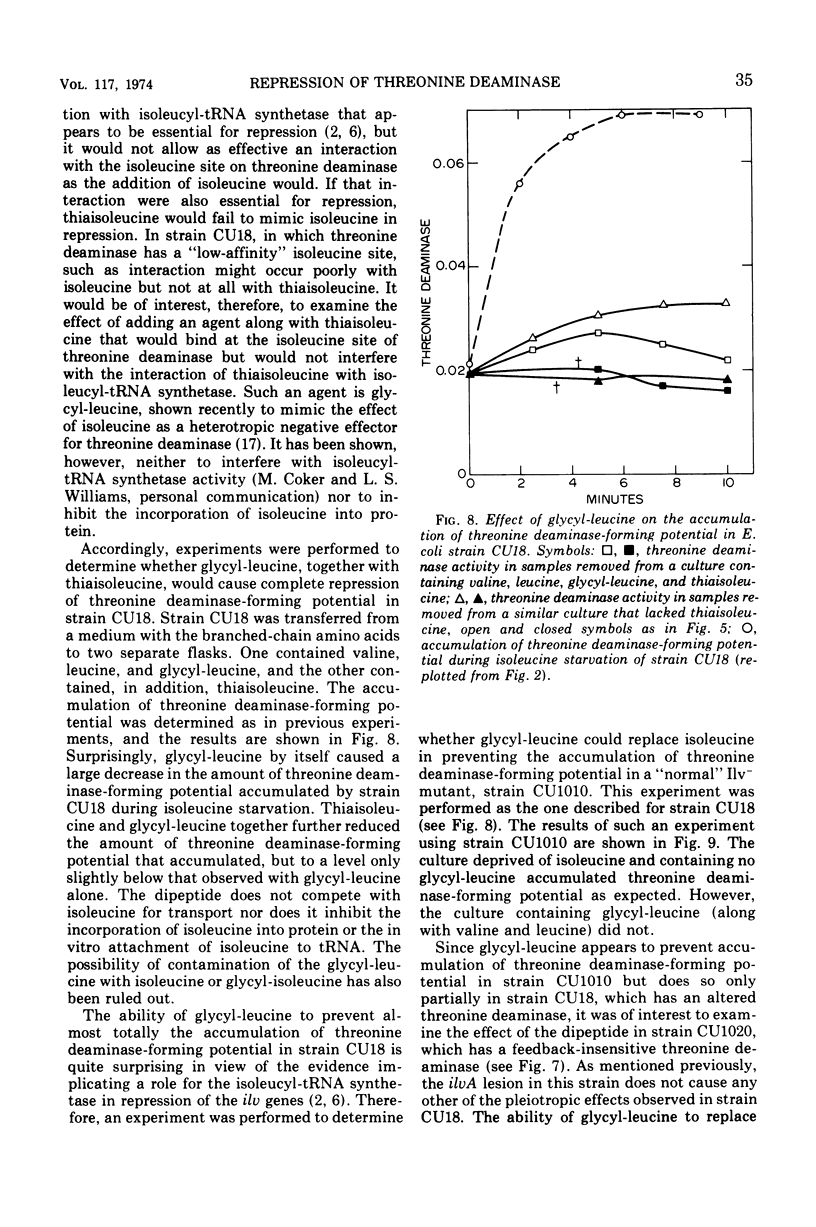
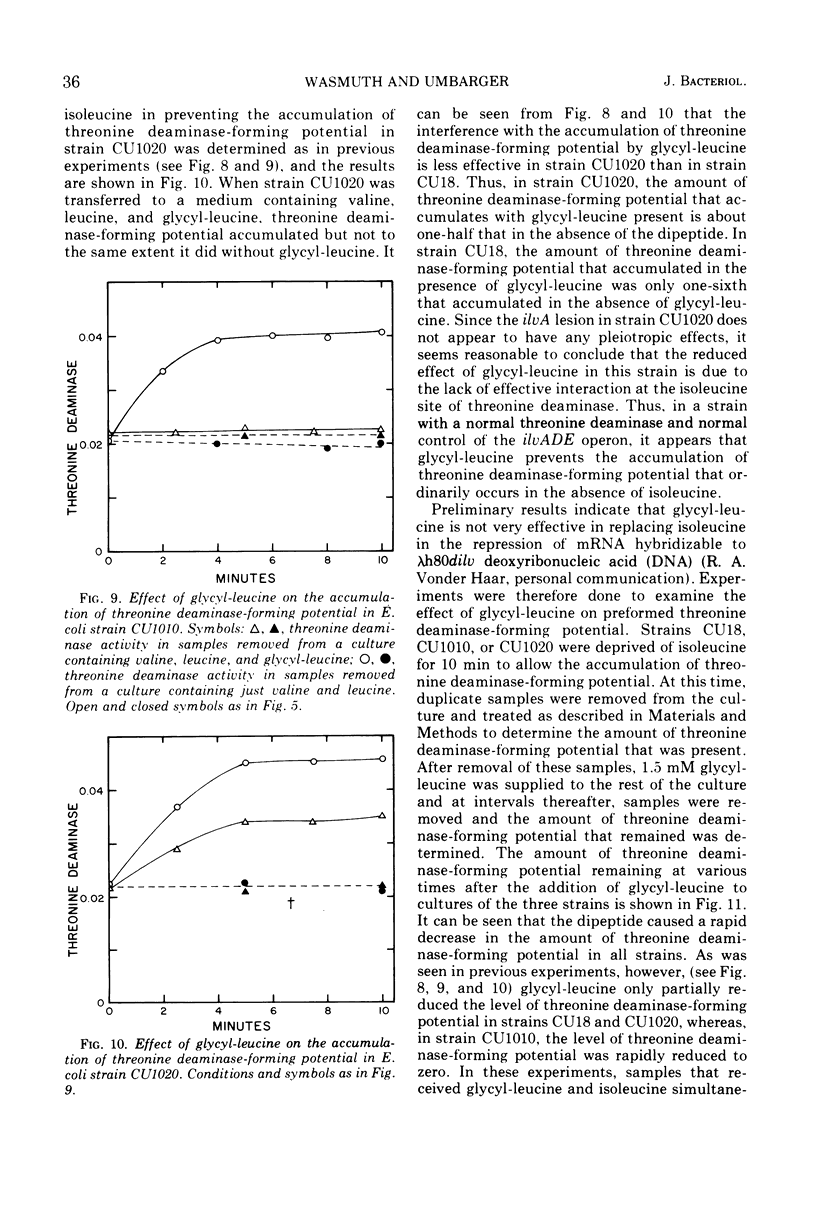
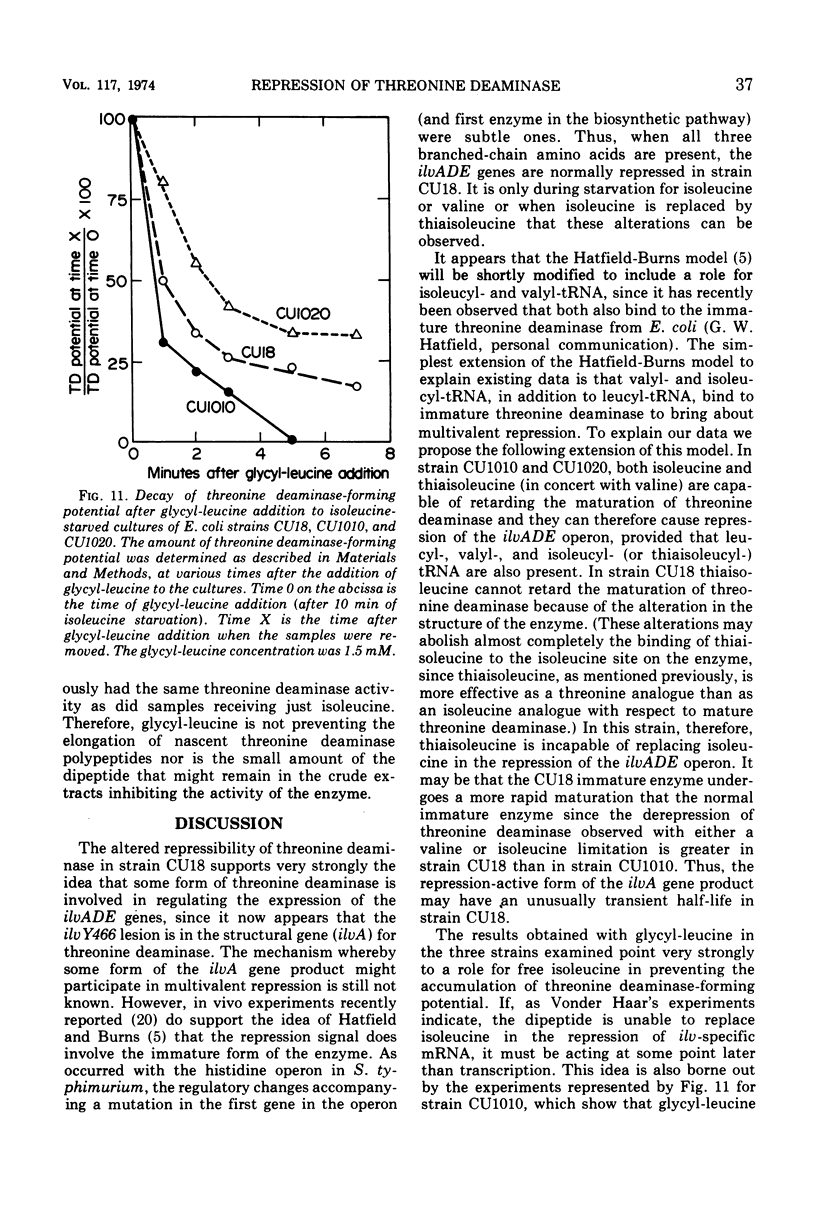
In Escherichia coli, the three branched-chain amino acid activating enzymes appear to be essential for multivalent repression of the isoleucine- and valine-forming enzymes. The results of experiments with a mutant, strain CU18, having an altered threonine deaminase, indicate that free isoleucine and some form of threonine deaminase (the product of the ilvA gene) are also involved in multivalent repression. This strain exhibits abnormally high derepressibility but normal repressibility of its ilv gene products, and its threonine deaminase is inhibited only by high concentrations of isoleucine. In strain CU18, the isoleucine analogue, thiaisoleucine, is incapable of replacing isoleucine in the multivalent repression of the ilv genes, whereas the analogue can fully replace the natural amino acid in repression in other strains examined. The dipeptide, glycyl-leucine, which, like isoleucine, is a heterotropic negative effector of threonine deaminase but is not a substrate for isoleucyl-transfer ribonucleic acid synthetase, can completely prevent the accumulation of threonine deaminase-forming potential during isoleucine starvation in strains with normal threonine deaminases. It can not, however, prevent such accumulation in strain CU18 or in other strains in which threonine deaminase is insensitive to any concentration of isoleucine.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blasi F., Barton R. W., Kovach J. S., Goldberger R. F. Interaction between the first enzyme for histidine biosynthesis and histidyl transfer ribonucleic acid. J Bacteriol. 1971 May;106(2):508–513. doi: 10.1128/jb.106.2.508-513.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt J. M., Umbarger H. E. On the role of isoleucyl-tRNA synthetase in multivalent repression. Biochem Genet. 1972 Apr;6(2):99–118. doi: 10.1007/BF00486395. [DOI] [PubMed] [Google Scholar]
- FREUNDLICH M., BURNS R. O., UMBARGER H. E. Control of isoleucine, valine, and leucine biosynthesis. I. Multivalent repression. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1804–1808. doi: 10.1073/pnas.48.10.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield G. W., Burns R. O. Specific binding of leucyl transfer RNA to an immature form of L-threonine deaminase: its implications in repression. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1027–1035. doi: 10.1073/pnas.66.4.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaccarino M., Berg P. Isoleucine auxotrophy as a consequence of a mutationally altered isoleucyl-transfer ribonucleic acid synthetase. J Bacteriol. 1971 Feb;105(2):527–537. doi: 10.1128/jb.105.2.527-537.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovach J. S., Ballesteros A. O., Meyers M., Soria M., Goldberger R. F. A cis-trans test of the effect of the first enzyme for histidine biosynthesis on regulation of the histidine operon. J Bacteriol. 1973 Apr;114(1):351–356. doi: 10.1128/jb.114.1.351-356.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovach J. S., Berberich M. A., Venetianer P., Goldberger R. F. Repression of the histidine operon: effect of the first enzyme on the kinetics of repression. J Bacteriol. 1969 Mar;97(3):1283–1290. doi: 10.1128/jb.97.3.1283-1290.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovach J. S., Phang J. M., Ference M., Goldberger R. F. Studies on repression of the histidine operon. II. The role of the first enzyme in control of the histidine system. Proc Natl Acad Sci U S A. 1969 Jun;63(2):481–488. doi: 10.1073/pnas.63.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVIN A. P., HARTMAN P. E. ACTION OF A HISTIDINE ANALOGUE, 1,2,4-TRIAZOLE-3-ALANINE, IN SALMONELLA TYPHIMURIUM. J Bacteriol. 1963 Oct;86:820–828. doi: 10.1128/jb.86.4.820-828.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOYED H. S. False feedback inhibition: inhibition of tryptophan biosynthesis by 5-methyltryptophan. J Biol Chem. 1960 Apr;235:1098–1102. [PubMed] [Google Scholar]
- Pledger W. J., Umbarger H. E. Isoleucine and valine metabolism in Escherichia coli. XXII. A pleiotropic mutation affecting induction of isomeroreductase activity. J Bacteriol. 1973 Apr;114(1):195–207. doi: 10.1128/jb.114.1.195-207.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOMERVILLE R. L., YANOFSKY C. STUDIES ON THE REGULATION OF TRYPTOPHAN BIOSYNTHESIS IN ESCHERICHIA COLI. J Mol Biol. 1965 Apr;11:747–759. doi: 10.1016/s0022-2836(65)80032-8. [DOI] [PubMed] [Google Scholar]
- Szentirmai A., Umbarger H. E. Isoleucine and valine metabolism of Escherichia coli. XIV. Effect of thiaisoleucine. J Bacteriol. 1968 May;95(5):1666–1671. doi: 10.1128/jb.95.5.1666-1671.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UMBARGER H. E. Evidence for a negative-feedback mechanism in the biosynthesis of isoleucine. Science. 1956 May 11;123(3202):848–848. doi: 10.1126/science.123.3202.848. [DOI] [PubMed] [Google Scholar]
- Vogel T., Meyers M., Kovach J. S., Goldberger R. F. Specificity of interaction between the first enzyme for histidine biosynthesis and aminoacylated histidine transfer ribonucleic acid. J Bacteriol. 1972 Oct;112(1):126–130. doi: 10.1128/jb.112.1.126-130.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonder Haar R. A., Umbarger H. E. Isoleucine and valine metabolism in Escherichia coli. XIX. Inhibition of isoleucine biosynthesis by glycyl-leucine. J Bacteriol. 1972 Oct;112(1):142–147. doi: 10.1128/jb.112.1.142-147.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasmuth J. J., Umbarger H. E. Effect of isoleucine, valine, or leucine starvation on the potential for formation of the branched-chain amino acid biosynthetic enzymes. J Bacteriol. 1973 Nov;116(2):548–561. doi: 10.1128/jb.116.2.548-561.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasmuth J. J., Umbarger H. E. Participation of branched-chain amino acid analogues in multivalent repression. J Bacteriol. 1973 Nov;116(2):562–570. doi: 10.1128/jb.116.2.562-570.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasmuth J., Umbarger H. E., Dempsey W. B. A role for a pyridoxne derivative in the multivalent repression of the isoleucine and valine biosynthetic enzymes. Biochem Biophys Res Commun. 1973 Mar 5;51(1):158–164. doi: 10.1016/0006-291x(73)90522-6. [DOI] [PubMed] [Google Scholar]


